Effect of Alpha-Glucosyl-Hesperidin Consumption on Lens Sclerosis and Presbyopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. G-Hsd Treatment for Animals
2.4. Measurement of Lens Elasticity
2.5. Antioxidant Levels
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
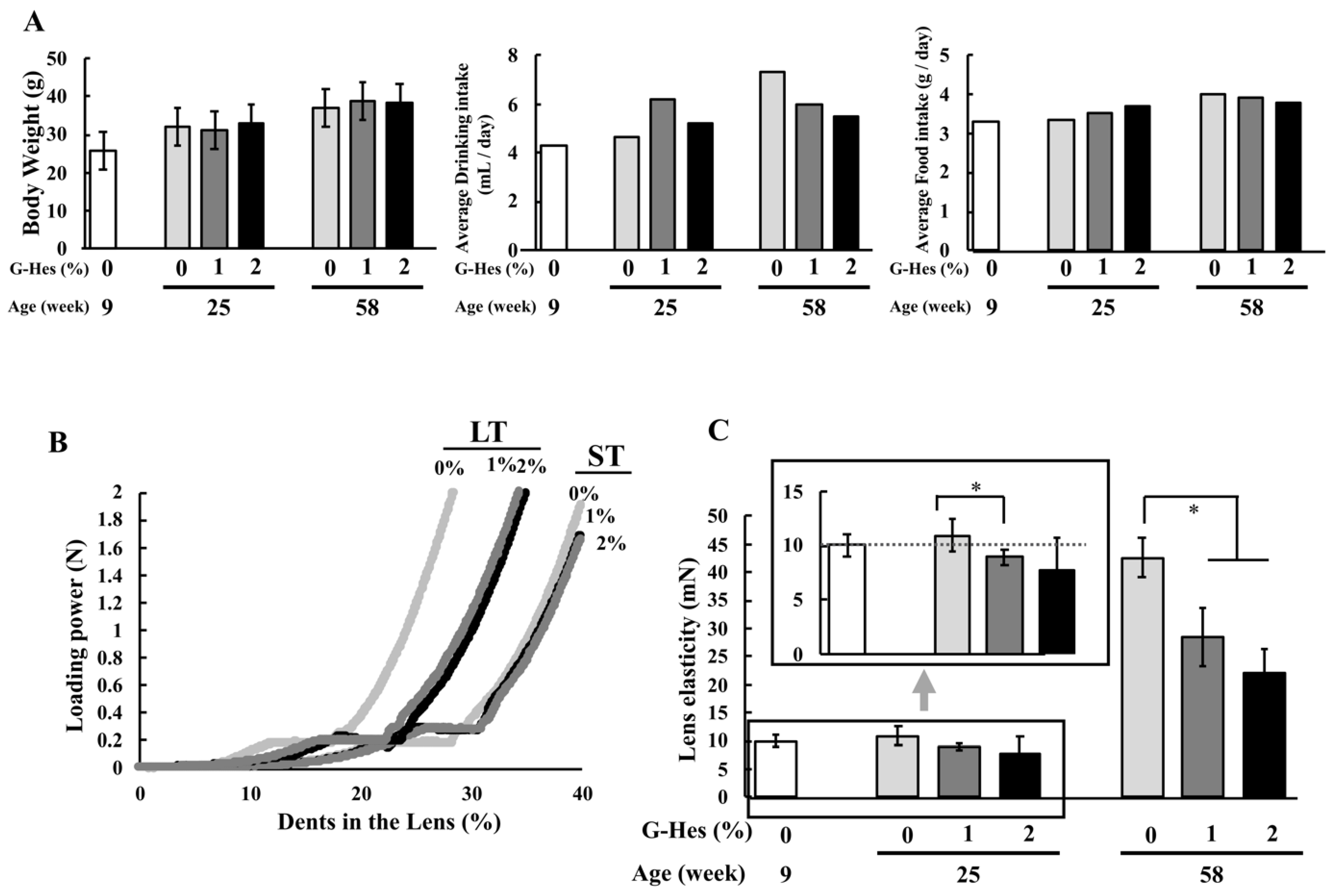
3.1. G-Hsd Consumption Ameliorated Lens Hardening
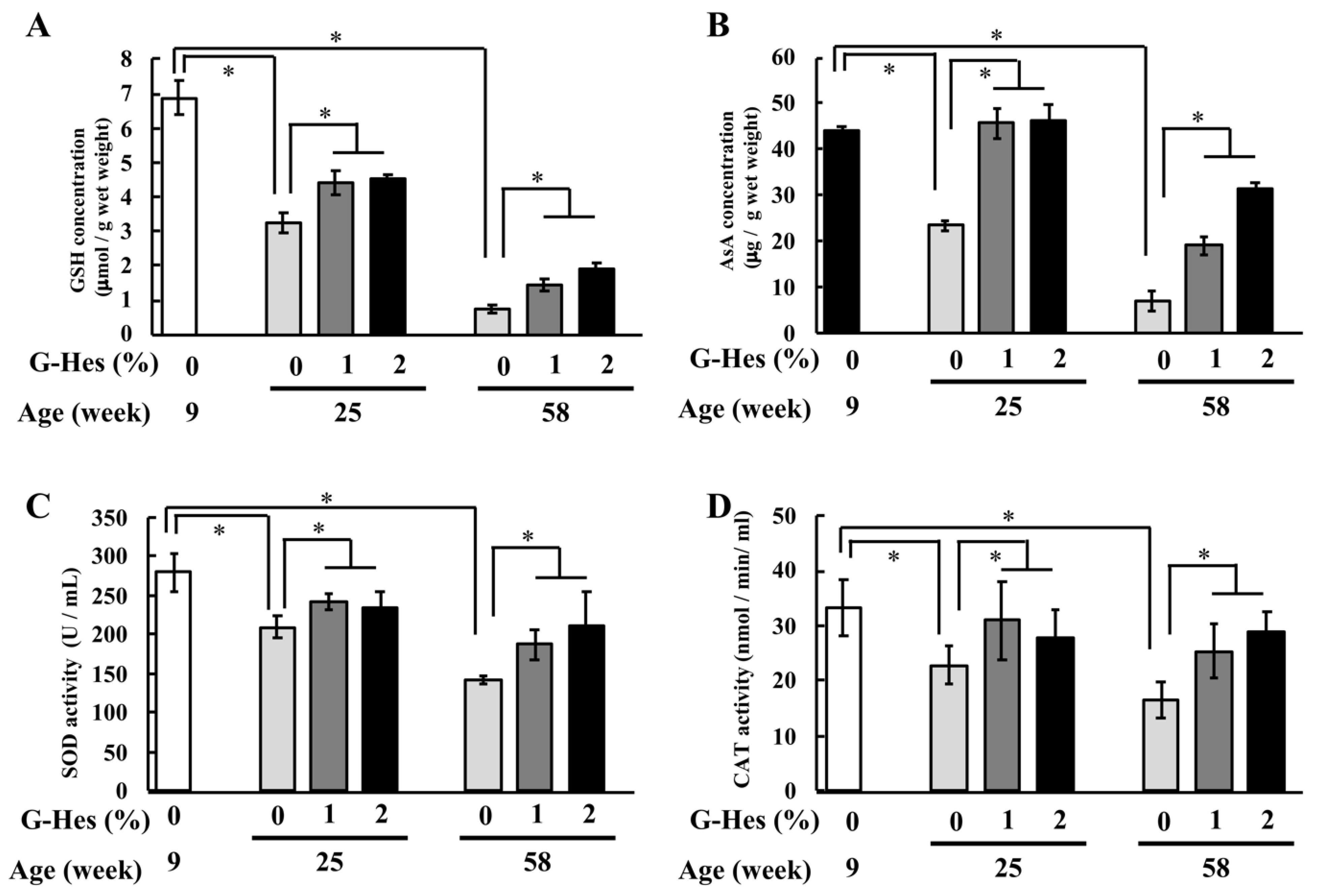
3.2. G-Hsd Treatment Affected the Antioxidant Ability
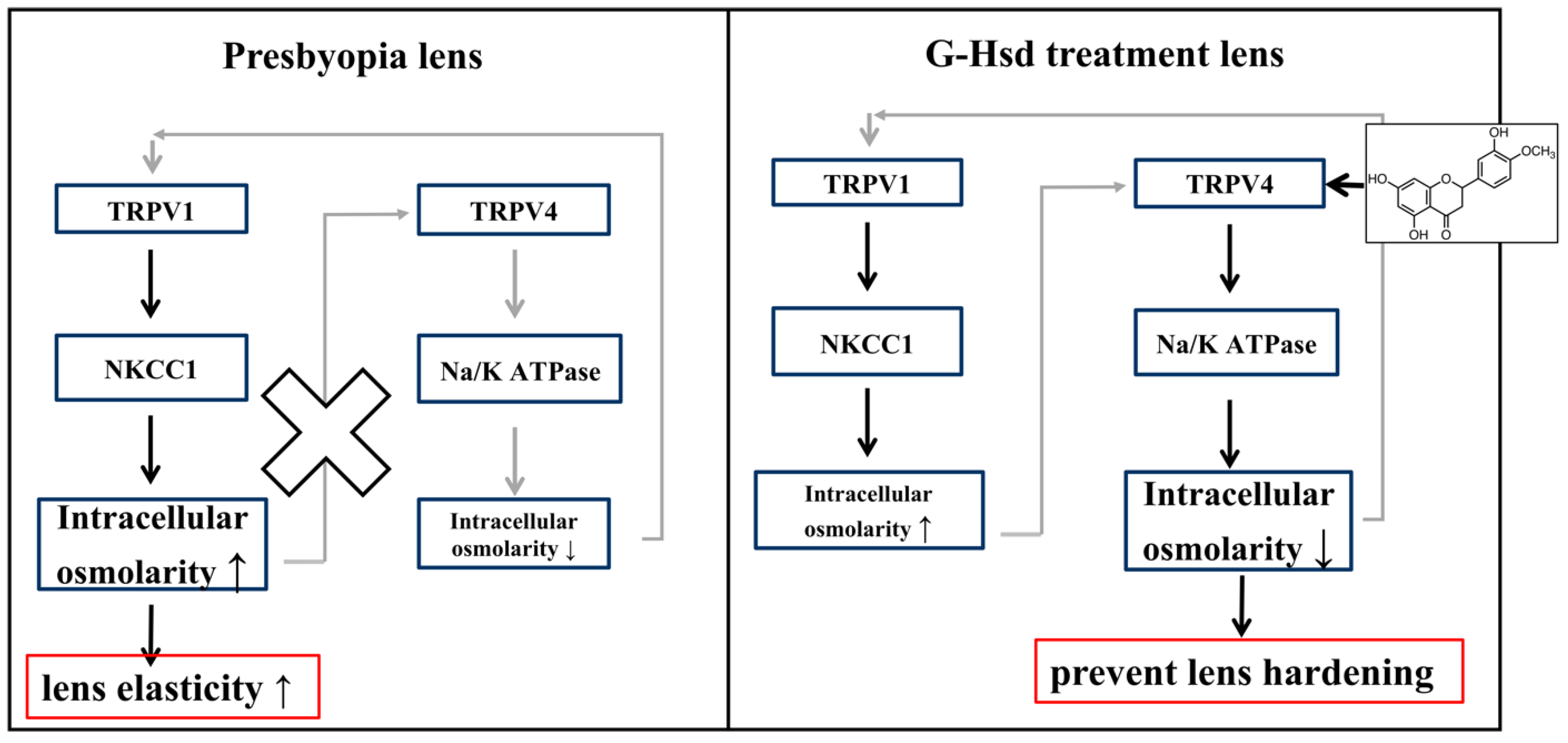
3.3. G-Hsd Treatment Altered TRPV4 Localization in the Peripheral Fibre Cells of the Lens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koopmans, S.A.; Terwee, T.; Barkhof, J.; Haitjema, H.J.; Kooijman, A.C. Polymer refilling of presbyopic human lenses in vitro restores the ability to undergo accommodative changes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 250–257. [Google Scholar] [CrossRef]
- McGinty, S.J.; Truscott, R.J. Presbyopia: The first stage of nuclear cataract? Ophthalmic Res. 2006, 38, 137–148. [Google Scholar] [CrossRef]
- Fernández, J.; Rodríguez-Vallejo, M.; Martínez, J.; Tauste, A.; Piñero, D.P. From Presbyopia to Cataracts: A Critical Review on Dysfunctional Lens Syndrome. J. Ophthalmol. 2018, 2018, 4318405. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Aoki, M.; Ishiwa, S.; Morishita, N.; Endo, S.; Nagai, N.; Yamamoto, N.; Funakoshi-Tago, M.; Tamura, H. Oral intake of alphaglucosylhesperidin ameliorates seleniteinduced cataract formation. Mol. Med. Rep. 2020, 21, 1258–1266. [Google Scholar] [CrossRef]
- Yamada, M.; Tanabe, F.; Arai, N.; Mitsuzumi, H.; Miwa, Y.; Kubota, M.; Chaen, H.; Kibata, M. Bioavailability of glucosyl hesperidin in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Yosuke Nakazawa, M.A.; Doki, Y.; Morishita, N.; Endo, S.; Nagai, N.; Funakoshi-Tago, M.; Tamura, H. Oral consumption of a-glucosyl-hesperidin could prevent lens hardening, which causes presbyopia. Biochem. Biophys. Rep. 2021. under review. [Google Scholar]
- Gao, J.; Wang, H.; Sun, X.; Varadaraj, K.; Li, L.; White, T.W.; Mathias, R.T. The effects of age on lens transport. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7174–7187. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A.; Mandal, A.; Shahidullah, M. The Significance of TRPV4 Channels and Hemichannels in the Lens and Ciliary Epithelium. J. Ocul. Pharmacol. Ther. 2016, 32, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sun, X.; White, T.W.; Delamere, N.A.; Mathias, R.T. Feedback Regulation of Intracellular Hydrostatic Pressure in Surface Cells of the Lens. Biophys. J. 2015, 109, 1830–1839. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Donaldson, P.J.; Petrova, R.S. Verification and spatial mapping of TRPV1 and TRPV4 expression in the embryonic and adult mouse lens. Exp. Eye Res. 2019, 186, 107707. [Google Scholar] [CrossRef] [PubMed]
- Petrova, R.S.; Bavana, N.; Zhao, R.; Schey, K.L.; Donaldson, P.J. Changes to Zonular Tension Alters the Subcellular Distribution of AQP5 in Regions of Influx and Efflux of Water in the Rat Lens. Investig. Ophthalmol. Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef]
- Ishimori, N.; Oguchi, J.; Nakazawa, Y.; Kobata, K.; Funakoshi-Tago, M.; Tamura, H. Roasting Enhances the Anti-Cataract Effect of Coffee Beans: Ameliorating Selenite-Induced Cataracts in Rats. Curr. Eye Res. 2017, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.D.; Donaldson, P.J.; Cannell, M.B.; Soeller, C. Resolving morphology and antibody labeling over large distances in tissue sections. Microsc. Res. Tech. 2003, 62, 83–91. [Google Scholar] [CrossRef]
- Petrova, R.S.; Schey, K.L.; Donaldson, P.J.; Grey, A.C. Spatial distributions of AQP5 and AQP0 in embryonic and postnatal mouse lens development. Exp. Eye Res. 2015, 132, 124–135. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Ho, S.M.; Wong, R.; Schlenther, G.; Cronje, S.; Burnett, A.; Papas, E.; Naidoo, K.S.; Frick, K.D. Global vision impairment due to uncorrected presbyopia. Arch. Ophthalmol. 2008, 126, 1731–1739. [Google Scholar] [CrossRef]
- Frick, K.D.; Joy, S.M.; Wilson, D.A.; Naidoo, K.S.; Holden, B.A. The Global Burden of Potential Productivity Loss from Uncorrected Presbyopia. Ophthalmology 2015, 122, 1706–1710. [Google Scholar] [CrossRef] [PubMed]
- Tsuneyoshi, Y.; Higuchi, A.; Negishi, K.; Tsubota, K. Suppression of presbyopia progression with pirenoxine eye drops: Experiments on rats and non-blinded, randomized clinical trial of efficacy. Sci. Rep. 2017, 7, 6819. [Google Scholar] [CrossRef]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-related changes in the kinetics of human lenses: Prevention of the cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [CrossRef]
- Xing, B.M.; Yang, Y.R.; Du, J.X.; Chen, H.J.; Qi, C.; Huang, Z.H.; Zhang, Y.; Wang, Y. Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J. Neurosci. 2012, 32, 14709–14721. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Almazi, J.G.; Darby, W.; Tovar-Lopez, F.J.; Mitchell, A.; McIntyre, P. Shear stress mediates exocytosis of functional TRPV4 channels in endothelial cells. Cell. Mol. Life Sci. 2016, 73, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; McNaughton, P.A. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008, 59, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Shahidullah, M.; Mandal, A.; Delamere, N.A. Activation of TRPV1 channels leads to stimulation of NKCC1 cotransport in the lens. Am. J. Physiol. Cell Physiol. 2018, 315, C793–C802. [Google Scholar] [CrossRef]
- Shahidullah, M.; Mandal, A.; Mathias, R.T.; Gao, J.; Krizaj, D.; Redmon, S.; Delamere, N.A. TRPV1 activation stimulates NKCC1 and increases hydrostatic pressure in the mouse lens. Am. J. Physiol. Cell Physiol. 2020, 318, C969–C980. [Google Scholar] [CrossRef] [PubMed]
- Pla-Paga, L.; Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Foguet-Romero, E.; Catalan, U.; Mayneris-Perxachs, J.; Canela, N.; Valls, R.M.; Arola, L.; et al. Proteomic Analysis of Heart and Kidney Tissues in Healthy and Metabolic Syndrome Rats after Hesperidin Supplementation. Mol. Nutr. Food Res. 2020, 64, e1901063. [Google Scholar] [CrossRef]
- Umran, N.S.S.; Mohamed, S.; Lau, S.F.; Mohd Ishak, N.I. Citrus hystrix leaf extract attenuated diabetic-cataract in STZ-rats. J. Food Biochem. 2020, 44, e13258. [Google Scholar] [CrossRef]
- Bassnett, S. Lens organelle degradation. Exp. Eye Res. 2002, 74, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morishita, H.; Mizushima, N. Autophagy in the lens. Exp. Eye Res. 2016, 144, 22–28. [Google Scholar] [CrossRef]



| Age | 9 weeks | 25 weeks (9 + 16) | 37 weeks (9 + 28) (#) | 58 weeks (9 + 49) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G-hsd | 0% (control) | 0% | 1% | 2% | 0% | 1% | 2% | 0% | 1% | 2% |
| Lens elasticity (mN) | 10.0 ± 1.08 | 11.0 ± 1.57 | 8.93 ± 0.66 | 7.75 ± 2.96 | 11.6 ± 0.39 | 7.30 ± 0.78 | 7.95 ± 0.44 | 42.6 ± 3.44 | 28.6 ± 5.14 | 22.1 ± 4.23 |
| GSH (mg/g) | 6.87 ± 0.50 | 3.50 ± 0.29 | 4.41 ± 0.33 | 4.53 ± 0.10 | 1.06 ± 0.11 | 3.24 ± 0.58 | 4.12 ± 0.42 | 0.73 ± 0.11 | 1.44 ± 0.17 | 1.91 ± 0.16 |
| AsA (mg/g) | 44.2 ± 0.80 | 23.4 ± 1.26 | 45.8 ± 3.26 | 46.1 ± 3.47 | 17.1 ± 1.20 | 24.4 ± 1.30 | 26.2 ± 4.00 | 7.01 ± 2.06 | 19.0 ± 2.00 | 31.4 ± 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakazawa, Y.; Doki, Y.; Sugiyama, Y.; Kobayashi, R.; Nagai, N.; Morisita, N.; Endo, S.; Funakoshi-Tago, M.; Tamura, H. Effect of Alpha-Glucosyl-Hesperidin Consumption on Lens Sclerosis and Presbyopia. Cells 2021, 10, 382. https://doi.org/10.3390/cells10020382
Nakazawa Y, Doki Y, Sugiyama Y, Kobayashi R, Nagai N, Morisita N, Endo S, Funakoshi-Tago M, Tamura H. Effect of Alpha-Glucosyl-Hesperidin Consumption on Lens Sclerosis and Presbyopia. Cells. 2021; 10(2):382. https://doi.org/10.3390/cells10020382
Chicago/Turabian StyleNakazawa, Yosuke, Yuri Doki, Yuki Sugiyama, Ryota Kobayashi, Noriaki Nagai, Naoki Morisita, Shin Endo, Megumi Funakoshi-Tago, and Hiroomi Tamura. 2021. "Effect of Alpha-Glucosyl-Hesperidin Consumption on Lens Sclerosis and Presbyopia" Cells 10, no. 2: 382. https://doi.org/10.3390/cells10020382
APA StyleNakazawa, Y., Doki, Y., Sugiyama, Y., Kobayashi, R., Nagai, N., Morisita, N., Endo, S., Funakoshi-Tago, M., & Tamura, H. (2021). Effect of Alpha-Glucosyl-Hesperidin Consumption on Lens Sclerosis and Presbyopia. Cells, 10(2), 382. https://doi.org/10.3390/cells10020382






