Understanding How Genetic Mutations Collaborate with Genomic Instability in Cancer
Abstract
1. Introduction
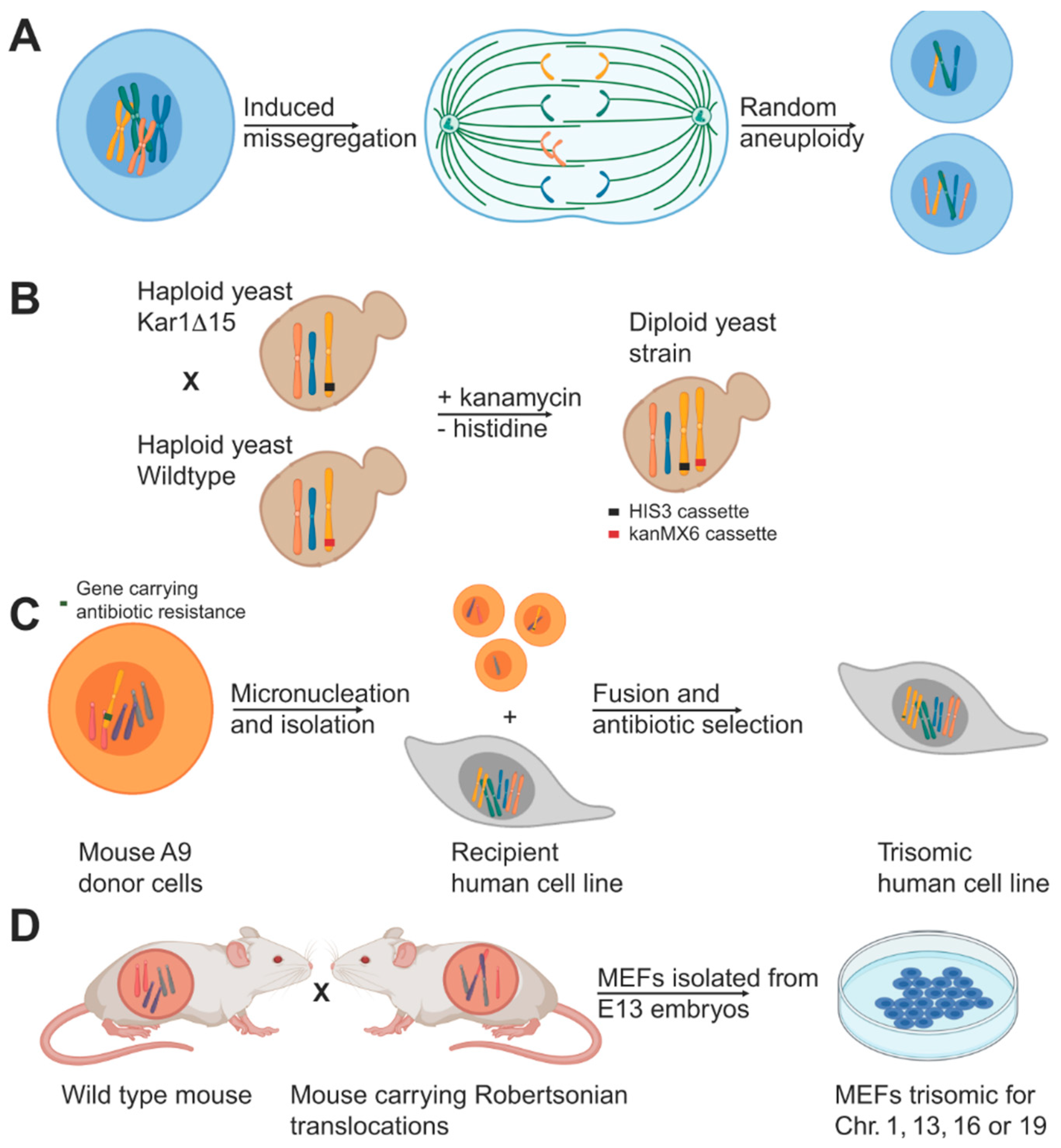
2. Model Systems for Stable Aneuploidy
2.1. Aneuploid Yeast Models
2.2. Stable Aneuploid Human Cell Lines
2.3. Stable Aneuploid Mouse Embryonic Fibroblasts
3. Models for Ongoing Chromosomal Instability
4. Early Findings from Model Systems
4.1. Gene Expression Changes
4.2. Proteotoxicity
4.3. Metabolic Stress
4.4. Inflammatory Response
5. Genetic Screens to Identify Cancer-Collaborating Hits
5.1. Chemical Mutagenesis
5.2. Retroviral Insertional Mutagenesis
5.3. Transposon Mutagenesis Screens
5.4. RNA interference Screens
5.5. CRISPR-Cas9 Screens
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Schukken, K.M.; Foijer, F. CIN and Aneuploidy: Different Concepts, Different Consequences. BioEssays 2017. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Abruzzo, M.; Adkins, K.; Griffin, D.; Merrill, M.; Millie, E.; Saker, D.; Shen, J.; Zaragoza, M. Human aneuploidy: Incidence, origin and etiology. Environ. Mol. Mutagen. 1996, 28, 167–175. [Google Scholar] [CrossRef]
- Torres, E.M.; Sokolsky, T.; Tucker, C.M.; Chan, L.Y.; Boselli, M.; Dunham, M.J.; Amon, A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 2007, 317, 916–924. [Google Scholar] [CrossRef]
- Williams, B.R.; Prabhu, V.R.; Hunter, K.E.; Glazier, C.M.; Whittaker, C.A.; Housman, D.E.; Amon, A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 2008, 322, 703–709. [Google Scholar] [CrossRef]
- Sheltzer, J.M.; Ko, J.H.; Replogle, J.M.; Passerini, V.; Storchova, Z.; Amon, A. Single-chromosome Gains Commonly Function as Tumor Suppressors. Cancer Cell 2017, 31, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Stingele, S.; Stoehr, G.; Peplowska, K.; Cox, J.; Mann, M.; Storchova, Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018. [Google Scholar] [CrossRef]
- Sheltzer, J.M.; Amon, A. The aneuploidy paradox: Costs and benefits of an incorrect karyotype. Trends Genet. 2011, 27, 446–453. [Google Scholar] [CrossRef]
- Fournier, R.E.K. A general high-efficiency procedure for production of microcell hybrids. Proc. Natl. Acad. Sci. USA 1981, 78, 6349–6353. [Google Scholar] [CrossRef] [PubMed]
- Saxon, P.J.; Srivatsan, E.S.; Stanbridge, E.J. Introduction of human chromosome 11 via microcell transfer controls tumorigenic expression of HeLa cells. EMBO J. 1986, 5, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Koi, M.; Morita, H.; Yamada, H.; Satoh, H.; Barrett, J.C.; Oshimura, M. Normal human chromosome 11 suppresses tumorigenicity of human cervical tumor cell line SiHa. Mol. Carcinog. 1989, 2, 12–21. [Google Scholar] [CrossRef]
- Dürrbaum, M.; Kuznetsova, A.Y.; Passerini, V.; Stingele, S.; Stoehr, G.; Storchová, Z. Unique features of the transcriptional response to model aneuploidy in human cells. BMC Genom. 2014, 15, 1–14. [Google Scholar] [CrossRef]
- Musacchio, A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015, 25, R1002–R1018. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Prifti, D.; Gui, P.; Liu, X.; Elowe, S.; Yao, X. Recent Progress on the Localization of the Spindle Assembly Checkpoint Machinery to Kinetochores. Cells 2019, 8, 278. [Google Scholar] [CrossRef]
- Hewitt, L.; Tighe, A.; Santaguida, S.; White, A.M.; Jones, C.D.; Musacchio, A.; Green, S.; Taylor, S.S. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J. Cell Biol. 2010, 190, 25–34. [Google Scholar] [CrossRef]
- Santaguida, S.; Tighe, A.; D’Alise, A.M.; Taylor, S.S.; Musacchio, A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 2010, 190, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Kastl, J.; Braun, J.; Prestel, A.; Möller, H.M.; Huhn, T.; Mayer, T.U. Mad2 Inhibitor-1 (M2I-1): A Small Molecule Protein-Protein Interaction Inhibitor Targeting the Mitotic Spindle Assembly Checkpoint. ACS Chem. Biol. 2015, 10, 1661–1666. [Google Scholar] [CrossRef]
- Resende, L.P.; Monteiro, A.; Brás, R.; Lopes, T.; Sunkel, C.E. Aneuploidy in intestinal stem cells promotes gut dysplasia in Drosophila. J. Cell Biol. 2018, 217, 3930–3946. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.; Coulter, J.; Woo, J.H.; Wilsbach, K.; Gabrielson, E. High levels of the Mps1 checkpoint protein are protective of aneuploidy in breast cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5384–5389. [Google Scholar] [CrossRef]
- Shi, Q.; Hu, M.; Luo, M.; Liu, Q.; Jiang, F.; Zhang, Y.; Wang, S.; Yan, C.; Weng, Y. Reduced expression of Mad2 and Bub1 proteins is associated with spontaneous miscarriages. Mol. Hum. Reprod. 2011, 17, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Basto, R.; Brunk, K.; Vinadogrova, T.; Peel, N.; Franz, A.; Khodjakov, A.; Raff, J.W. Centrosome Amplification Can Initiate Tumorigenesis in Flies. Cell 2008, 133, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Gogendeau, D.; Siudeja, K.; Gambarotto, D.; Pennetier, C.; Bardin, A.J.; Basto, R. Aneuploidy causes premature differentiation of neural and intestinal stem cells. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Levine, M.S.M.S.; Bakker, B.; Boeckx, B.; Moyett, J.; Lu, J.; Vitre, B.; Spierings, D.C.D.C.; Lansdorp, P.M.P.M.; Cleveland, D.W.D.W.; Lambrechts, D.; et al. Centrosome Amplification Is Sufficient to Promote Spontaneous Tumorigenesis in Mammals. Dev. Cell 2017, 40, 313–322.e5. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Genovese, G.; Compton, D. a Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr. Biol. 2009, 19, 1937–1942. [Google Scholar] [CrossRef]
- Pezic, D.; Weeks, S.L.; Hadjur, S. More to cohesin than meets the eye: Complex diversity for fine-tuning of function. Curr. Opin. Genet. Dev. 2017, 43, 93–100. [Google Scholar] [CrossRef]
- Schukken, K.M.; Lin, Y.-C.; Bakker, P.; Schubert, M.; Preuss, S.F.; Simon, J.E.; Van den Bos, H.; Storchova, Z.; Colome-Tatche, M.; Bastians, H.; et al. Altering microtubule dynamics is synergistically toxic with spindle assembly checkpoint inhibition. Life Sci. Alliance 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Tijhuis, A.E.; Johnson, S.C.; McClelland, S.E. The emerging links between chromosomal instability (CIN), metastasis, inflammation and tumour immunity. Mol. Cytogenet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.J.; Sharpe, Z.; Heng, H.H. Origins and consequences of chromosomal instability: From cellular adaptation to genome chaos-mediated system survival. Genes 2020, 1162. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, M.; Guilgur, L.G.; Tavares, A.; Passagem-Santos, D.; Oliveira, R.A. Induced aneuploidy in neural stem cells triggers a delayed stress response and impairs adult life span in flies. PLoS Biol. 2019, 17, e3000016. [Google Scholar] [CrossRef]
- Zhou, L.; Jilderda, L.J.; Foijer, F. Exploiting aneuploidy-imposed stresses and coping mechanisms to battle cancer. Open Biol. 2020, 10. [Google Scholar] [CrossRef]
- Foijer, F.; DiTommaso, T.; Donati, G.; Hautaviita, K.; Xie, S.Z.; Heath, E.; Smyth, I.; Watt, F.M.; Sorger, P.K.; Bradley, A. Spindle checkpoint deficiency is tolerated by murine epidermal cells but not hair follicle stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2928–2933. [Google Scholar] [CrossRef]
- Foijer, F.; Xie, S.Z.; Simon, J.E.; Bakker, P.L.; Conte, N.; Davis, S.H.; Kregel, E.; Jonkers, J.; Bradley, A.; Sorger, P.K. Chromosome instability induced by Mps1 and p53 mutation generates aggressive lymphomas exhibiting aneuploidy-induced stress. Proc. Natl. Acad. Sci. USA 2014, 111, 13427–13432. [Google Scholar] [CrossRef]
- Mao, R.; Zielke, C.L.; Ronald Zielke, H.; Pevsner, J. Global up-regulation of chromosome 21 gene expression in the developing down syndrome brain. Genomics 2003, 81, 457–467. [Google Scholar] [CrossRef]
- Foijer, F.; Albacker, L.A.; Bakker, B.; Spierings, D.C.; Yue, Y.; Xie, S.Z.; Davis, S.; Lutum-Jehle, A.; Takemoto, D.; Hare, B.; et al. Deletion of the MAD2L1 spindle assembly checkpoint gene is tolerated in mouse models of acute T-cell lymphoma and hepatocellular carcinoma. Elife 2017, 6. [Google Scholar] [CrossRef]
- Kahlem, P. Transcript Level Alterations Reflect Gene Dosage Effects Across Multiple Tissues in a Mouse Model of Down Syndrome. Genome Res. 2004, 14, 1258–1267. [Google Scholar] [CrossRef]
- Lyle, R. Gene Expression From the Aneuploid Chromosome in a Trisomy Mouse Model of Down Syndrome. Genome Res. 2004, 14, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Kazuki, Y.; Oshimura, M.; Ikeo, K.; Gojobori, T. Gene dosage imbalance of human chromosome 21 in mouse embryonic stem cells differentiating to neurons. Gene 2011, 481, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, N.; Rancati, G.; Zhu, J.; Bradford, W.D.; Saraf, A.; Florens, L.; Sanderson, B.W.; Hattem, G.L.; Li, R. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 2010, 468, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Vaites, L.P.; Wells, J.N.; Santaguida, S.; Paulo, J.A.; Storchova, Z.; Harper, J.W.; Marsh, J.A.; Amon, A. Protein aggregation mediates stoichiometry of protein complexes in aneuploid cells. Genes Dev. 2019, 33, 1031–1047. [Google Scholar] [CrossRef]
- Donnelly, N.; Passerini, V.; Dürrbaum, M.; Stingele, S.; Storchová, Z. HSF 1 deficiency and impaired HSP 90-dependent protein folding are hallmarks of aneuploid human cells. EMBO J. 2014. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Williams, B.R.; Siegel, J.J.; Amon, A. Identification of aneuploidy-selective antiproliferation compounds. Cell 2011, 144, 499–512. [Google Scholar] [CrossRef]
- Stingele, S.; Stoehr, G.; Storchova, Z. Activation of autophagy in cells with abnormal karyotype. Autophagy 2013, 9, 246–248. [Google Scholar] [CrossRef][Green Version]
- Oromendia, A.B.; Dodgson, S.E.; Amon, A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012, 26, 2696–2708. [Google Scholar] [CrossRef]
- Oromendia, A.B.; Amon, A. Aneuploidy: Implications for protein homeostasis and disease. DMM Dis. Model. Mech. 2014, 7, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.M.; Dephoure, N.; Panneerselvam, A.; Tucker, C.M.; Whittaker, C.A.; Gygi, S.P.; Dunham, M.J.; Amon, A. Identification of aneuploidy-tolerating mutations. Cell 2010. [Google Scholar] [CrossRef]
- Dodgson, S.E.; Kim, S.; Costanzo, M.; Baryshnikova, A.; Morse, D.L.; Kaiser, C.A.; Boone, C.; Amon, A. Chromosome-specific and global effects of aneuploidy in Saccharomyces cerevisiae. Genetics 2016, 202, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, S.E.; Santaguida, S.; Kim, S.; Sheltzer, J.; Amon, A. The pleiotropic deubiquitinase ubp3 confers aneuploidy tolerance. Genes Dev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Vasile, E.; White, E.; Amon, A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 2015, 29, 2010–2021. [Google Scholar] [CrossRef]
- Cai, Y.; Crowther, J.; Pastor, T.; Abbasi Asbagh, L.; Baietti, M.F.; De Troyer, M.; Vazquez, I.; Talebi, A.; Renzi, F.; Dehairs, J.; et al. Loss of Chromosome 8p Governs Tumor Progression and Drug Response by Altering Lipid Metabolism. Cancer Cell 2016, 29, 751–766. [Google Scholar] [CrossRef]
- Tang, Y.C.; Yuwen, H.; Wang, K.; Bruno, P.M.; Bullock, K.; Deik, A.; Santaguida, S.; Trakala, M.; Pfau, S.J.; Zhong, N.; et al. Aneuploid cell survival relies upon sphingolipid homeostasis. Cancer Res. 2017, 77, 5272–5286. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Gustafsson, H.T.; O’Sullivan, C.; Bisceglia, G.; Huang, X.; Klose, C.; Schevchenko, A.; Dickson, R.C.; Cavaliere, P.; Dephoure, N.; et al. Serine-Dependent Sphingolipid Synthesis Is a Metabolic Liability of Aneuploid Cells. Cell Rep. 2017, 21, 3807–3818. [Google Scholar] [CrossRef]
- Viganó, C.; Von Schubert, C.; Ahrné, E.; Schmidt, A.; Lorber, T.; Bubendorf, L.; De Vetter, J.R.F.; Zaman, G.J.R.; Storchova, Z.; Nigg, E.A. Quantitative proteomic and phosphoproteomic comparison of human colon cancer DLD-1 cells differing in ploidy and chromosome stability. Mol. Biol. Cell 2018, 29, 1031–1047. [Google Scholar] [CrossRef]
- Santaguida, S.; Richardson, A.; Iyer, D.R.; M’Saad, O.; Zasadil, L.; Knouse, K.A.; Wong, Y.L.; Rhind, N.; Desai, A.; Amon, A. Chromosome Mis-segregation Generates Cell-Cycle-Arrested Cells with Complex Karyotypes that Are Eliminated by the Immune System. Dev. Cell 2017, 41, 638–651.e5. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, K.J.; Carroll, P.; Martin, C.A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A.; et al. CGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017, 548, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G. cGAS produces a 2 ′ -5 ′ -linked cyclic dinucleotide second messenger that activates STING. Nature 2014, 498, 380–384. [Google Scholar] [CrossRef]
- Xia, T.; Konno, H.; Ahn, J.; Barber, G.N. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates with Tumorigenesis. Cell Rep. 2016, 14, 282–297. [Google Scholar] [CrossRef]
- Song, S.; Peng, P.; Tang, Z.; Zhao, J.; Wu, W.; Li, H.; Shao, M.; Li, L.; Yang, C.; Duan, F.; et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Hong, C.; Tijhuis, A.E.; Foijer, F. The cGAS Paradox: Contrasting Roles for cGAS-STING Pathway in Chromosomal Instability. Cells 2019, 8, 1228. [Google Scholar] [CrossRef]
- Russell, W.L.; Kelly, E.M.; Hunsicker, P.R.; Bangham, J.W.; Maddux, S.C.; Phipps, E.L. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc. Natl. Acad. Sci. USA 1979, 76, 5818–5819. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Arozena, A.; Wells, S.; Potter, P.; Kelly, M.; Cox, R.D.; Brown, S.D.M. ENU Mutagenesis, a Way Forward to Understand Gene Function. Annu. Rev. Genomics Hum. Genet. 2008, 9, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Brammeld, J.S.; Petljak, M.; Martincorena, I.; Williams, S.P.; Alonso, L.G.; Dalmases, A.; Bellosillo, B.; Robles-Espinoza, C.D.; Price, S.; Barthorpe, S.; et al. Genome-wide chemical mutagenesis screens allow unbiased saturation of the cancer genome & identification of drug resistance mutations. Genome Res. 2017, 27, 613–625. [Google Scholar]
- Shima, N.; Hartford, S.A.; Duffy, T.; Wilson, L.A.; Schimenti, K.J.; Schimenti, J.C. Phenotype-based identification of mouse chromosome instability mutants. Genetics 2003, 163, 1031–1040. [Google Scholar]
- Gaiano, N.; Amsterdam, A.; Kawakami, K.; Allende, M.; Becker, T.; Hopkins, N. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 1996, 383, 829–832. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003, 300, 1749–1751. [Google Scholar] [CrossRef]
- Dudley, J.P. Tag, you’re hit: Retroviral insertions identify genes involved in cancer. Trends Mol. Med. 2003, 9, 43–45. [Google Scholar] [CrossRef]
- Jonkers, J.; Berns, A. Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochim. Biophys. Acta 1996, 1287, 29–57. [Google Scholar] [CrossRef]
- Mikkers, H.; Berns, A. Retroviral insertional mutagenesis: Tagging cancer pathways. Adv. Cancer Res. 2003, 88, 53–99. [Google Scholar] [PubMed]
- Suzuki, T.; Shen, H.; Akagi, K.; Morse, H.C.; Malley, J.D.; Naiman, D.Q.; Jenkins, N.A.; Copeland, N.G. New genes involved in cancer identified by retroviral tagging. Nat. Genet. 2002, 32, 166–174. [Google Scholar] [CrossRef]
- Mikkers, H.; Allen, J.; Knipscheer, P.; Romeyn, L.; Hart, A.; Vink, E.; Berns, A. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat. Genet. 2002, 32, 153–159. [Google Scholar] [CrossRef]
- Lund, A.H.; Turner, G.; Trubetskoy, A.; Verhoeven, E.; Wientjens, E.; Hulsman, D.; Russell, R.; DePinho, R.A.; Lenz, J.; Van Lohuizen, M. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat. Genet. 2002, 32, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, V.; Kimm, M.A.; Boer, M.; Wessels, L.; Theelen, W.; Jonkers, J.; Hilkens, J. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer. Nat. Genet. 2007, 39, 759–769. [Google Scholar] [CrossRef]
- Goodier, J.L.; Davidson, W.S. Tc1 Transposon-like Sequences are Widely Distributed in Salmonids. J. Mol. Biol. 1994, 241, 26–34. [Google Scholar] [CrossRef]
- Ivics, Z.N.; Hackett, P.B.; Plasterk, R.H.; Izsvá, Z. Molecular Reconstruction of Sleeping Beauty, a Tc1-like Transposon from Fish, and Its Transposition in Human Cells its original location and promotes its reintegration else- where in the genome (Plasterk, 1996). Autonomous mem- bers of a transposon. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef]
- Luo, G.; Ivics, Z.; Izsvák, Z.; Bradley, A. Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 1998, 95, 10769–10773. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wu, X.; Li, G.; Han, M.; Zhuang, Y.; Xu, T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 2005, 122, 473–483. [Google Scholar] [CrossRef]
- Rad, R.; Rad, L.; Wang, W.; Cadinanos, J.; Vassiliou, G.; Rice, S.; Campos, L.S.; Yusa, K.; Banerjee, R.; Li, M.A.; et al. PiggyBac transposon mutagenesis: A tool for cancer gene discovery in mice. Science 2010. [Google Scholar] [CrossRef]
- Fraser, M.J.; Ciszczon, T.; Elick, T.; Bauser, C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol. Biol. 1996, 5, 141–151. [Google Scholar] [CrossRef]
- Collier, L.S.; Carlson, C.M.; Ravimohan, S.; Dupuy, A.J.; Largaespada, D.A. Largaespada Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 2005, 436, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.J.; Akagi, K.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005, 436, 221–226. [Google Scholar] [CrossRef]
- Dupuy, A.J.; Rogers, L.M.; Kim, J.; Nannapaneni, K.; Starr, T.K.; Liu, P.; Largaespada, D.A.; Scheetz, T.E.; Jenkins, N.A.; Copeland, N.G. A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res. 2009, 69, 8150–8156. [Google Scholar] [CrossRef]
- Starr, T.K.; Sarver, A.L.; Bergemann, T.L.; Gupta, M.; Sullivan, M.G.O.; Asmann, Y.W.; Thibodeau, S.N.; Tessarollo, L.; Copeland, N.G. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 2009, 323, 1747–1750. [Google Scholar] [CrossRef]
- Keng, V.W.; Villanueva, A.; Chiang, D.Y.; Dupuy, A.J.; Ryan, B.J.; Matise, I.; Silverstein, K.A.T.; Sarver, A.; Starr, T.K.; Akagi, K.; et al. A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nat. Biotechnol. 2009, 27, 264–274. [Google Scholar] [CrossRef]
- O’Donnell, K.A. Advances in functional genetic screening with transposons and CRISPR/Cas9 to illuminate cancer biology. Curr. Opin. Genet. Dev. 2018, 49, 85–94. [Google Scholar] [CrossRef]
- Mann, M.B.; Jenkins, N.A.; Copeland, N.G.; Mann, K.M. Sleeping Beauty mutagenesis: Exploiting forward genetic screens for cancer gene discovery. Curr. Opin. Genet. Dev. 2014, 24, 16–22. [Google Scholar] [CrossRef]
- Beckmann, P.J.; Largaespada, D.A. Transposon insertion mutagenesis in mice for modeling human cancers: Critical insights gained and new opportunities. Int. J. Mol. Sci. 2020, 21, 1172. [Google Scholar] [CrossRef]
- Moriarity, B.S.; Otto, G.M.; Rahrmann, E.P.; Rathe, S.K.; Wolf, N.K.; Weg, M.T.; Manlove, L.A.; Larue, R.S.; Temiz, N.A.; Molyneux, S.D.; et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat. Genet. 2015, 47, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Wei, Z.; Koso, H.; Rust, A.G.; Yew, C.C.K.; Mann, M.B.; Ward, J.M.; Adams, D.J.; Copeland, N.G.; Jenkins, N.A. Transposon mutagenesis identifies genes and evolutionary forces driving gastrointestinal tract tumor progression. Nat. Genet. 2015, 47, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Rangel, R.; Lee, S.C.; Ban, K.H.K.; Guzman-Rojas, L.; Mann, M.B.; Newberg, J.Y.; Kodama, T.; McNoe, L.A.; Selvanesan, L.; Ward, J.M.; et al. Transposon mutagenesis identifies genes that cooperate with mutant Pten in breast cancer progression. Proc. Natl. Acad. Sci. USA 2016, 113, E7749–E7758. [Google Scholar] [CrossRef] [PubMed]
- Chapeau, E.A.; Gembarska, A.; Durand, E.Y.; Mandon, E.; Estadieu, C.; Romanet, V.; Wiesmann, M.; Tiedt, R.; Lehar, J.; De Weck, A.; et al. Resistance mechanisms to TP53-MDM2 inhibition identified by in vivo piggyBac transposon mutagenesis screen in an Arf−/−mouse model. Proc. Natl. Acad. Sci. USA 2017, 114, 3151–3156. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef]
- Abubaker, S.; Abdalla, S.; Mahmud, S.; Wilkie, B. Antiviral innate immune response of RNA interference. J. Infect. Dev. Ctries. 2014, 8, 804–810. [Google Scholar]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef]
- Ameres, S.L.; Martinez, J.; Schroeder, R. Molecular Basis for Target RNA Recognition and Cleavage by Human RISC. Cell 2007, 130, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Siomi, H.; Siomi, M.C. On the road to reading the RNA-interference code. Nature 2009, 457, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Brummelkamp, T.R.; Bernards, R.; Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002, 296, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Fellmann, C.; Lowe, S.W. Stable RNA interference rules for silencing. Nat. Cell Biol. 2014, 16, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Pipkin, M.E. In vivo RNAi screens: Concepts and applications. Trends Immunol. 2015, 36, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Saraç, H.; Morova, T.; Pires, E.; McCullagh, J.; Kaplan, A.; Cingöz, A.; Bagci-Onder, T.; Önder, T.; Kawamura, A.; Lack, N.A. Systematic characterization of chromatin modifying enzymes identifies KDM3B as a critical regulator in castration resistant prostate cancer. Oncogene 2020, 39, 2187–2201. [Google Scholar] [CrossRef]
- Bailey, M.L.; Singh, T.; Mero, P.; Moffat, J.; Hieter, P. Dependence of Human Colorectal Cells Lacking the FBW7 Tumor Suppressor on the Spindle Assembly Checkpoint. Genetics 2015, 201, 885–895. [Google Scholar] [CrossRef]
- Bric, A.; Miething, C.; Bialucha, C.U.; Scuoppo, C.; Zender, L.; Krasnitz, A.; Xuan, Z.; Zuber, J.; Wigler, M.; Hicks, J.; et al. Functional Identification of Tumor-Suppressor Genes through an In Vivo RNA Interference Screen in a Mouse Lymphoma Model. Cancer Cell 2009, 16, 324–335. [Google Scholar] [CrossRef]
- Wang, X.; Fredericksen, Z.S.; Vierkant, R.A.; Kosel, M.L.; Pankratz, V.S.; Cerhan, J.R.; Justenhoven, C.; Brauch, H.; Olson, J.; GENICA Consortium; et al. Association of genetic variation in mitotic kinases with breast cancer risk. Breast Cancer Res. Treat. 2010, 119, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, Z.; Wong, H.W.S.; Nicolson, S.; Saint, R.B.; Gregory, S.L. A Screen for Selective Killing of Cells with Chromosomal Instability Induced by a Spindle Checkpoint Defect. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Kwon, M.; Godinho, S.A.; Chandhok, N.S.; Ganem, N.J.; Azioune, A.; Thery, M.; Pellman, D. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008, 22, 2189–2203. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Dusko Ehrlich, S. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.J.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.H.; Snijders, A.P.L.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; Van der Oost, J. Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pinera, P.; Kocak, D.D.; Vockley, C.M.; Adler, A.F.; Kabadi, A.M.; Polstein, L.R.; Thakore, P.I.; Glass, K.A.; Ousterout, D.G.; Leong, K.W.; et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 2013, 10, 973–976. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Kurata, M.; Yamamoto, K.; Moriarity, B.S.; Kitagawa, M.; Largaespada, D.A. CRISPR/Cas9 library screening for drug target discovery. J. Hum. Genet. 2018, 63, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Thu, K.L.; Silvester, J.; Elliott, M.J.; Ba-Alawi, W.; Duncan, M.H.; Elia, A.C.; Mer, A.S.; Smirnov, P.; Safikhani, Z.; Haibe-Kains, B.; et al. Disruption of the anaphase-promoting complex confers resistance to TTK inhibitors in triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E1570–E1577. [Google Scholar] [CrossRef]
- Chen, S.; Sanjana, N.E.; Zheng, K.; Shalem, O.; Lee, K.; Shi, X.; Scott, D.A.; Song, J.; Pan, J.Q.; Weissleder, R.; et al. Genome-wide CRISPR Screen in a Mouse Model of Tumor Growth and Metastasis. Cell 2015, 160, 1246–1260. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Boettcher, M.; McManus, M.T. Choosing the Right Tool for the Job: RNAi, TALEN, or CRISPR. Mol. Cell 2015, 58, 575–585. [Google Scholar] [CrossRef]
- Simon, J.; Bakker, B.; Foijer, F. CINcere modelling: What have mouse models for chromosome instability taught us? Results Cancer Res. 2015, 200, 39–60. [Google Scholar]
- Schvartzman, J.-M.; Sotillo, R.; Benezra, R. Mitotic chromosomal instability and cancer: Mouse modelling of the human disease. Nat. Rev. Cancer 2010, 10, 102–115. [Google Scholar] [CrossRef]
- Hoevenaar, W.H.M.; Janssen, A.; Quirindongo, A.I.; Ma, H.; Klaasen, S.J.; Teixeira, A.; Van Gerwen, B.; Lansu, N.; Morsink, F.H.M.; Offerhaus, G.J.A.; et al. Degree and site of chromosomal instability define its oncogenic potential. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Baker, D.J.; Jin, F.; Jeganathan, K.B.; Van Deursen, J.M. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell 2009, 16, 475–486. [Google Scholar] [CrossRef]
- Shoshani, O.; Bakker, B.; Wang, Y.; Kim, D.H.; Maldonado, M.; Demarest, M.A.; Artates, J.; Zhengyu, O.; Mark, A.; Wardenaar, R.; et al. Transient genomic instability drives tumorigenesis through accelerated clonal evolution. bioRxiv 2020. [Google Scholar] [CrossRef]
| Mutagenesis System | Advantages | Disadvantages |
|---|---|---|
| Chemical |
|
|
| Retrovirus |
|
|
| Transposon |
|
|
| RNA interference |
|
|
| CRISPR-Cas9 |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jilderda, L.J.; Zhou, L.; Foijer, F. Understanding How Genetic Mutations Collaborate with Genomic Instability in Cancer. Cells 2021, 10, 342. https://doi.org/10.3390/cells10020342
Jilderda LJ, Zhou L, Foijer F. Understanding How Genetic Mutations Collaborate with Genomic Instability in Cancer. Cells. 2021; 10(2):342. https://doi.org/10.3390/cells10020342
Chicago/Turabian StyleJilderda, Laura J., Lin Zhou, and Floris Foijer. 2021. "Understanding How Genetic Mutations Collaborate with Genomic Instability in Cancer" Cells 10, no. 2: 342. https://doi.org/10.3390/cells10020342
APA StyleJilderda, L. J., Zhou, L., & Foijer, F. (2021). Understanding How Genetic Mutations Collaborate with Genomic Instability in Cancer. Cells, 10(2), 342. https://doi.org/10.3390/cells10020342






