Oligomeric Aβ1-42 Induces an AMD-Like Phenotype and Accumulates in Lysosomes to Impair RPE Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Oligomeric Aβ1-42
2.2. Animal Housing and Husbandry
2.3. Animal Preparation and Recovery
2.4. Transscleral Subretinal Injection Surgery
2.5. Funduscopy
2.6. Full-Field Electroretinography (ERG)
2.7. Optical Coherence Tomography (OCT)
2.8. Histological Analysis
2.9. Cell Culture
2.10. Endothelial Cell Migration Assay
2.11. Enzyme-Linked Immunosorbent Assays (ELISA)
2.12. FITC-Dextran Diffusion Assay
2.13. Confocal Immunofluorescence
2.14. Live-Cell Imaging
2.15. Quantitative PCR
2.16. Isolation of Photoreceptor Outer Segments (POS)
2.17. Determination of POS Phagocytosis
2.18. Statistical Methods
3. Results
3.1. Funduscopy and Electrophysiology Studies in Mouse Eyes Exposed to Aβ Showed Signs of Retinal Pathology without Evidence of Impaired Function
3.2. Non-Invasive Scans of Living Mouse Eyes Revealed Evidence of Dynamic Aβ-Induced Pathology in Localized Areas
3.3. In Vitro Studies Revealed Aβ Effects in Choroidal Endothelial Cells and in the RPE Monolayer
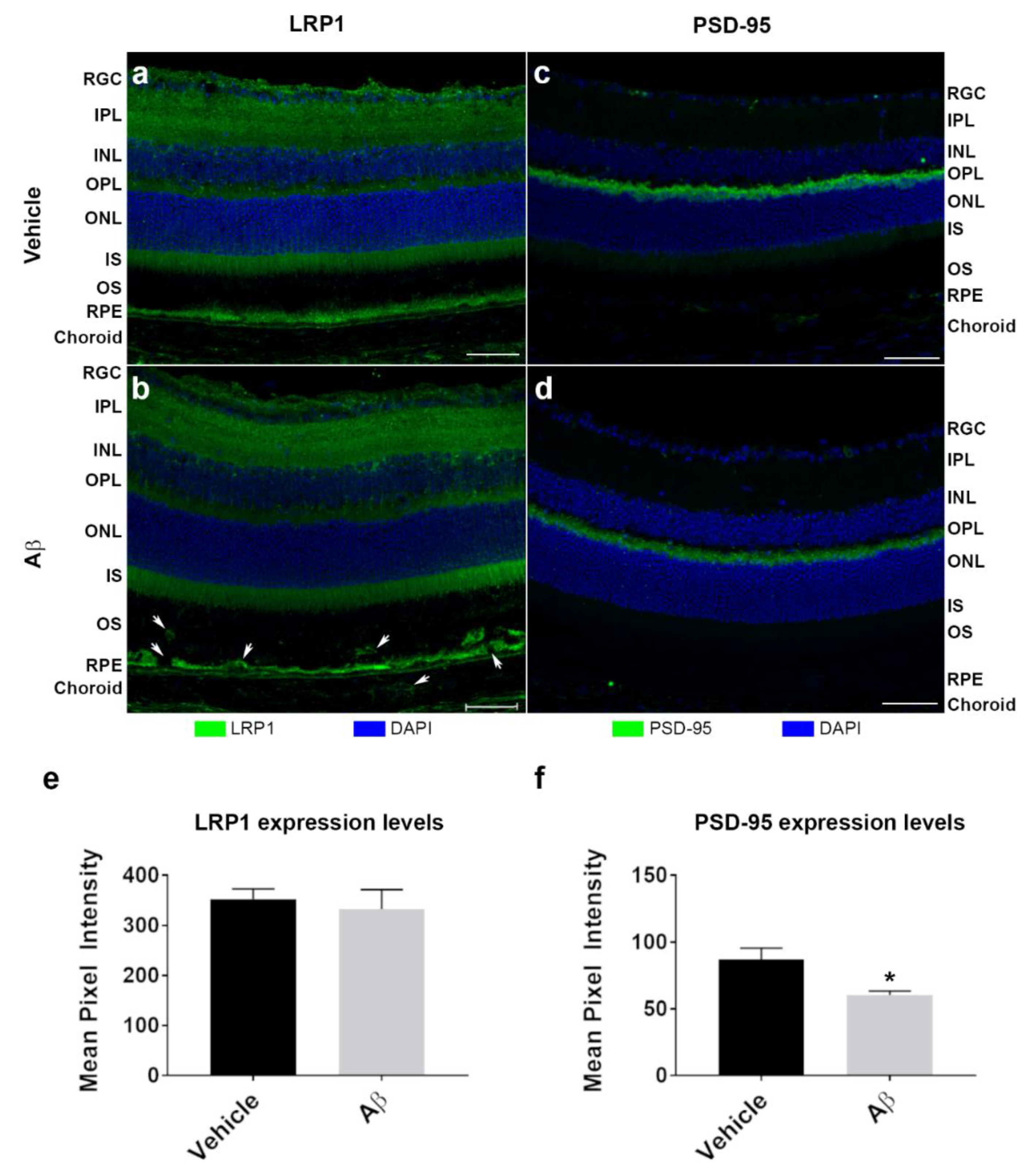
3.4. The Intracellular Fate of Aβ and Cellular Response to Its Accumulation
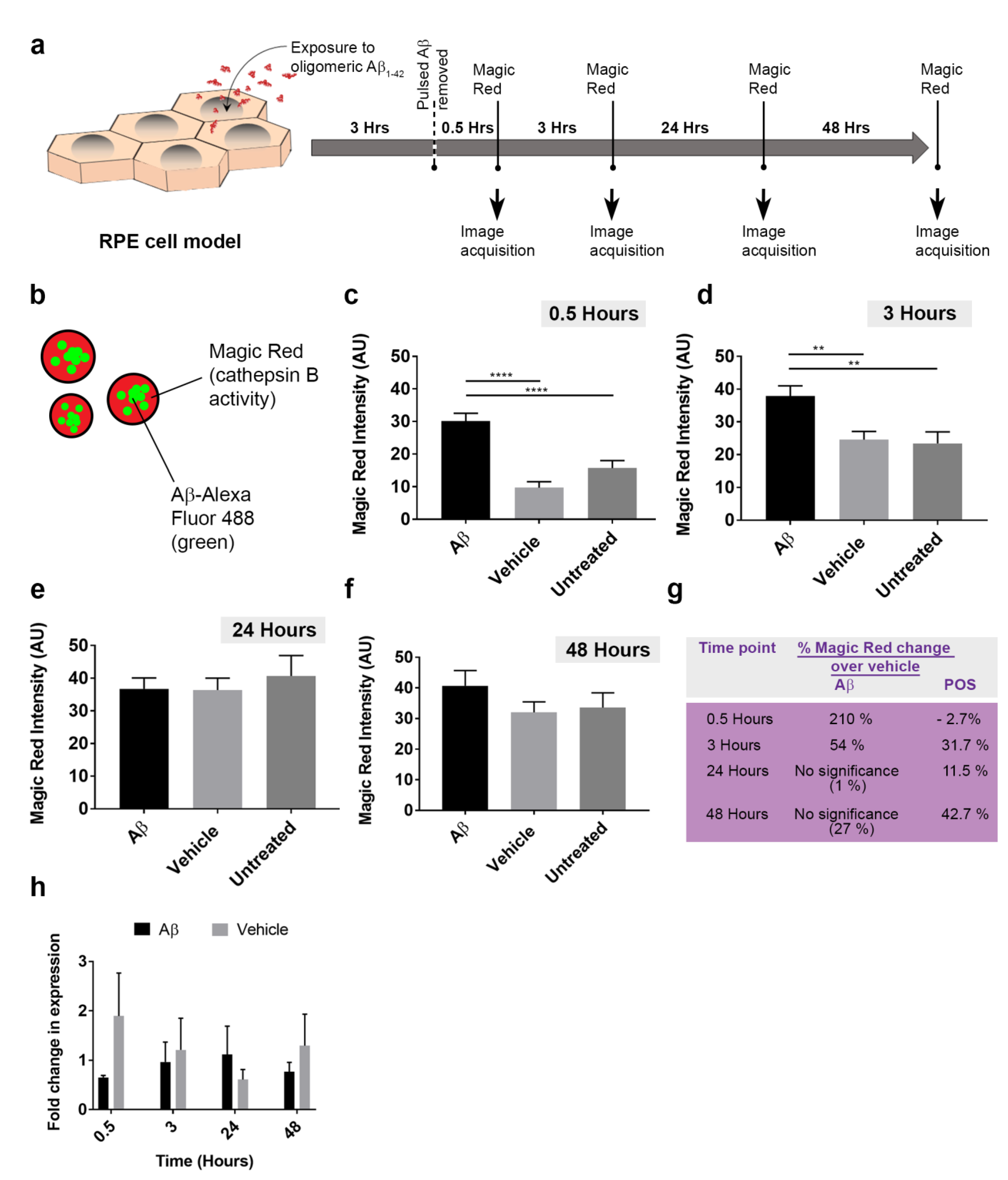
3.5. The Dynamics of Aβ Internalization and Consequences to Cargo Trafficking Capability of RPE Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrington, D.A.; Sinha, D.; Kaarniranta, K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin. Eye Res. 2016, 51, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Sadda, S.R.; Staurenghi, G.; Lindner, M.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; Csaky, K.; et al. Imaging Protocols in Clinical Studies in Advanced Age-Related Macular Degeneration: Recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology 2017, 124, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Bindewald-Wittich, A.; Fleckenstein, M.; Dreyhaupt, J.; Scholl, H.P.; Schmitz-Valckenberg, S. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 463–472. [Google Scholar] [CrossRef]
- Rudolf, M.; Clark, M.E.; Chimento, M.F.; Li, C.M.; Medeiros, N.E.; Curcio, C.A. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1200–1209. [Google Scholar] [CrossRef]
- Lynn, S.A.; Keeling, E.; Munday, R.; Gabha, G.; Griffiths, H.; Lotery, A.J.; Ratnayaka, J.A. The complexities underlying age-related macular degeneration: Could amyloid beta play an important role? Neural Regen. Res. 2017, 12, 538–548. [Google Scholar] [CrossRef]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A.J. Dementia of the eye: The role of amyloid beta in retinal degeneration. Eye 2015, 29, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Matsui, K. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog. Retin. Eye Res. 2011, 30, 217–238. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. The Alzheimer’s A beta -peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 11830–11835. [Google Scholar] [CrossRef]
- Anderson, D.H.; Talaga, K.C.; Rivest, A.J.; Barron, E.; Hageman, G.S.; Johnson, L.V. Characterization of beta amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef]
- Thompson, R.B.; Reffatto, V.; Bundy, J.G.; Kortvely, E.; Flinn, J.M.; Lanzirotti, A.; Jones, E.A.; McPhail, D.S.; Fearn, S.; Boldt, K.; et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc. Natl. Acad. Sci. USA 2015, 112, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Isas, J.M.; Luibl, V.; Johnson, L.V.; Kayed, R.; Wetzel, R.; Glabe, C.G.; Langen, R.; Chen, J. Soluble and mature amyloid fibrils in drusen deposits. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Luibl, V.; Isas, J.M.; Kayed, R.; Glabe, C.G.; Langen, R.; Chen, J. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J. Clin. Investig. 2006, 116, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dentchev, T.; Milam, A.H.; Lee, V.M.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 9, 184–190. [Google Scholar]
- Hoh, K.J.; Lenassi, E.; Jeffery, G. Viewing ageing eyes: Diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Malek, G.; Johnson, L.V.; Mace, B.E.; Saloupis, P.; Schmechel, D.E.; Rickman, D.W.; Toth, C.A.; Sullivan, P.M.; Bowes, R.C. Apolipoprotein E allele-dependent pathogenesis: A model for age-related retinal degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 11900–11905. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, J.H.; Mook-Jung, I.; Kim, K.W.; Park, W.J.; Park, K.H.; Kim, J.H. Intracellular amyloid beta alters the tight junction of retinal pigment epithelium in 5XFAD mice. Neurobiol. Aging 2014, 35, 2013–2020. [Google Scholar] [CrossRef]
- Catchpole, I.; Germaschewski, V.; Hoh, K.J.; von Leithner, P.L.; Ford, S.; Gough, G.; Adamson, P.; Overend, P.; Hilpert, J.; Lopez, F.J.; et al. Systemic Administration of Abeta mAb Reduces Retinal Deposition of Abeta and Activated Complement C3 in Age-Related Macular Degeneration Mouse Model. PLoS ONE 2013, 8, e65518. [Google Scholar] [CrossRef]
- Ding, J.D.; Johnson, L.V.; Herrmann, R.; Farsiu, S.; Smith, S.G.; Groelle, M.; Mace, B.E.; Sullivan, P.; Jamison, J.A.; Kelly, U.; et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, E279–E287. [Google Scholar] [CrossRef]
- Shi, H.; Koronyo, Y.; Rentsendorj, A.; Regis, G.C.; Sheyn, J.; Fuchs, D.T.; Kramerov, A.A.; Ljubimov, A.V.; Dumitrascu, O.M.; Rodriguez, A.R.; et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020. [Google Scholar] [CrossRef]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef] [PubMed]
- Broersen, K.; Jonckheere, W.; Rozenski, J.; Vandersteen, A.; Pauwels, K.; Pastore, A.; Rousseau, F.; Schymkowitz, J. A standardized and biocompatible preparation of aggregate-free amyloid beta peptide for biophysical and biological studies of Alzheimer’s disease. Protein Eng. Des. Sel. 2011, 24, 743–750. [Google Scholar] [CrossRef]
- Soura, V.; Stewart-Parker, M.; Williams, T.L.; Ratnayaka, A.; Atherton, J.; Gorringe, K.; Tuffin, J.; Darwent, E.; Rambaran, R.; Klein, W.; et al. Visualization of co-localization in Abeta42-administered neuroblastoma cells reveals lysosome damage and autophagosome accumulation related to cell death. Biochem. J. 2012, 441, 579–590. [Google Scholar] [CrossRef]
- Dumitrescu, A.V.; Drack, A.V. Gene Therapy for Blinding Pediatric Eye Disorders. Adv. Pediatr. 2015, 62, 185–210. [Google Scholar] [CrossRef] [PubMed]
- Lynn, S.A.; Ward, G.; Keeling, E.; Scott, J.A.; Cree, A.J.; Johnston, D.A.; Page, A.; Cuan-Urquizo, E.; Bhaskar, A.; Grossel, M.C.; et al. Ex-vivo models of the Retinal Pigment Epithelium (RPE) in long-term culture faithfully recapitulate key structural and physiological features of native RPE. Tissue Cell 2017, 49, 447–460. [Google Scholar] [CrossRef]
- Lynn, S.A.; Keeling, E.; Dewing, J.M.; Johnston, D.A.; Page, A.; Cree, A.J.; Tumbarello, D.A.; Newman, T.A.; Lotery, A.J.; Ratnayaka, J.A. A convenient protocol for establishing a human cell culture model of the outer retina. F1000Research 2018, 7, 1107. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Westenskow, P.D.; Moreno, S.K.; Krohne, T.U.; Kurihara, T.; Zhu, S.; Zhang, Z.N.; Zhao, T.; Xu, Y.; Ding, S.; Friedlander, M. Using flow cytometry to compare the dynamics of photoreceptor outer segment phagocytosis in iPS-derived RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6282–6290. [Google Scholar] [CrossRef] [PubMed]
- Keeling, E.; Chatelet, D.S.; Johnston, D.A.; Page, A.; Tumbarello, D.A.; Lotery, A.J.; Ratnayaka, J.A. Oxidative Stress and Dysfunctional Intracellular Traffic Linked to an Unhealthy Diet Results in Impaired Cargo Transport in the Retinal Pigment Epithelium (RPE). Mol. Nutr. Food Res. 2019, 63, 1800951. [Google Scholar] [CrossRef] [PubMed]
- Costes, S.V.; Daelemans, D.; Cho, E.H.; Dobbin, Z.; Pavlakis, G.; Lockett, S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys. J. 2004, 86, 3993–4003. [Google Scholar] [CrossRef]
- Krohne, T.U.; Holz, F.G.; Kopitz, J. Apical-to-basolateral transcytosis of photoreceptor outer segments induced by lipid peroxidation products in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 553–560. [Google Scholar] [CrossRef]
- Schraermeyer, U.; Enzmann, V.; Kohen, L.; Addicks, K.; Wiedemann, P.; Heimann, K. Porcine iris pigment epithelial cells can take up retinal outer segments. Exp. Eye Res. 1997, 65, 277–287. [Google Scholar] [CrossRef]
- Mao, Y.; Finnemann, S.C. Analysis of photoreceptor outer segment phagocytosis by RPE cells in culture. Methods Mol. Biol. 2013, 935, 285–295. [Google Scholar] [CrossRef]
- Finnemann, S.C. Role of avb5 integrin in regulating phagocytosis by the retinal pigment epithelium. In Retinal Degenerations; Advances in Experimental Medicine and Biology; LaVail, M.M., Hollyfield, J.G., Anderson, R.E., Eds.; Springer: Boston, MA, USA, 2003; Volume 533. [Google Scholar]
- Finnemann, S.C.; Silverstein, R.L. Differential Roles of CD36 and αvβ5 Integrin in Photoreceptor Phagocytosis by the Retinal Pigment Epithelium. J. Exp. Med. 2001, 194, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, F.; Safa, H.; Finnemann, S.C. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Exp. Eye Res. 2014, 126, 51–60. [Google Scholar] [CrossRef]
- Prakasam, A.; Muthuswamy, A.; Ablonczy, Z.; Greig, N.H.; Fauq, A.; Rao, K.J.; Pappolla, M.A.; Sambamurti, K. Differential accumulation of secreted AbetaPP metabolites in ocular fluids. J. Alzheimers Dis 2010, 20, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ohno-Matsui, K.; Ichinose, S.; Sato, T.; Iwata, N.; Saido, T.C.; Hisatomi, T.; Mochizuki, M.; Morita, I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J. Clin. Investig. 2005, 115, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Chow, K.M.; Derafshi, Z.; Fautsch, M.P.; Hetling, J.R.; Rodgers, D.W.; Hersh, L.B.; Pepperberg, D.R. Reduction of amyloid-beta levels in mouse eye tissues by intra-vitreally delivered neprilysin. Exp. Eye Res. 2015, 138, 134–144. [Google Scholar] [CrossRef]
- Keeling, E.; Lotery, A.J.; Tumbarello, D.A.; Ratnayaka, J.A. Impaired Cargo Clearance in the Retinal Pigment Epithelium (RPE) Underlies Irreversible Blinding Diseases. Cells 2018, 7, 16. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Penna, I.; Vella, S.; Gigoni, A.; Russo, C.; Cancedda, R.; Pagano, A. Selection of candidate housekeeping genes for normalization in human postmortem brain samples. Int. J. Mol. Sci. 2011, 12, 5461–5470. [Google Scholar] [CrossRef]
- Guymer, R.; Cipriani, T.; Rittenhouse, K.D.; Lim, L.; Robman, L.D.; Li, W.; Wang, W.; Deng, S.; Banerjee, P. Plasma levels of amyloid beta and other proinflammatory mediators in patients with age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1347–1354. [Google Scholar] [CrossRef]
- Alexandrov, P.N.; Pogue, A.; Bhattacharjee, S.; Lukiw, W.J. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer’s disease. Neuroreport 2011, 22, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, C.; Cerri, E.; Fabiani, C.; Capsoni, S.; Cattaneo, A.; Domenici, L. The retina as a window to early dysfunctions of Alzheimer’s disease following studies with a 5xFAD mouse model. Neurobiol. Aging 2018, 67, 181–188. [Google Scholar] [CrossRef]
- Park, S.W.; Im, S.; Jun, H.O.; Lee, K.; Park, Y.J.; Kim, J.H.; Park, W.J.; Lee, Y.H.; Kim, J.H. Dry age-related macular degeneration like pathology in aged 5XFAD mice: Ultrastructure and microarray analysis. Oncotarget 2017, 8, 40006–40018. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; van Lookeren, C.M. Geographic atrophy: Clinical features and potential therapeutic approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Berrow, E.J.; Bartlett, H.E.; Eperjesi, F.; Gibson, J.M. The electroretinogram: A useful tool for evaluating age-related macular disease? Doc. Ophthalmol. 2010, 121, 51–62. [Google Scholar] [CrossRef]
- Liu, C.; Cao, L.; Yang, S.; Xu, L.; Liu, P.; Wang, F.; Xu, D. Subretinal injection of amyloid-beta peptide accelerates RPE cell senescence and retinal degeneration. Int. J. Mol. Med. 2015, 35, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.; Shankar, G.M.; Mehta, T.; Walsh, D.M.; Selkoe, D.J. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: A potent role for trimers. J. Physiol. 2006, 572, 477–492. [Google Scholar] [CrossRef]
- Ramanathan, A.; Nelson, A.; Sagare, A.; Zlokovic, B. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: The role, regulation and restoration of LRP1. Front. Aging Neurosci. 2015, 7. [Google Scholar] [CrossRef]
- Ibbett, P.; Goverdhan, S.V.; Pipi, E.; Chouhan, J.K.; Keeling, E.; Angus, E.M.; Scott, J.A.; Gatherer, M.; Page, A.; Teeling, J.L.; et al. A lasered mouse model of retinal degeneration displays progressive outer retinal pathology providing insights into early geographic atrophy. Sci. Rep. 2019, 9, 7475. [Google Scholar] [CrossRef]
- Ratnayaka, J.A.; Lotery, A.J. Challenges in studying geographic atrophy (GA) age-related macular degeneration: The potential of a new mouse model with GA-like features. Neural Regen. Res. 2020, 15, 863–864. [Google Scholar] [CrossRef]
- Bruban, J.; Glotin, A.L.; Dinet, V.; Chalour, N.; Sennlaub, F.; Jonet, L.; An, N.; Faussat, A.M.; Mascarelli, F. Amyloid-beta(1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell 2009, 8, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; Ogura, S.; Baldeosingh, R.; McLeod, D.S.; Lutty, G.A.; Edwards, M.M. An Acute Injury Model for the Phenotypic Characteristics of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2018, 59. [Google Scholar] [CrossRef]
- Do, K.V.; Kautzmann, M.I.; Jun, B.; Gordon, W.C.; Nshimiyimana, R.; Yang, R.; Petasis, N.A.; Bazan, N.G. Elovanoids counteract oligomeric beta-amyloid-induced gene expression and protect photoreceptors. Proc. Natl. Acad. Sci. USA 2019, 116, 24317–24325. [Google Scholar] [CrossRef]
- Cunvong, K.; Huffmire, D.; Ethell, D.W.; Cameron, D.J. Amyloid-beta increases capillary bed density in the adult zebrafish retina. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1516–1521. [Google Scholar] [CrossRef]
- Cao, L.; Wang, H.; Wang, F.; Xu, D.; Liu, F.; Liu, C. Abeta-induced senescent retinal pigment epithelial cells create a proinflammatory microenvironment in AMD. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3738–3750. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, J.H.; Park, S.M.; Moon, M.; Lee, K.H.; Park, K.H.; Park, W.J.; Kim, J.H. RAGE mediated intracellular Abeta uptake contributes to the breakdown of tight junction in retinal pigment epithelium. Oncotarget 2015, 6, 35263–35273. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J. Senescence in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 2020, 77, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ohno-Matsui, K.; Morita, I. Elevated amyloid beta production in senescent retinal pigment epithelium, a possible mechanism of subretinal deposition of amyloid beta in age-related macular degeneration. Biochem. Biophys. Res. Commun. 2012, 423, 73–78. [Google Scholar] [CrossRef]
- Tarau, I.S.; Berlin, A.; Curcio, C.A.; Ach, T. The Cytoskeleton of the Retinal Pigment Epithelium: From Normal Aging to Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3578. [Google Scholar] [CrossRef] [PubMed]
- Chafekar, S.M.; Baas, F.; Scheper, W. Oligomer-specific Abeta toxicity in cell models is mediated by selective uptake. Biochim. Biophys. Acta 2008, 1782, 523–531. [Google Scholar] [CrossRef]
- Edgar, J.R.; Willen, K.; Gouras, G.K.; Futter, C.E. ESCRTs regulate amyloid precursor protein sorting in multivesicular bodies and intracellular amyloid-beta accumulation. J. Cell Sci. 2015, 128, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Crick, S.L.; Bu, G.; Frieden, C.; Pappu, R.V.; Lee, J.M. Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc. Natl. Acad. Sci. USA 2009, 106, 20324–20329. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Q.; Zhou, Q.H.; Ji, S.R.; Zhou, Q.; Feng, D.; Wu, Y.; Sui, S.F. Membrane localization of beta-amyloid 1-42 in lysosomes: A possible mechanism for lysosome labilization. J. Biol. Chem. 2010, 285, 19986–19996. [Google Scholar] [CrossRef]
- Xu, H.; Ren, D. Lysosomal physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Cyphersmith, A.; Zapata, J.A.; Kim, Y.J.; Payne, C.K. Lysosome transport as a function of lysosome diameter. PLoS ONE 2014, 9, e86847. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Piippo, N.; Korhonen, E.; Hytti, M.; Kinnunen, K.; Kaarniranta, K.; Kauppinen, A. Oxidative Stress is the Principal Contributor to Inflammasome Activation in Retinal Pigment Epithelium Cells with Defunct Proteasomes and Autophagy. Cell. Physiol. Biochem. 2018, 49, 359–367. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, H.; He, D.; Wang, Y.; Cai, B.; Chen, J.; Ma, J.; Liu, Z.; Wu, Y. Retinal Pigment Epithelium Cell Death Is Associated With NLRP3 Inflammasome Activation by All-trans Retinal. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3034–3045. [Google Scholar] [CrossRef]
- Marshall, K.E.; Vadukul, D.M.; Staras, K.; Serpell, L.C. Misfolded amyloid-beta-42 impairs the endosomal-lysosomal pathway. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef]
- Bosch, E.; Horwitz, J.; Bok, D. Phagocytosis of outer segments by retinal pigment epithelium: Phagosome-lysosome interaction. J. Histochem. Cytochem. 1993, 41, 253–263. [Google Scholar] [CrossRef]
- Kayser, L.; Perrild, H.; Thomsen, J.; Hoyer, P.E. Microfluorometric kinetic analysis of cathepsin B activity in single human thyroid follicular epithelial cells using image analysis and continuous monitoring. Histochem. J. 1996, 28, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Shoji, A.; Kabeya, M.; Ishida, Y.; Yanagida, A.; Shibusawa, Y.; Sugawara, M. Evaluation of cathepsin B activity for degrading collagen IV using a surface plasmon resonance method and circular dichroism spectroscopy. J. Pharm. Biomed. Anal. 2014, 95, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, P.; Smit-McBride, Z.; Oltjen, S.L.; Hjelmeland, L.M. Regulation of cysteine cathepsin expression by oxidative stress in the retinal pigment epithelium/choroid of the mouse. Exp. Eye Res. 2006, 83, 679–687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Notomi, S.; Ishihara, K.; Efstathiou, N.E.; Lee, J.J.; Hisatomi, T.; Tachibana, T.; Konstantinou, E.K.; Ueta, T.; Murakami, Y.; Maidana, D.E.; et al. Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc. Natl. Acad. Sci. USA 2019, 116, 23724–23734. [Google Scholar] [CrossRef] [PubMed]







| Product Name | Company | Catalogue Number | Clone/Isotype | Species | Dilution | RRID |
|---|---|---|---|---|---|---|
| ZO-1 Antibody | Invitrogen | 40–2200 | IgG, pAb | Rabbit | 1:100 | AB_2533456 |
| Anti-human Amyloid β (N) (82E1) | IBL | 10323 | 82E1, IgG1, mAb | Mouse | 1:100 | AB_10707424 |
| Anti-β3-Tubulin | Abcam | ab18207 | IgG, pAb | Rabbit | 1:200 | AB_444319 |
| Anti-LAMP1 | Abcam | ab24170 | IgG, pAb | Rabbit | 1:1000 | AB_775978 |
| Anti-Rhodopsin [RET-P1] | Abcam | ab3267 | IgG1,mAb | Mouse | 1:100 | AB_303655 |
| Anti-PSD-95 | Cell Signaling Technologies | 2507s | pAb | Rabbit | 1:100 | AB_561221 |
| Anti-LRP1 [EPR3724] | Abcam | ab92544 | IgG, mAb | Rabbit | 1:500 | AB_2234877 |
| Anti-Rabbit Alexa Fluor® 594 | Life Technologies | A11072 | IgG, pAb | Goat | 1:200 | AB_142057 |
| Anti-Mouse Alexa Fluor® 594 | Life Technologies | A11020 | IgG, pAb | Goat | 1:200 | AB_141974 |
| Gene | Accession | Sequence (5′–3′) | Tm (°C) | GC Content (%) | Amplicon Length (bp) |
|---|---|---|---|---|---|
| CTSB | NM_001908.4 | F: GGGCCGGGAGGGTACTTA | 60.0 | 66.7 | 145 |
| R: GATCCTAGATCCACCCAGCG | 59.4 | 60.0 | |||
| ACTB | NM_001101.3 | F: ACAGAGCCTCGCCTTTGCC | 62.9 | 63.2 | 70 |
| R: GATATCATCATCCATGGTGAGCTGG | 61.2 | 48.0 | |||
| GAPDH | NM_001289745.1 | F: GAAGACGGGCGGAGAGAAAC | 60.7 | 60.0 | 151 |
| R: CGACCAAATCCGTTGACTCC | 58.9 | 55.0 | |||
| CYC1 | NM_001916.4 | F: TACGGACACCTCAGGCAGT | 60.2 | 57.9 | 183 |
| R: CACGGTGAGACCACGGATAG | 59.9 | 60.0 | |||
| EIF4A2 | NM_001967.3 | F: GGTCAGGGTCAAGTCGTGTT | 59.9 | 55.0 | 136 |
| R: CCCCCTCTGCCAATTCTGTG | 60.7 | 60.0 |
| ERG Component | Week 1 | Week 2 | ||
|---|---|---|---|---|
| Vehicle | Aβ | Vehicle | Aβ | |
| A Wave | 39.3 ± 40.2 | 25.7 ± 24.6 | 100.2 ± 71.3 | 98.8 ± 63.1 |
| B Wave | −7.1 ± 19.6 | 4.7 ± 12.0 | 34.8 ± 32.1 | 53.5 ± 30.7 |
| T(A) | −10.1 ± 9.4 | 7.2 ± 12.2 | −17.4 ± 7.0 | −0.8 ± 6.0 |
| T(B) | −41.3 ± 19.3 | −49.3 ± 14.3 | −37.6 ± 19.9 | −52.3 ± 15.2 |
| Retinal Layer | Change in Layer Thickness (%) | |||
|---|---|---|---|---|
| Week 1 | Week 2 | |||
| Vehicle | Aβ | Vehicle | Aβ | |
| RNFL | −9.2 ± 5.9 | −22.9 ± 5.3 | −21.9 ± 4.4 | −31.8 ± 6.8 |
| GCL/IPL | −7.0 ± 3 | −4.4 ± 2.6 | −10.7 ± 3.2 | −7.2 ± 2.5 |
| INL | 7.1 ± 4.5 | 14.6 ± 4.8 | 2.5 ± 4.1 | −4.7 ± 2.9 |
| OPL | 5.4 ± 9.4 | −12.9 ± 10.4 | −18.9 ± 10.9 | 13.0 ± 31.6 |
| ONL | −2.1 ± 6.0 | −0.06 ± 7.9 | 0.007 ± 8.0 | −2.0 ± 6.2 |
| IS | 23.5 ± 14.7 | 3.65 ± 9.0 | −4.0 ± 10.4 | 8.6 ± 10.6 |
| OS | −2.8 ± 8.3 | −9.6 ± 6.3 | 1.3 ± 9.5 | −10.7 ± 8.0 |
| ETPRS | −4.1 ± 13.1 | −7.1 ± 7.6 | −4.5 ± 13.8 | −7.3 ± 7.7 |
| RPE | 10.3 ± 21.5 | −14.2 ± 18.8 | −5.5 ± 14.2 | −22.4 ± 8.4 |
| RT | −2.2 ± 2.4 | −3.6 ± 2.1 | −2.9 ± 1.6 | −3.1 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynn, S.A.; Johnston, D.A.; Scott, J.A.; Munday, R.; Desai, R.S.; Keeling, E.; Weaterton, R.; Simpson, A.; Davis, D.; Freeman, T.; et al. Oligomeric Aβ1-42 Induces an AMD-Like Phenotype and Accumulates in Lysosomes to Impair RPE Function. Cells 2021, 10, 413. https://doi.org/10.3390/cells10020413
Lynn SA, Johnston DA, Scott JA, Munday R, Desai RS, Keeling E, Weaterton R, Simpson A, Davis D, Freeman T, et al. Oligomeric Aβ1-42 Induces an AMD-Like Phenotype and Accumulates in Lysosomes to Impair RPE Function. Cells. 2021; 10(2):413. https://doi.org/10.3390/cells10020413
Chicago/Turabian StyleLynn, Savannah A., David A. Johnston, Jenny A. Scott, Rosie Munday, Roshni S. Desai, Eloise Keeling, Ruaridh Weaterton, Alexander Simpson, Dillon Davis, Thomas Freeman, and et al. 2021. "Oligomeric Aβ1-42 Induces an AMD-Like Phenotype and Accumulates in Lysosomes to Impair RPE Function" Cells 10, no. 2: 413. https://doi.org/10.3390/cells10020413
APA StyleLynn, S. A., Johnston, D. A., Scott, J. A., Munday, R., Desai, R. S., Keeling, E., Weaterton, R., Simpson, A., Davis, D., Freeman, T., Chatelet, D. S., Page, A., Cree, A. J., Lee, H., Newman, T. A., Lotery, A. J., & Ratnayaka, J. A. (2021). Oligomeric Aβ1-42 Induces an AMD-Like Phenotype and Accumulates in Lysosomes to Impair RPE Function. Cells, 10(2), 413. https://doi.org/10.3390/cells10020413