Abstract
Surgery remains an essential therapeutic approach for most solid malignancies. Although for more than a century accumulating clinical and experimental data have indicated that surgical procedures themselves may promote the appearance and progression of recurrent and metastatic lesions, only in recent years has renewed interest been taken in the mechanism by which metastasizing of cancer occurs following operative procedures. It is well proven now that surgery constitutes a risk factor for the promotion of pre-existing, possibly dormant micrometastases and the acceleration of new metastases through several mechanisms, including the release of neuroendocrine and stress hormones and wound healing pathway-associated immunosuppression, neovascularization, and tissue remodeling. These postoperative consequences synergistically facilitate the establishment of new metastases and the development of pre-existing micrometastases. While only in recent years the role of the peripheral nervous system has been recognized as another contributor to cancer development and metastasis, little is known about the contribution of tumor-associated neuronal and neuroglial elements in the metastatic disease related to surgical trauma and wound healing. Specifically, although numerous clinical and experimental data suggest that biopsy- and surgery-induced wound healing can promote survival and metastatic spread of residual and dormant malignant cells, the involvement of the tumor-associated neuroglial cells in the formation of metastases following tissue injury has not been well understood. Understanding the clinical significance and underlying mechanisms of neuroimmune regulation of surgery-associated metastasis will not only advance the field of neuro–immuno–oncology and contribute to basic science and translational oncology research but will also produce a strong foundation for developing novel mechanism-based therapeutic approaches that may protect patients against the oncologically adverse effects of primary tumor biopsy and excision.
1. Introduction
About 1.9 million new cancer cases are projected to occur in the USA and almost 20 million cases worldwide in 2021 [1,2]. It was estimated earlier that almost fifty percent of malignant tumors in humans might metastasize prior to clinical presentation [3,4], although the number may be lower and is certainly cancer dependent. While the 5-year survival rate for all cancers combined has increased substantially since the early 1960s, from ~40% to ~70%, outcomes for patients with metastatic cancer have largely remained stagnant [1,5]. Approximately 70–90% of all cancer-related deaths are due to metastases [6,7]. Importantly, observations of accelerated metastatic tumor growth after excision of the primary tumor have been repeatedly reported for several cancers, including colorectal, ovarian, NSCLC, breast, pancreatic, and other cancers (Table 1) [8,9,10,11,12,13,14,15,16,17]. Even the incisional biopsies, including the diagnostic core needle biopsy, might increase local tumor recurrence, lymph node metastases, and distant metastases [18,19,20,21]. However, our understanding of the cellular and molecular pathways of this phenomenon is still limited. Although accumulating evidence unequivocally demonstrates that an intratumoral cellular network actively supports metastatic dissemination of malignant cells [22], clinical data suggest that not all stromal elements and factors regulating migratory and invasive potential of malignant cells have been elucidated. This markedly limits the development and comparative testing of innovative antimetastatic approaches. It is increasingly critical to understand the principles of surgery-induced tumor progression and dissemination to conceive perioperative or adjuvant strategies to further improve long-term tumor control [23]. Understanding the mechanisms by which cancer progression is accelerated as a result of surgery may also provide pharmacologic interventions [24].

Table 1.
Observations of accelerated postoperative metastatic tumor growth.
Tumor-associated immune cells, fibroblasts, epithelial cells, pericytes, and adipocytes can enhance metastasis by regulating extracellular matrix remodeling and neovascularization, and by promoting the epithelial-mesenchymal transition of the malignant cells [22,59]. Recently, intratumoral neurofilaments have been recognized as important constituents of the tumor milieu [60,61,62], and the degree of tumor innervation has been correlated with metastases and patient survival [63,64,65], Although the involvement of neurotransmitters and neuropeptides in nerve-mediated metastases has been proposed, the role of the neuroglia of the peripheral nervous system (PNS) in promoting metastases of solid tumors remains unconsidered. With the exception of perineural invasion (i.e., locoregional invasion of cancer into the space surrounding a nerve), the extent to which the Schwann cells, the principal glia of the PNS, participate in the formation of metastases has not been elucidated. A recent surge in studies of Schwann cell biology has revealed their expansive functions in neurodegenerative diseases, pain syndrome, autoimmune and inflammatory neuropathies, nerve and tissue repair, tissue regeneration, and cell-based therapy for spinal cord injury and autoimmune neurological diseases. However, the pro-metastatic activity of Schwann cells during surgery/biopsy-associated wound healing has not yet been considered.
New data demonstrates that nerves/Schwann cells are present in human and animal tumor specimens, and that nerves/Schwann cells may accelerate tumor growth and progression in mouse tumor models [66,67,68,69,70,71]. It is conceivable to suggest that this effect is due to cancer cell-Schwann cell-nerve cell crosstalk, which induces the so-called “repair-like” phenotype of Schwann cells and results in: (i) attraction and activation of immune regulatory cells, (ii) reorganization of the extracellular matrix, and (iii) enhanced migratory and invasive potential of malignant cells. New in vivo data suggest that these consequences of tumor-nerve-Schwann cell interactions might increase cancer’s metastatic potential. We can speculate that neuroglial failure occurs when adaptive strategies developed by tumor-activated Schwann cells fail, and, in some settings, when the program associated with Schwann cell-driven tissue repair culminates in a maladaptive response that contributes to metastatic disease. It is possible that tumor-associated “repair-like” Schwann cells promote metastasis during surgery/biopsy-associated wound healing even more strongly since surgical stress and wound healing pathways contribute to the higher level of Schwann cell activation, dedifferentiation, and proliferation in the regenerating tumor microenvironment. However, this has never been experimentally tested.
2. The Neuronal Regulation of Tumor Progression
The crosstalk between malignant cells and the stromal and infiltrating cells is fundamental in the tumorigenesis process. Nerve endings are also detected within solid tumors [72,73]. Their communication with tumor cells is believed to represent so-called “neuro-neoplastic synapses” or “tumor-nervous connections” [74,75]. The role of the nerve filaments seen within the tumor mass was first believed to be mechanical, providing “paths” for the migration of the perineural invading cells [76,77]. Perineural invasion, also called perineural spread or neurotropic carcinomatous spread, is malignant cell invasion in, around, and through nerves, which is histologically observed as cancer cells within the layers of the nerve sheath including epineurium, perineurium, and endoneurium. However, it is now clear that the PNS, as a functionally pertinent association of cells and factors at the tumor milieu, regulates tumor development, growth, and dissemination [75,78,79]. Neurons within and at the tumor periphery release neurotransmitters, neuropeptides, and other biologically active substances acting on specific receptors on cancerous cells, stromal elements, and infiltrating immune cells, and altering cell function and cellular interactions in the tumor milieu.
A growing body of evidence shows that cancerous cells can utilize the benefit of the factors released by the nerves to generate a positive environment for survival, proliferation, and spreading [64,80,81,82]. Neural-related factors can alter the progression of metastasis, affecting the base membranes’ degradation and cancerous cell invasiveness, motility, extravasation, and colonization. They also modulate angiogenesis, the tumor stroma, immune cell functions, bone marrow activity, and local and systemic inflammatory pathways to impact metastases [74,83]. These neurotransmitters, neurotrophins, and neuropeptides include acetylcholine, catecholamines, γ-aminobutyric acid, serotonin, substance P, neurokinin A, bombesin, neuropeptide Y, vasoactive intestinal polypeptide, opioids, neurotensin, and other neuromodulating molecules. For instance, activation of β-adrenoceptors on tumor cells and tumor-associated macrophages can promote metastasis in animal models of breast, pancreatic, colon, neuroblastoma, ovarian, and prostate cancers [64,65,84,85,86,87]. Interestingly, in the head and neck cancer model, the crosstalk between malignant cells and neurons represents a mechanism by which tumor-associated neurons can be reprogrammed towards an adrenergic phenotype that augments tumor progression [88]. Although impediment of signaling pathways of the sympathetic nervous system with β-blockers or genetic deletion of β-adrenergic receptors primarily terminates metastasis of different types of tumors, α-adrenergic receptors’ function in cancer metastasis is yet to be clarified [89]. Acetylcholine secreted by tumor-infiltrating nerves from the parasympathetic nervous system was reported to target stromal cells expressing muscarinic receptors and to play a role in modulating tumor cell invasion and migration [64]. Neuropeptide Y, which can be released from sympathetic neurons, is generally accepted to be a powerful angiogenic factor [90]. Substance P and its receptors, at least in breast cancer, have been implicated in bone marrow metastasis formation [91]. Methionine-enkephalin expression in colorectal carcinomas may be associated with nodal and liver metastasis [92], while expression of bombesin/gastrin-releasing peptide in prostate cancer may be related to the lymph node metastases [93].
Although a broad understanding of the complex and multifaceted tumor-regulating role of neuronal factors has been reached [75,94], little is known about the role of tumor innervation in metastasis development in response to surgery and therapy. This gap in our knowledge is important because understanding the role of the PNS in cancer progression and metastasis formation after surgical procedures (tumor biopsy or resection) has a high significance in the clinical management of patients with cancer. In spite of exciting evidence that the removal of nerves from the tumor microenvironment is sufficient to terminate or decelerate disease progression, chemical or surgical denervation is unlikely to be of clinical use [79]. On the other hand, novel clinical studies emphasize cancer patient vulnerability to disease recurrence and metastasis formation following diagnostic and surgical interventions: for instance, excisional or incisional biopsy. The potential magnitude of perioperative vulnerability is underscored by the fact that greater than 60% of nearly 18–20 million patients diagnosed with cancer each year worldwide will require surgical resection [95]. As such, any opportunity to abrogate the risk of cancer progression arising during or after the vulnerable perioperative period could provide substantial benefit to patients. Thus, understanding the role of the PNS in surgery-induced metastasis should support the development of novel clinical approaches to reduce or limit metastasis formation.
3. Tissue Damage and Wounding Affect Tumor Progression
For more than a century accumulating clinical and experimental data have indicated that surgical procedures themselves may promote the appearance and progression of malignant lesions (Table 1) [25,29,30,36,40,42,96]. For example, investigations bearing on the occurrence of metastases in mice from which implanted tumors had been removed by operation were made by Clunet at the beginning of the last century [97,98]. Excision of the primary tumor mass might involve obvious dangers because it can release malignant cells into the systemic circulation or lymphatics [99,100,101], up-regulate expression of angiogenic and growth factors [26,102,103,104,105], and inhibit cell-mediated immunity [106,107,108]. The cytokine cascade activated in response to surgical trauma consists of a complex biochemical network with diverse effects, which are essential for wound healing, on the injured host [109,110]. Together, these postoperative outcomes have been suggested to synergistically accelerate the establishment of new distant metastases and the advancement of pre-existing micrometastases [111].
Biopsy remains the gold standard for the diagnosis of many cancers. However, this approach does not address the presence of microscopic residual or “in-transit” malignant cells, which may give rise to metastatic foci [112,113,114,115]. For instance, in patients with residual melanoma after the initial biopsy, 5-year survival is significantly lower, and recurrence is documented in ~25% of patients, with distant metastases being the most common form (40%) [20,53]. Importantly, observations of accelerated metastatic tumor growth after excision of the primary tumor have been repeatedly reported for colorectal, ovarian, lung, breast, gastric, bladder, testicular, and pancreatic cancers (Table 1) [8,9,10,11,12,13,14,15,16,17,31,40,50,54,116,117,118]. Even when complete locoregional control was thought to have been achieved, postoperative disease recurrence occurred in up to one-third of patients [119].
The local tissue response to surgery and biopsy involves the initiation of wound healing, which includes inflammation, neuronal regeneration, extracellular matrix reorganization, and neoangiogenesis (Figure 1). At the same time, these processes are known to be associated with the promotion of tumor growth and metastasis [16,30,36,42,109,120]. For instance, in a zebrafish larval model of Ras(G12V)-driven neoplasia, wound-associated neutrophils increase the proliferation of pre-neoplastic cells, and chronic wounding leads to a higher incidence of melanoma [121]. Furthermore, tumor growth is enhanced in healing wounds but not in the surrounding normal tissues: the probability of a tumor cell leading to a deposit in a wound is increased 1000-fold compared to normal tissue [42,103]. The degree of surgical tissue wounding correlates with the metastatic disease burden in mouse models of colon and breast cancers [48,122], and surgery-induced immune regulatory cells, such as MDSC and Treg cells, accelerate the formation of pulmonary metastases [123]. Macrophages have also been shown to contribute both to post-surgical tumor relapse and growth of metastases, probably by stimulating a population of tumor-initiating cells [52]. Postoperative NK-cell suppression has been reported to correlate with augmented metastatic burden in animal models; in cancer patients, reduced NK-cell activity during the postoperative period has been associated with a high rate of disease recurrence and mortality [49,124,125]. Furthermore, surgery-induced inflammation may potentiate colon cancer stem cell involvement in the metastatic process [126].
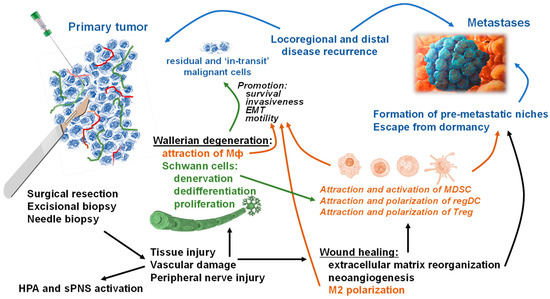
Figure 1.
Pathways and mechanisms of accelerated locoregional and distant tumor recurrence after surgical interventions on primary tumors. Resection and biopsy cause unavoidable tissue distraction, damage of blood and lymph vasculature, and injury and trauma of the peripheral neurons. Central systemic effects of surgical stress, together with its psychological components, result in the hypothalamic-pituitary-adrenal (HPA) axis activation and stimulation of the sympathetic branch of the peripheral nervous system (sPNS). Locally, tissue injury and regional hemorrhage, followed by different levels of hypoxemic cellular stress, inflammation, and ischemia, initiate a cascade of tissue repair pathways including wound healing and Wallerian degeneration. The wound healing, after the initial hemostasis and inflammation phases, incorporates extracellular matrix reorganization for remaking new tissue, neoangiogenesis/lymphogenesis for a new network of blood/lymph vessels, and attraction and polarization of regulatory immune cells, such as macrophages (alternatively activated type 2 or M2), for resolving inflammation and augmentation of tissue and vasculature restoration. These pathways phenotypically and functionally resemble the tumor microenvironment characteristics and thus may promote reactivation of dormant malignant cells, the formation of premetastatic niches and the survival and motility of residual and “in-transit” cancerous cells. Wallerian degeneration, prompted by the axonal injury, involves Schwann cell activation–denervation, de-differentiation, and proliferation (‘repair’ phenotype), which is required for the attraction of macrophages (Mф), cleaning the myelin and dead neuronal debris, and axonal regeneration. Resent data revealed that the repair Schwann cells functionally resemble the tumor-activated Schwann cells that can attract and activate myeloid-derived suppressor cells (MDSC), attract conventional dendritic cells (DC) and polarize them into regulatory immunosuppressive DC (regDC), and attract and polarize T cells into the regulatory phenotype (Treg). This pathway also supports local and systemic tumor-associated environments, which favor the establishment and growth of local and distant micrometastases. In addition, activated Schwann cells have been reported to induce the epithelial-mesenchymal transition (EMT) of malignant cells suggesting that tumor pre-activated, as well as axon injury-activated, Schwann cells may boost motility and invasiveness of residual and “in-transit” cancerous cells supporting the formation of the locoregional and distant metastases after surgical excision of primary tumors. (Cancerous cells and their migratory pathways are shown in blue font and blue arrows; immune cells and their effects on malignant cells are shown in brown font and brown arrows; neuroglial Schwann cells and their effects on immune and malignant cells are shown in green font and green arrows. Common pathways connected with primary tumor invasive procedures and tissue injury and repair, and their influences on malignant, neuroglial, and immune cells are shown in black font and black arrows.).
These clinical and experimental data suggest that biopsy or surgery-induced wound healing can accelerate the growth of pre-existing micrometastases (i.e., escape from dormancy) or promote survival and invasiveness of residual cancerous cells leading to metastasis [16,127]. Determining mechanisms of cancer spread during wound healing and understanding how peri- and postoperative care should be adapted to reduce the risk of local and distal disease recurrence is an important clinical issue [127,128]. This knowledge is pivotal for the development of therapeutic strategies to prevent or reduce surgery-associated metastasis.
Recently, peripheral nerves and neuroglia have also been implicated in wound healing and cancer spreading [129,130]. For instance, tumor progression after surgical procedures has, in part, been linked to high levels of β-adrenoreceptor signaling [10,48,131]. However, the mechanisms of nerve/glia involvement in an accelerated metastatic disease after excision of the primary tumor have never been explored.
4. PNS Functioning in Surgery-Associated Metastasis Formation
Surgical resection is still the most valuable procedure to eliminate primary tumors and involved lymph nodes. However, residual cancerous cells may remain after surgery, and the tumor dislodged during excision may spread via lymphovascular vessels [132,133]. For instance, identification of tumor cells in the blood and peritoneal lavage fluid after surgery has been linked with significantly shorter disease-free survival in patients with colorectal cancer [132]. Similarly, circulating tumor cell numbers expand following surgery for gastric, lung, breast, and hepatocellular cancers and are associated with poor survival [55,57,58,134]. In addition, postoperative wound healing may lead to locoregional and distal disease recurrence by accelerating the growth of pre-existing micrometastases (i.e., escape from dormancy) or by enabling residual tumor cells to spread faster (Figure 1) [16,127,135]. Furthermore, surgery-induced or anesthesia-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system may facilitate metastasis through the release of neuroendocrine mediators such as catecholamines and prostaglandins, which, in turn, increase immunosuppressive cytokines (e.g., IL-4, IL-10, TGF-β) and VEGF, as well as proinflammatory cytokines (e.g., IL-6 and IL-8), which promote tumor angiogenesis and spreading or creation of the pre-metastatic niches [23,136,137,138,139]. While necessary for normal tissue repair, the local and systemic changes of the innate and adaptive immune responses are able to inspire cancerous cell proliferation, migration, and survival, as well as intratumoral angiogenesis and extravasation of disseminating malignant cells [140]. In addition to the immune cells in the tumor milieu, neurons are now known to be involved in the complex interactions between the formation of cancers, inflammation, wound healing, and metastasis formation.
Surgical tumor removal is commonly associated with a traumatic injury of neuronal axons within the resected tumor or within the surrounding or peripheral tumor tissue. Following peripheral nerve injury, a cascade of molecular and cellular events is initiated at the proximal and distal ends of the lesion site. The distal end is disconnected from the neural body and undergoes Wallerian degeneration [141,142]. Schwann cells (or neurolemmocytes), the principal glial cells of the PNS [143], play a crucial role in the repair of peripheral nerves and represent the major players providing a specialized local extracellular microenvironment for regrowth of injured axons and rebuilding of myelin sheaths on regenerating axons. Distal Schwann cells denervate, i.e., detach from the axon, break down their myelin sheaths, and attract and polarize macrophages to remove axonal and myelin debris [144]. This is associated with Schwann cell de-differentiation/transdifferentiation, proliferation, and production of neurotrophic factors (“repair” Schwann cells) that support and guide the re-growing axon [145,146].
In addition to neurotrauma, new findings suggest that injury can activate a repair program in adult Schwann cells [147], which promotes the repair and regeneration of different tissues [148,149]. Recognition of versatile Schwann cell functions allows us to speculate that tissue wounding, induced by tumor excision or biopsy, may augment the ‘repair’ phenotype of Schwann cells, which is associated with the promotion of malignant cell motility, invasiveness, and metastasis (Figure 1). Interestingly, it was recently discovered that the local neurodegenerative response to cutaneous melanoma growth is quite similar to the neuronal repair pathway induced by skin injury [70,150]. Specifically, genetic, molecular, phenotypic, and functional similarities between tumor-activated, or “repair-like” Schwann cells at the tumor site and nerve injury-induced “repair” Schwann cells were revealed. Furthermore, a new unpublished work has revealed that ex vivo generated tumor-activated and resident PNS-injury-induced (“repair”) Schwann cells accelerated tumor growth and formation of metastases in vivo, while the absence of Schwann cells significantly decelerated tumor growth and metastasis (Y.Bunimovich, UPMC, personal communication). These results, as well as an almost ubiquitous presence of Schwann cells throughout the body, raise an important question: How are Schwann cells involved in the adjustment of the tumor environment after surgery/biopsy? Which mechanisms of tumor-Schwann-immune cell interactions that promote metastasis during wound healing may be involved in this phenomenon? Both in vitro and in vivo studies are needed to answer these questions and uncover the role of neuronal and neuroglial cells in the regulation of metastasis formation after surgical removal of primary tumor tissue.
Interestingly, a novel, recently reported findings demonstrates that Schwann cells can accelerate metastasis in several cancer models [67,68,69]. Although the main function of Schwann cells is to maintain axonal integrity, Schwann cells have been shown to stimulate pancreatic and prostate cancer cell invasion in an integrin-dependent manner and to promote perineural invasion via neural cell adhesion molecule 1 (NCAM1) signaling [151,152]. Moreover, tumor-activated Schwann cells have been shown to activate the CXCL5/CXCR2/PI3K/AKT/GSK-3β/Snail-Twist pathway to promote the epithelial-to-mesenchymal transition, motility, invasiveness, and metastatic potential of lung cancer cells both in vitro and in vivo in immunocompetent mice [68]. In addition to this direct effect of Schwann cells on tumor cells, new data have revealed that in the tumor microenvironment, Schwann cells display a strong ability to chemoattract immature myeloid cells and conventional dendritic cells (DC) and to polarize them into MDSC and regulatory DC that express robust immunosuppressive properties. [69,150] Our new data demonstrate that in addition to affecting myeloid regulatory cells, tumor-activated Schwann cells can also chemoattract T cells and up-regulate their exhaustion phenotype (Shurin et al., unpublished data). Thus, results showing that tumor-activated Schwann cells participate in the development of the immunosuppressive tumor microenvironment, together with the data revealing phenotypic and functional similarities between tumor-activated, i.e., “repair-like,’ Schwann cells and neurotrauma-induced, i.e., “repair,” Schwann cells [70], allow the following speculation. Surgical procedures associated with cancer treatment may provide additional polarization of Schwann cells in the cancer milieu that directly and indirectly (via the immune system) support tumor spreading and dissemination (Figure 1). In other words, the local and systemic crosstalk between the PNS and immune elements and factors is an important regulator not only of cancer development but also of cancer progression and metastasis.
However, the involvement of Schwann cells in metastasis formation as a consequence of wound healing has never been suggested. More experimental results are needed as they can provide a new target for safe and accelerated wound healing and tissue regeneration after oncology-associated surgical procedures.
5. Future Directions
Only in recent years has the role of the PNS been recognized as a new contributor to cancer development and metastasis. At the same time, old data showing that the removal of human and experimental animal tumors may be followed by an abrupt increase in metastatic growth (Table 1) [31,50,118,153,154,155,156] have attracted new attention. As 45–60% of patients diagnosed with common cancer require surgery to remove their tumor, as part of their primary cancer treatment [95,157], new questions should now be answered: How may PNS elements be involved in surgery-induced metastasis spreading, and how can they be targeted for improving patients’ survival and wellbeing? Answering these questions will allow us to design innovative approaches to alleviate undesired impacts of surgical procedures and, at the same time, to expand the clinical benefit of these interventions.
Another important point is that in spite of growing data showing some undesired effects of surgical intervention on tumor progression and spread, this does not imply that surgical resection or biopsy should be restricted since these side effects may only affect a subset of patients. The benefit of these surgical procedures for correct diagnosis and expanded survival is indisputable and outweighs the potential negative side effects, justifying their continued use versus their cessation [158]. It remains crucial to completely recognize their mechanistic impact on tumor neuroimmunopathology with the aim of averting these effects and of developing adjuvant remedies that can make these surgical interventions much safer and more beneficial for the patients [158].
The perioperative period is now recognized as crucial in influencing the incidence of postoperative metastases and long-standing cancer outcomes [131,159]. Therefore, numerous perioperative preventive interventions are currently being evaluated. Importantly, new studies provide hope that tumor resection and the wound-healing response can be uncoupled, resulting in attenuation of the outgrowth of distant cancerous cells, especially if this outgrowth is controlled by the immune responses. Although the impact of this approach on prolonging the clinical outcome after surgery has not yet been determined, the potential benefit is supported by data showing a correlation between the use of COX-2 inhibitors and β-blockers with progression-free survival in patients with breast cancer [160,161]. For instance, it was demonstrated in these patients that the peri- and postoperative treatment with anti-inflammatory agents attenuates the impact of surgical wounding on the outgrowth of distant tumors [162]. One week of β-blockade with oral propranolol prior to surgery in patients with breast cancer reduced expression of genes associated with the metastatic potential of tumor cells [163]. A benefit to perioperative β-blockade during surgery-induced stress with respect to breast cancer recurrence and metastases has been also suggested [164]. Similarly, a significant reduction in liver metastases of colon cancer in the context of surgery via β-adrenoceptor blockade and COX-2 inhibition was confirmed in different animal models [165]. CpG-C, a TLR-9 agonist, also markedly improved resistance to colon cancer-associated hepatic metastases in postoperative mice [159]. Other pre-clinical findings have revealed that phosphodiesterase-5 inhibitors could reduce postoperative metastasis by blocking the capacity of surgery-induced MDSC to suppress NK cell cytotoxicity [166]. Although there are not many studies that have tested the impact of targeting the neuro-immune axis in cancer patients before or after surgical procedures, a novel therapeutic paradigm was proposed on the basis of the evidence presented [111]. It declares that the postoperative period represents a window of opportunity during which the patient may be additionally protected against the oncological effects of tumor removal. Upcoming progress in this field should provide effective solutions that would make required unavoidable surgical interventions safer and more useful for the patients.
6. Conclusions
Clinical and experimental animal data that show that surgical interventions may boost the emergence of local and distant cancer recurrence have been collected for several decades. However, the notion that surgery-induced wound healing and tissue repair pathways may constitute a risk factor for re-activation of pre-existing dormant malignant cells and the acceleration of growth of distant metastases is not yet widely accepted. Despite this, ongoing investigation of the mechanism of surgery-associated metastatic disease continues revealing new pathways involved in this clinically important and not fully understood phenomenon. In addition to the proven role of surgical stress-induced neurotransmitters and neuromediators, neuroendocrine and stress hormones, inflammatory and tissue remodeling cytokines and chemokines, and angiogenic growth factors, the involvement of new pathways, like Wallerian degeneration, has also been considered. Understanding the biological significance and primary mechanisms of neuroimmune regulation of surgery-accompanying metastasis should advance the field of neuro-immuno-oncology and create a strong foundation for developing novel mechanism-based perioperative prophylactic interventions that protect cancer patients against the adverse effects of surgery and biopsy procedures.
Author Contributions
All authors contributed equally. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Non applicable.
Informed Consent Statement
Non applicable.
Data Availability Statement
Non applicable.
Acknowledgments
This work was in part supported by UPMC Hillman Cancer Center Development Funding Pilot Project Award (to M.R.S.) that was supported in part by award number P30CA047904 from the National Cancer Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Cancer Society Official Page. Cancer Facts and Figures 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed on 1 November 2020).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Simpson-Herren, L.; Sanford, A.H.; Holmquist, J.P. Effects of surgery on the cell kinetics of residual tumor. Cancer Treat. Rep. 1976, 60, 1749–1760. [Google Scholar] [PubMed]
- Horg, S.A.; Rubin, P.; DeWys, W.D. Metastasis and disseminated disease. In Clinical Oncology for Medical Students and Physicians-A Multidisciplinary Approach, 6th ed.; American Cancer Society: New York, NY, USA, 1983; pp. 498–499. [Google Scholar]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Dillekas, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Pommier, A.; Anaparthy, N.; Memos, N.; Kelley, Z.L.; Gouronnec, A.; Yan, R.; Auffray, C.; Albrengues, J.; Egeblad, M.; Iacobuzio-Donahue, C.A.; et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science 2018, 360. [Google Scholar] [CrossRef]
- Al-Sahaf, O.; Wang, J.H.; Browne, T.J.; Cotter, T.G.; Redmond, H.P. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann. Surg. 2010, 252, 1037–1043. [Google Scholar] [CrossRef]
- Lee, J.W.; Shahzad, M.M.; Lin, Y.G.; Armaiz-Pena, G.; Mangala, L.S.; Han, H.D.; Kim, H.S.; Nam, E.J.; Jennings, N.B.; Halder, J.; et al. Surgical stress promotes tumor growth in ovarian carcinoma. Clin. Cancer Res. 2009, 15, 2695–2702. [Google Scholar] [CrossRef]
- Demicheli, R.; Valagussa, P.; Bonadonna, G. Does surgery modify growth kinetics of breast cancer micrometastases? Br. J. Cancer 2001, 85, 490–492. [Google Scholar] [CrossRef]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.; Baum, M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat. Clin. Pract. Oncol. 2007, 4, 699–710. [Google Scholar] [CrossRef]
- Kelsey, C.R.; Fornili, M.; Ambrogi, F.; Higgins, K.; Boyd, J.A.; Biganzoli, E.; Demicheli, R. Metastasis dynamics for non-small-cell lung cancer: Effect of patient and tumor-related factors. Clin. Lung Cancer 2013, 14, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Oosterling, S.J.; van der Bij, G.J.; van Egmond, M.; van der Sijp, J.R. Surgical trauma and peritoneal recurrence of colorectal carcinoma. Eur. J. Surg. Oncol. 2005, 31, 29–37. [Google Scholar] [CrossRef]
- van der Bij, G.J.; Oosterling, S.J.; Beelen, R.H.; Meijer, S.; Coffey, J.C.; van Egmond, M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann. Surg. 2009, 249, 727–734. [Google Scholar] [CrossRef]
- Retsky, M.; Demicheli, R.; Hrushesky, W.; Baum, M.; Gukas, I. Surgery triggers outgrowth of latent distant disease in breast cancer: An inconvenient truth? Cancers 2010, 2, 305–337. [Google Scholar] [CrossRef]
- Retsky, M.; Demicheli, R.; Hrushesky, W.J.; Forget, P.; De Kock, M.; Gukas, I.; Rogers, R.A.; Baum, M.; Sukhatme, V.; Vaidya, J.S. Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: New findings and a review. Curr. Med. Chem. 2013, 20, 4163–4176. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Torosian, M.H.; Boraas, M.C.; Sigurdson, E.R.; Hoffman, J.P.; Eisenberg, B.L.; Fowble, B. Local recurrence of breast cancer in the stereotactic core needle biopsy site: Case reports and review of the literature. Breast J. 2001, 7, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.; Ye, X.; Grube, B.J.; Giuliano, A.E. Manipulation of the primary breast tumor and the incidence of sentinel node metastases from invasive breast cancer. Arch. Surg. 2004, 139, 634–639; discussion 639–640. [Google Scholar] [CrossRef] [PubMed]
- Hocevar, M.; Dragonja, Z.; Pilko, G.; Gazic, B.; Zgajnar, J. Residual melanoma after an excisional biopsy is an independent prognostic factor for local recurrence and overall survival. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014, 40, 1271–1275. [Google Scholar] [CrossRef]
- Mathenge, E.G.; Dean, C.A.; Clements, D.; Vaghar-Kashani, A.; Photopoulos, S.; Coyle, K.M.; Giacomantonio, M.; Malueth, B.; Nunokawa, A.; Jordan, J.; et al. Core needle biopsy of breast cancer tumors increases distant metastases in a mouse model. Neoplasia 2014, 16, 950–960. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Govaert, K.M.; Jongen, J.M.J.; Kranenburg, O.; Borel Rinkes, I.H.M. Surgery-induced tumor growth in (metastatic) colorectal cancer. Surg. Oncol. 2017, 26, 535–543. [Google Scholar] [CrossRef]
- Perry, J.A.; Douglas, H. Immunomodulatory Effects of Surgery, Pain, and Opioids in Cancer Patients. The Veterinary clinics of North America. Small Anim. Pract. 2019, 49, 981–991. [Google Scholar] [CrossRef]
- Ryall, C. Cancer infection and cancer recurrence: A anger to avoid in cancer operations. Lancet 1907, 2, 1311–1316. [Google Scholar] [CrossRef]
- Tyzzer, E.E. Factors in the Production and Growth of tumor Metastases. J. Med Res. 1913, 28, 309–332-1. [Google Scholar] [PubMed]
- Jones, F.S.; Rous, P. On the cause of the localization of secondary tumors at points of injury. J. Exp. Med. 1914, 20, 404–412. [Google Scholar] [CrossRef]
- Schatten, W.E. An experimental study of postoperative tumor metastases. I. Growth of pulmonary metastases following total removal of primary leg tumor. Cancer 1958, 11, 455–459. [Google Scholar] [CrossRef]
- Lewis, M.R.; Cole, W.H. Experimental increase of lung metastases after operative trauma (amputation of limb with tumor). AMA Arch. Surg. 1958, 77, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, L.; Yamakawa, T.; Seltzer, D. Carcinoma of the gastric stump. Am. J. Surg. 1973, 125, 29–38. [Google Scholar] [CrossRef]
- Lange, P.H.; Hekmat, K.; Bosl, G.; Kennedy, B.J.; Fraley, E.E. Acclerated growth of testicular cancer after cytoreductive surgery. Cancer 1980, 45, 1498–1506. [Google Scholar] [CrossRef]
- Moore, G.E. Debunking debulking. Surg. Gynecol. Obs. 1980, 150, 395–396. [Google Scholar]
- Hughes, E.S.; McDermott, F.T.; Polglase, A.L.; Johnson, W.R. Tumor recurrence in the abdominal wall scar tissue after large-bowel cancer surgery. Dis. Colon Rectum 1983, 26, 571–572. [Google Scholar] [CrossRef]
- Baker, D.G.; Masterson, T.M.; Pace, R.; Constable, W.C.; Wanebo, H. The influence of the surgical wound on local tumor recurrence. Surgery 1989, 106, 525–532. [Google Scholar] [PubMed]
- Hoskins, W.J. The influence of cytoreductive surgery on progression-free interval and survival in epithelial ovarian cancer. Baillieres Clin. Obs. Gynaecol. 1989, 3, 59–71. [Google Scholar] [CrossRef]
- Schuh, A.C.; Keating, S.J.; Monteclaro, F.S.; Vogt, P.K.; Breitman, M.L. Obligatory wounding requirement for tumorigenesis in v-jun transgenic mice. Nature 1990, 346, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Asakura, T.; Nemir, P., Jr. Effect of local tumor removal and retained oncolysate on lung metastasis. J. Surg. Res. 1992, 53, 30–38. [Google Scholar] [CrossRef]
- Ramos, J.M.; Gupta, S.; Anthone, G.J.; Ortega, A.E.; Simons, A.J.; Beart, R.W., Jr. Laparoscopy and colon cancer. Is the port site at risk? A preliminary report. Arch. Surg. 1994, 129, 897–899; discussion 900. [Google Scholar] [CrossRef]
- Lee, J.Y.; Murphy, S.M.; Scanlon, E.F. Effect of trauma of implantation of metastatic tumor in bone in mice. J. Surg. Oncol. 1994, 56, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.I. Abdominal wall metastasis after laparoscopic gastroenterostomy. Med. J. Aust. 1995, 163, 106–107. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Nishioka, K.; Maruyama, R.; Saitoh, G.; Hamatake, M.; Fukuyama, Y.; Yaita, H.; Ishida, T.; Sugimachi, K. Kinetic analysis of recurrence and survival after potentially curative resection of nonsmall cell lung cancer. J. Surg. Oncol. 1996, 63, 159–165. [Google Scholar] [CrossRef]
- Hofer, S.O.; Shrayer, D.; Reichner, J.S.; Hoekstra, H.J.; Wanebo, H.J. Wound-induced tumor progression: A probable role in recurrence after tumor resection. Arch. Surg. 1998, 133, 383–389. [Google Scholar] [CrossRef]
- Karrison, T.G.; Ferguson, D.J.; Meier, P. Dormancy of mammary carcinoma after mastectomy. J. Natl. Cancer Inst. 1999, 91, 80–85. [Google Scholar] [CrossRef]
- Pidgeon, G.P.; Harmey, J.H.; Kay, E.; Da Costa, M.; Redmond, H.P.; Bouchier-Hayes, D.J. The role of endotoxin/lipopolysaccharide in surgically induced tumour growth in a murine model of metastatic disease. Br. J. Cancer 1999, 81, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, Y.; Kanki, M.; Okita, Y. Importance of the control of lung recurrence soon after surgery of pulmonary metastases. Am. J. Surg. 2000, 179, 122–125. [Google Scholar] [CrossRef]
- Da Costa, M.L.; Redmond, H.P.; Bouchier-Hayes, D.J. Taurolidine improves survival by abrogating the accelerated development and proliferation of solid tumors and development of organ metastases from circulating tumor cells released following surgery. J. Surg. Res. 2001, 101, 111–119. [Google Scholar] [CrossRef]
- Li, T.S.; Kaneda, Y.; Ueda, K.; Hamano, K.; Zempo, N.; Esato, K. The influence of tumour resection on angiostatin levels and tumour growth--an experimental study in tumour-bearing mice. Eur. J. Cancer 2001, 37, 2283–2288. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef]
- Shakhar, G.; Abudarham, N.; Melamed, R.; Schwartz, Y.; Rosenne, E.; Ben-Eliyahu, S. Amelioration of operation-induced suppression of marginating pulmonary NK activity using poly IC: A potential approach to reduce postoperative metastasis. Ann. Surg. Oncol. 2007, 14, 841–852. [Google Scholar] [CrossRef]
- Beecken, W.D.; Engl, T.; Jonas, D.; Blaheta, R.A. Expression of angiogenesis inhibitors in human bladder cancer may explain rapid metastatic progression after radical cystectomy. Int. J. Mol. Med. 2009, 23, 261–266. [Google Scholar]
- Goldfarb, Y.; Shapiro, H.; Singer, P.; Kalderon, Y.; Levi, B.; Glasner, A.; Benish, M.; Ben-Eliyahu, S. Fish oil attenuates surgery-induced immunosuppression, limits post-operative metastatic dissemination and increases long-term recurrence-free survival in rodents inoculated with cancer cells. Clin. Nutr. 2012, 31, 396–404. [Google Scholar] [CrossRef]
- Tham, M.; Khoo, K.; Yeo, K.P.; Kato, M.; Prevost-Blondel, A.; Angeli, V.; Abastado, J.P. Macrophage depletion reduces postsurgical tumor recurrence and metastatic growth in a spontaneous murine model of melanoma. Oncotarget 2015, 6, 22857–22868. [Google Scholar] [CrossRef]
- O’Connell, E.P.; O’Leary, D.P.; Fogarty, K.; Khan, Z.J.; Redmond, H.P. Predictors and patterns of melanoma recurrence following a negative sentinel lymph node biopsy. Melanoma Res. 2016, 26, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Pinson, H.; Cosyns, S.; Ceelen, W.P. The impact of surgical resection of the primary tumor on the development of synchronous colorectal liver metastasis: A systematic review. Acta Chir. Belg. 2018, 118, 203–211. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, F.; Li, Z.; Gao, J.; Li, Y.; Shen, L.; Ji, J.; Lu, M. A prospective study on the changes and clinical significance of pre-operative and post-operative circulating tumor cells in resectable gastric cancer. J. Transl. Med. 2018, 16, 171. [Google Scholar] [CrossRef]
- Grewal, S.; Korthouwer, R.; Bögels, M.; Braster, R.; Heemskerk, N.; Budding, A.E.; Pouw, S.M.; van Horssen, J.; Ankersmit, M.; Meijerink, J.; et al. Spillage of bacterial products during colon surgery increases the risk of liver metastases development in a rat colon carcinoma model. Oncoimmunology 2018, 7, e1461302. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Huang, Y.; Xiang, L.; Chen, Z.; Fang, Y.; Lin, Y.; Cui, Z.; Yu, S.; Li, X.; Yang, D. Circulating Tumor Cell Phenotype Indicates Poor Survival and Recurrence After Surgery for Hepatocellular Carcinoma. Dig. Dis. Sci. 2018, 63, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhu, Y.; Cui, Y.; Yang, Z.; Zhou, S.; Han, Y.; Yu, D.; Xiao, N.; Cao, X.; Li, Y.; et al. Circulating tumor cells in the pulmonary vein increase significantly after lobectomy: A prospective observational study. Thorac. Cancer 2019, 10, 163–169. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Seifert, P.; Benedic, M.; Effert, P. Nerve fibers in tumors of the human urinary bladder. Virchows Arch. Int. J. Pathol. 2002, 440, 291–297. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef]
- Mravec, B.; Gidron, Y.; Hulin, I. Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Semin. Cancer Biol. 2008, 18, 150–163. [Google Scholar] [CrossRef]
- Seifert, P.; Spitznas, M. Axons in human choroidal melanoma suggest the participation of nerves in the control of these tumors. Am. J. Ophthalmol. 2002, 133, 711–713. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Fernandez, E.V.; Price, D.K.; Figg, W.D. Prostate cancer progression attributed to autonomic nerve development: Potential for therapeutic prevention of localized and metastatic disease. Cancer Biol. Ther. 2013, 14, 1005–1006. [Google Scholar] [CrossRef] [PubMed]
- Keskinov, A.A.; Tapias, V.; Watkins, S.C.; Ma, Y.; Shurin, M.R.; Shurin, G.V. Impact of the Sensory Neurons on Melanoma Growth In Vivo. PLoS ONE 2016, 11, e0156095. [Google Scholar] [CrossRef] [PubMed]
- Bunimovich, Y.L.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Schwann cells: A new player in the tumor microenvironment. Cancer Immunol. Immunother. 2017, 66, 959–968. [Google Scholar] [CrossRef]
- Zhou, Y.; Shurin, G.V.; Zhong, H.; Bunimovich, Y.L.; Han, B.; Shurin, M.R. Schwann Cells Augment Cell Spreading and Metastasis of Lung Cancer. Cancer Res. 2018, 78, 5927–5939. [Google Scholar] [CrossRef]
- Martyn, G.V.; Shurin, G.V.; Keskinov, A.A.; Bunimovich, Y.L.; Shurin, M.R. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol. Immunother. 2019, 68, 1819–1829. [Google Scholar] [CrossRef]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D., Jr.; et al. Melanoma-Induced Reprogramming of Schwann Cell Signaling Aids Tumor Growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Shurin, M.R.; Shurin, G.V.; Zlotnikov, S.B.; Bunimovich, Y.L. The Neuroimmune Axis in the Tumor Microenvironment. J. Immunol. 2020, 204, 280–285. [Google Scholar] [CrossRef]
- Seifert, P.; Spitznas, M. Tumours may be innervated. Virchows Arch. Int. J. Pathol. 2001, 438, 228–231. [Google Scholar] [CrossRef]
- Ondicova, K.; Mravec, B. Role of nervous system in cancer aetiopathogenesis. Lancet. Oncol. 2010, 11, 596–601. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Gao, D. Role of the nervous system in cancer metastasis. Oncol. Lett. 2013, 5, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl. Med. 2018, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.O.; Demir, I.E.; Altintas, B.; Rauch, U.; Thiel, G.; Müller, M.W.; Giese, N.A.; Friess, H.; Schäfer, K.-H. Neural invasion in pancreatic cancer: A mutual tropism between neurons and cancer cells. Biochem. Biophys. Res. Commun. 2008, 374, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Cole, S.W. Nervous system regulation of the cancer genome. Brain Behav. Immun. 2013, 30, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef]
- Rabben, H.L.; Zhao, C.M.; Hayakawa, Y.; Wang, T.C.; Chen, D. Vagotomy and Gastric Tumorigenesis. Curr. Neuropharmacol. 2016, 14, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Cancer–neuronal crosstalk and the startups working to silence it. Nat. Biotechnol. 2020, 38, 115–117. [Google Scholar] [CrossRef]
- Entschladen, F.; Drell, T.L.t.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet. Oncol. 2004, 5, 254–258. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef]
- Mancino, M.; Ametller, E.; Gascon, P.; Almendro, V. The neuronal influence on tumor progression. Biochim. Biophys. Acta 2011, 1816, 105–118. [Google Scholar] [CrossRef]
- Pimentel, M.; Chai, M.; Le, C.; Cole, S.; Sloan, E. Sympathetic nervous system regulation of metastasis. In Metastatic Cancer: Clinical and Biological Perspectives; Jandial, R., Ed.; Landes Bioscience: Austin, TX, USA, 2013; pp. 169–179. [Google Scholar]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pouya, F.D.; Rasmi, Y.; Asl, E.R. Role of Neurotransmitters and Neuropeptides in Breast Cancer Metastasis. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2020, 14, 107–116. [Google Scholar] [CrossRef]
- Tilan, J.; Kitlinska, J. Neuropeptide Y (NPY) in tumor growth and progression: Lessons learned from pediatric oncology. Neuropeptides 2016, 55, 55–66. [Google Scholar] [CrossRef]
- Rao, G.; Patel, P.S.; Idler, S.P.; Maloof, P.; Gascon, P.; Potian, J.A.; Rameshwar, P. Facilitating role of preprotachykinin-I gene in the integration of breast cancer cells within the stromal compartment of the bone marrow: A model of early cancer progression. Cancer Res. 2004, 64, 2874–2881. [Google Scholar] [CrossRef]
- Ohmori, H.; Fujii, K.; Sasahira, T.; Luo, Y.; Isobe, M.; Tatsumoto, N.; Kuniyasu, H. Methionine-enkephalin secreted by human colorectal cancer cells suppresses T lymphocytes. Cancer Sci. 2009, 100, 497–502. [Google Scholar] [CrossRef]
- Ishimaru, H.; Kageyama, Y.; Hayashi, T.; Nemoto, T.; Eishi, Y.; Kihara, K. Expression of matrix metalloproteinase-9 and bombesin/gastrin-releasing peptide in human prostate cancers and their lymph node metastases. Acta Oncol. 2002, 41, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 5. [Google Scholar] [CrossRef]
- Alkire, B.C.; Raykar, N.P.; Shrime, M.G.; Weiser, T.G.; Bickler, S.W.; Rose, J.A.; Nutt, C.T.; Greenberg, S.L.; Kotagal, M.; Riesel, J.N.; et al. Global access to surgical care: A modelling study. Lancet Glob. Health 2015, 3, e316–e323. [Google Scholar] [CrossRef]
- Griffiths, J.D. The dissemination of cancer cells during operative procedures. Ann. R. Coll. Surg. Engl. 1960, 27, 14–44. [Google Scholar] [PubMed]
- Marie, P.; Clunet, J. Frequence des m6tastases visc6rale chez les souris cancereuses apr6s ablation chirurgicale de leur tumneur. Bulletin L’association Francaise Pour l’etude Cancer 1910, 3, 19–23. [Google Scholar]
- Clunet, J. Recherches Expdrimentales Sur Les Tumeurs Malignes; Steinheil: Paris, French, 1910. [Google Scholar]
- Ryall, C. The technique of cancer operations with reference to the danger of caneer infection. Br. Med. J. 1908, 2, 1005. [Google Scholar]
- Yamaguchi, K.; Takagi, Y.; Aoki, S.; Futamura, M.; Saji, S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann. Surg. 2000, 232, 58–65. [Google Scholar] [CrossRef]
- Ebenezer, G.J.; McArthur, J.C.; Thomas, D.; Murinson, B.; Hauer, P.; Polydefkis, M.; Griffin, J.W. Denervation of skin in neuropathies: The sequence of axonal and Schwann cell changes in skin biopsies. Brain 2007, 130, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.; Marikovsky, M.; Meir, G.; Neeman, M. Stimulation of tumour growth by wound-derived growth factors. Br. J. Cancer 1999, 79, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Skipper, D.; Jeffrey, M.J.; Cooper, A.J.; Alexander, P.; Taylor, I. Enhanced growth of tumour cells in healing colonic anastomoses and laparotomy wounds. Int. J. Colorectal Dis. 1989, 4, 172–177. [Google Scholar] [CrossRef]
- Jiang, W.G.; Puntis, M.C.; Hallett, M.B. Molecular and cellular basis of cancer invasion and metastasis: Implications for treatment. Br. J. Surg. 1994, 81, 1576–1590. [Google Scholar] [CrossRef]
- Svendsen, M.N.; Werther, K.; Nielsen, H.J.; Kristjansen, P.E. VEGF and tumour angiogenesis. Impact of surgery, wound healing, inflammation and blood transfusion. Scand. J. Gastroenterol. 2002, 37, 373–379. [Google Scholar] [CrossRef]
- Berguer, R.; Bravo, N.; Bowyer, M.; Egan, C.; Knolmayer, T.; Ferrick, D. Major surgery suppresses maximal production of helper T-cell type 1 cytokines without potentiating the release of helper T-cell type 2 cytokines. Arch. Surg. 1999, 134, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Herbert, T.B. Health psychology: Psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu. Rev. Psychol. 1996, 47, 113–142. [Google Scholar] [CrossRef]
- Moynihan, J.A.; Ader, R. Psychoneuroimmunology: Animal models of disease. Psychosom. Med. 1996, 58, 546–558. [Google Scholar] [CrossRef]
- Neeman, E.; Ben-Eliyahu, S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav. Immun. 2013, 30, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Pattyn, P.; Mareel, M. Surgery, wound healing, and metastasis: Recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 2014, 89, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Nan Tie, E.; Henderson, M.A.; Gyorki, D.E. Management of in-transit melanoma metastases: A review. ANZ J. Surg. 2019, 89, 647–652. [Google Scholar] [CrossRef]
- Blakely, A.M.; Comissiong, D.S.; Vezeridis, M.P.; Miner, T.J. Suboptimal Compliance with National Comprehensive Cancer Network Melanoma Guidelines: Who Is at Risk? Am. J. Clin. Oncol. 2018, 41, 754–759. [Google Scholar] [CrossRef]
- Mangold, A.R.; Skinner, R.; Dueck, A.C.; Sekulic, A.; Pockaj, B.A. Risk Factors Predicting Positive Margins at Primary Wide Local Excision of Cutaneous Melanoma. Dermatol. Surg. 2016, 42, 646–652. [Google Scholar] [CrossRef]
- Mathew, G.; Watson, D.I.; Rofe, A.M.; Baigrie, C.F.; Ellis, T.; Jamieson, G.G. Wound metastases following laparoscopic and open surgery for abdominal cancer in a rat model. Br. J. Surg. 1996, 83, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Spelt, L.; Andersson, B.; Nilsson, J.; Andersson, R. Prognostic models for outcome following liver resection for colorectal cancer metastases: A systematic review. Eur. J. Surg. Oncol. 2012, 38, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Dillekas, H.; Straume, O.; Biganzoli, E. Distant metastasis dynamics following subsequent surgeries after primary breast cancer removal. Breast Cancer Res. 2019, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Qadri, S.S.; Wang, J.H.; Coffey, J.C.; Alam, M.; O’Donnell, A.; Aherne, T.; Redmond, H.P. Can surgery for cancer accelerate the progression of secondary tumors within residual minimal disease at both local and systemic levels? Ann. Thorac. Surg. 2005, 80, 1046–1050; discussion 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Goto, T. Links between Inflammation and Postoperative Cancer Recurrence. J. Clin. Med. 2021, 10, 228. [Google Scholar] [CrossRef]
- Antonio, N.; Bonnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef]
- Murthy, S.M.; Goldschmidt, R.A.; Rao, L.N.; Ammirati, M.; Buchmann, T.; Scanlon, E.F. The influence of surgical trauma on experimental metastasis. Cancer 1989, 64, 2035–2044. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Yu, L.; Wang, Y.Y.; Chen, R.; Qian, J.; Hong, Z.P.; Su, X.S. Surgery-induced monocytic myeloid-derived suppressor cells expand regulatory T cells in lung cancer. Oncotarget 2017, 8, 17050–17058. [Google Scholar] [CrossRef]
- Tai, L.H.; Zhang, J.; Auer, R.C. Preventing surgery-induced NK cell dysfunction and cancer metastases with influenza vaccination. Oncoimmunology 2013, 2, e26618. [Google Scholar] [CrossRef]
- Tai, L.H.; de Souza, C.T.; Bélanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.P.; O’Leary, E.; Foley, N.; Cotter, T.G.; Wang, J.H.; Redmond, H.P. Effects of surgery on the cancer stem cell niche. Eur. J. Surg. oncol. 2016, 42, 319–325. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nature reviews. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Sullivan, R.; Peppercorn, J.; Sikora, K.; Zalcberg, J.; Meropol, N.J.; Amir, E.; Khayat, D.; Boyle, P.; Autier, P.; Tannock, I.F.; et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011, 12, 933–980. [Google Scholar] [CrossRef]
- Chéret, J.; Lebonvallet, N.; Buhé, V.; Carre, J.L.; Misery, L.; Le Gall-Ianotto, C. Influence of sensory neuropeptides on human cutaneous wound healing process. J. Dermatol. Sci. 2014, 74, 193–203. [Google Scholar] [CrossRef]
- Blais, M.; Mottier, L.; Germain, M.A.; Bellenfant, S.; Cadau, S.; Berthod, F. Sensory neurons accelerate skin reepithelialization via substance P in an innervated tissue-engineered wound healing model. Tissue Eng. Part A 2014, 20, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Ricon, I.; Hanalis-Miller, T.; Haldar, R.; Jacoby, R.; Ben-Eliyahu, S. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of beta-adrenergic and cyclooxygenase 2 signaling. Cancer 2019, 125, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.M.; McIver, C.M.; Stephenson, S.A.; Hewett, P.J.; Rieger, N.; Hardingham, J.E. Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin. Cancer Res. 2006, 12, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef]
- Brown, D.C.; Purushotham, A.D.; Birnie, G.D.; George, W.D. Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery 1995, 117, 95–101. [Google Scholar] [CrossRef]
- Retsky, M.W.; Demicheli, R.; Hrushesky, W.J.; Baum, M.; Gukas, I.D. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 2008, 116, 730–741. [Google Scholar] [CrossRef]
- Baum, M.; Demicheli, R.; Hrushesky, W.; Retsky, M. Does surgery unfavourably perturb the "natural history" of early breast cancer by accelerating the appearance of distant metastases? Eur. J. Cancer 2005, 41, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J. Transl. Med. 2018, 16, 8. [Google Scholar] [CrossRef]
- Sood, A.K.; Bhatty, R.; Kamat, A.A.; Landen, C.N.; Han, L.; Thaker, P.H.; Li, Y.; Gershenson, D.M.; Lutgendorf, S.; Cole, S.W. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006, 12, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.V.; Kim, S.J.; Donovan, E.L.; Chen, M.; Gross, A.C.; Webster Marketon, J.I.; Barsky, S.H.; Glaser, R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav. Immun. 2009, 23, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.; Whited, J.L. Parallels between wound healing, epimorphic regeneration and solid tumors. Development 2020, 147, dev181636. [Google Scholar] [CrossRef]
- Conforti, L.; Gilley, J.; Coleman, M.P. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 2014, 15, 394–409. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Whalley, K. Glia: Schwann cells provide life support for axons. Nat. Rev. Neurosci. 2014, 15, 698–699. [Google Scholar] [CrossRef]
- Stratton, J.A.; Shah, P.T. Macrophage polarization in nerve injury: Do Schwann cells play a role? Neural Regen. Res. 2016, 11, 53–57. [Google Scholar] [CrossRef]
- Wong, K.M.; Babetto, E.; Beirowski, B. Axon degeneration: Make the Schwann cell great again. Neural Regen. Res. 2017, 12, 518–524. [Google Scholar] [CrossRef]
- Qu, W.R.; Zhu, Z.; Liu, J.; Song, D.B.; Tian, H.; Chen, B.P.; Li, R.; Deng, L.X. Interaction between Schwann cells and other cells during repair of peripheral nerve injury. Neural Regen. Res. 2021, 16, 93–98. [Google Scholar] [CrossRef]
- Parfejevs, V.; Antunes, A.T.; Sommer, L. Injury and stress responses of adult neural crest-derived cells. Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Parfejevs, V.; Debbache, J.; Shakhova, O.; Schaefer, S.M.; Glausch, M.; Wegner, M.; Suter, U.; Riekstina, U.; Werner, S.; Sommer, L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236. [Google Scholar] [CrossRef]
- Johnston, A.P. Schwann cells: An emerging player in tissue regeneration. Stem Cell Investig. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, S.H.; Shurin, G.V.; Khosravi, H.; Kazi, R.; Kruglov, O.; Shurin, M.R.; Bunimovich, Y.L. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 2020, 69, 245–253. [Google Scholar] [CrossRef]
- Sroka, I.C.; Chopra, H.; Das, L.; Gard, J.M.; Nagle, R.B.; Cress, A.E. Schwann Cells Increase Prostate and Pancreatic Tumor Cell Invasion Using Laminin Binding A6 Integrin. J. Cell. Biochem. 2016, 117, 491–499. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.Y.; Barajas, F.; Chen, C.H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Peeters, C.F.; de Waal, R.M.; Wobbes, T.; Ruers, T.J. Metastatic dormancy imposed by the primary tumor: Does it exist in humans? Ann. Surg. Oncol. 2008, 15, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Di Gianni, P.; Franco, M.; Meiss, R.P.; Vanzulli, S.; Piazzon, I.; Pasqualini, C.D.; Bustuoabad, O.D.; Ruggiero, R.A. Inhibition of metastases by a serum factor associated to concomitant resistance induced by unrelated murine tumors. Oncol. Rep. 1999, 6, 1073–1084. [Google Scholar] [CrossRef]
- Bonfil, R.D.; Ruggiero, R.A.; Bustuoabad, O.D.; Meiss, R.P.; Pasqualini, C.D. Role of concomitant resistance in the development of murine lung metastases. Int. J. Cancer 1988, 41, 415–422. [Google Scholar] [CrossRef]
- Day, S.; Myers, W.; Stansly, P.; Garattini, S.; Lewis, M.; Sugarbaker, E.; Thornthwaite, J.; Ketcham, A. Inhibitory effect of a primary tumor on metastasis. In Cancer Invasion and Metastasis. Biological Mechanisms and Therapy; Raven Press: New York, NY, USA, 1977; pp. 227–240. [Google Scholar]
- NCRAS. Chemotherapy, Radiotherapy and Surgical Tumour Resections in England; The National Cancer Registration and Analysis Service: London, UK, 2020.
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Sorski, L.; Melamed, R.; Levi, B.; Matzner, P.; Lavon, H.; Rosenne, E.; Shaashua, L.; Ricon, I.; Sandbank, E.; Benbenishty, A.; et al. Prevention of liver metastases through perioperative acute CpG-C immune stimulation. Cancer Immunol. Immunother. 2020, 69, 2021–2031. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Vandenhende, J.; Berliere, M.; Machiels, J.P.; Nussbaum, B.; Legrand, C.; De Kock, M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth. Analg. 2010, 110, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Krall, J.A.; Reinhardt, F.; Mercury, O.A.; Pattabiraman, D.R.; Brooks, M.W.; Dougan, M.; Lambert, A.W.; Bierie, B.; Ploegh, H.L.; Dougan, S.K.; et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018, 10, eaan3464. [Google Scholar] [CrossRef]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Sorski, L.; Melamed, R.; Matzner, P.; Lavon, H.; Shaashua, L.; Rosenne, E.; Ben-Eliyahu, S. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through β-adrenoceptors blockade and COX2 inhibition. Brain Behav. Immun. 2016, 58, 91–98. [Google Scholar] [CrossRef]
- Tai, L.H.; Alkayyal, A.A.; Leslie, A.L.; Sahi, S.; Bennett, S.; Tanese de Souza, C.; Baxter, K.; Angka, L.; Xu, R.; Kennedy, M.A.; et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology 2018, 7, e1431082. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).