The NFKB1 Promoter Polymorphism (-94ins/delATTG) Is Associated with Susceptibility to Cytomegalovirus Infection after Kidney Transplantation and Should Have Implications on CMV Prophylaxis Regimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatments
2.3. Clinical Definitions
2.4. DNA Genotyping
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
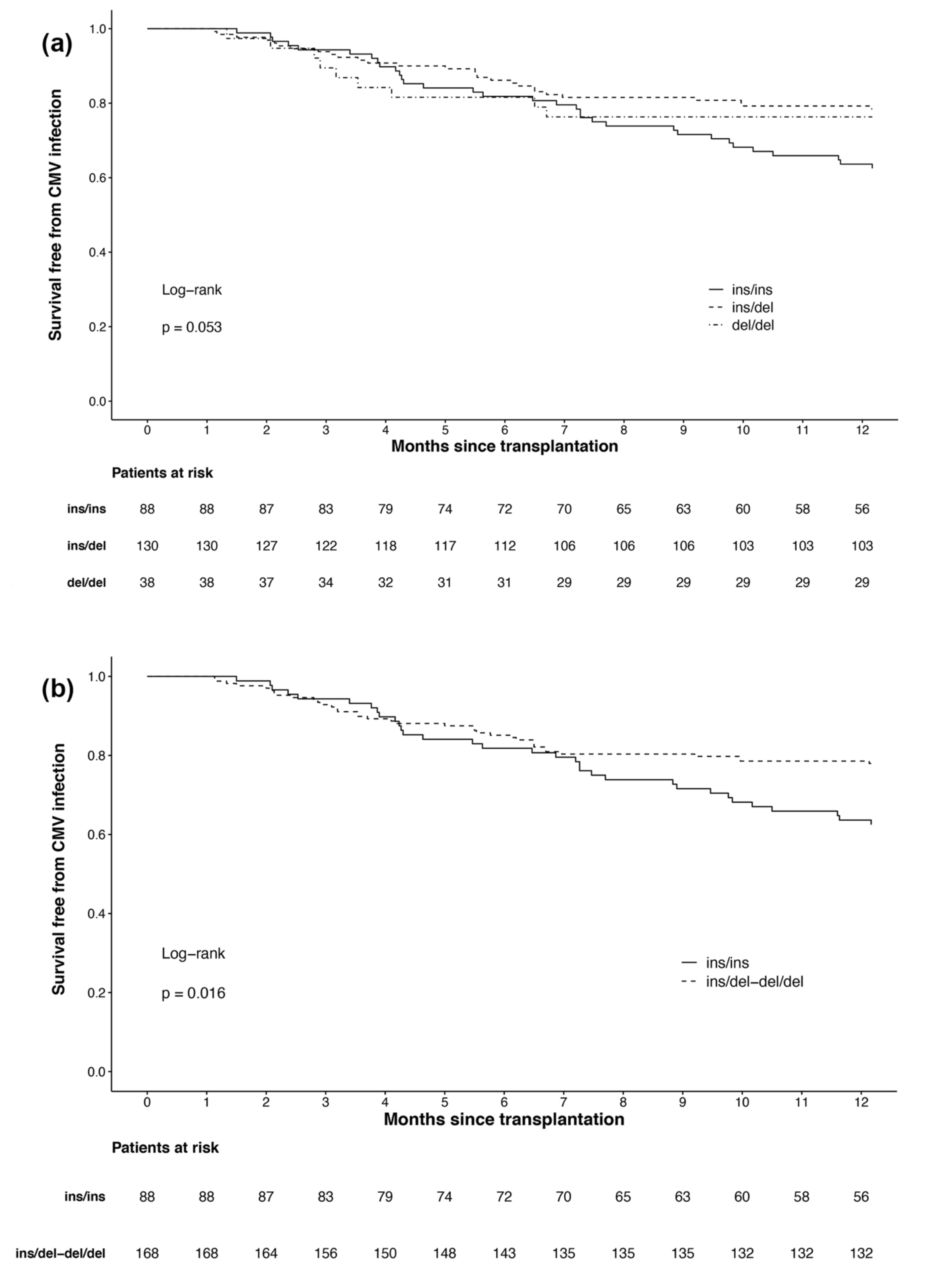
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fishman, J.A.; Rubin, R.H. Infection in organ-transplant recipients. N. Engl. J. Med. 1998, 338, 1741–1751. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Asberg, A.; Chou, S.; Danziger-Isakov, L.; Humar, A.; The Transplantation Society International CMV Consensus Group. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013, 96, 333–360. [Google Scholar] [CrossRef]
- Hartmann, A.; Sagedal, S.; Hjelmesaeth, J. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation 2006, 82, S15–S17. [Google Scholar] [CrossRef]
- Gjertson, D.W. Look-up survival tables for living-donor renal transplants: OPTN/UNOS data 1995-2002. Clin. Transpl. 2003, 337–386. Available online: https://pubmed.ncbi.nlm.nih.gov/15387123/ (accessed on 9 February 2021).
- Fehr, T.; Cippa, P.E.; Mueller, N.J. Cytomegalovirus post kidney transplantation: Prophylaxis versus pre-emptive therapy? Transpl. Int. 2015, 28, 1351–1356. [Google Scholar] [CrossRef]
- Witzke, O.; Hauser, I.A.; Bartels, M.; Wolf, G.; Wolters, H.; Nitschke, M.; Group, V.S. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 2012, 93, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.A.; Storch, G.A.; Bohl, D.L.; Schuessler, R.M.; Torrence, S.M.; Lockwood, M.; Gaudreault-Keener, M.; Koch, M.J.; Miller, B.W.; Hardinger, K.L.; et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am. J. Transplant. 2006, 6, 2134–2143. [Google Scholar] [CrossRef]
- Reischig, T.; Jindra, P.; Hes, O.; Svecova, M.; Klaboch, J.; Treska, V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am. J. Transplant. 2008, 8, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, M.; Corrales, I.; Arias, M.; Campistol, J.M.; Gimenez, E.; Crespo, J.; Lopez-Oliva, M.O.; Beneyto, I.; Martin-Moreno, P.L.; Llamas-Fuente, F.; et al. Association between individual and combined SNPs in genes related to innate immunity and incidence of CMV infection in seropositive kidney transplant recipients. Am. J. Transplant. 2015, 15, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Rahmel, T.; Nowak, H.; Rump, K.; Koos, B.; Schenker, P.; Viebahn, R.; Adamzik, M.; Bergmann, L. The Aquaporin 5 -1364A/C Promoter Polymorphism Is Associated With Cytomegalovirus Infection Risk in Kidney Transplant Recipients. Front. Immunol. 2019, 10, 2871. [Google Scholar] [CrossRef]
- Ono, G.; Medina Pestana, J.O.; Aranha Camargo, L.F. Late cytomegalovirus (CMV) infections after kidney transplantation under the preemptive strategy: Risk factors and clinical aspects. Transpl. Infect. Dis. 2019, 21, e13035. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Hancock, M.H.; Nelson, J.A. Modulation of the NFkappab Signalling Pathway by Human Cytomegalovirus. Virology 2017, 1, 104. [Google Scholar] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFkappaB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Adamzik, M.; Frey, U.H.; Rieman, K.; Sixt, S.; Beiderlinden, M.; Siffert, W.; Peters, J. Insertion/deletion polymorphism in the promoter of NFKB1 influences severity but not mortality of acute respiratory distress syndrome. Intensive Care Med. 2007, 33, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Wang, F.; Feng, X.L.; Tao, J.H.; Zhu, J.M.; Pan, F.M.; Su, H. Association of NFKB1 -94ins/delATTG promoter polymorphism with susceptibility to autoimmune and inflammatory diseases: A meta-analysis. Tissue Antigens 2011, 77, 9–17. [Google Scholar] [CrossRef]
- Schafer, S.T.; Gessner, S.; Scherag, A.; Rump, K.; Frey, U.H.; Siffert, W.; Westendorf, A.M.; Steinmann, J.; Peters, J.; Adamzik, M. Hydrocortisone fails to abolish NF-kappaB1 protein nuclear translocation in deletion allele carriers of the NFKB1 promoter polymorphism (-94ins/delATTG) and is associated with increased 30-day mortality in septic shock. PLoS ONE 2014, 9, e104953. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Wang, D.; Gong, T.; Zhu, J. An updated meta-analysis of 37 case-control studies on the association between NFKB1 -94ins/del ATTG promoter polymorphism and cancer susceptibility. Oncotarget 2016, 7, 58659–58670. [Google Scholar] [CrossRef]
- Leone, F.; Gigliotti, P.; La Russa, A.; Lofaro, D.; Perri, A.; Vizza, D.; Lupinacci, S.; Toteda, G.; Bonofiglio, M.; Presta, P.; et al. NFKB1 promoter polymorphism: A new predictive marker of cytomegalovirus infection after kidney transplantation. Transpl. Infect. Dis. 2019, 21, e13027. [Google Scholar] [CrossRef] [PubMed]
- Valachis, A.; Mauri, D.; Neophytou, C.; Polyzos, N.P.; Tsali, L.; Garras, A.; Papanikolau, E.G. Translational medicine and reliability of single-nucleotide polymorphism studies: Can we believe in SNP reports or not? Int. J. Med. Sci. 2011, 8, 492–500. [Google Scholar] [CrossRef][Green Version]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D.; et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. 2017, 64, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Fryer, J.F.; Heath, A.B.; Anderson, R.; Minor, P.D.; World Health Organization Biologicals Unit. Collaborative Study to Evaluate the Proposed 1st [first] WHO International Standard for Human Cytomegalovirus (HCMV) for Nucleic Acid Amplification (NAT)-Based Assays. Available online: https://apps.who.int/iris/handle/10665/70521 (accessed on 18 June 2020).
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Peralvarez, M.; Rico-Juri, J.M.; Tsochatzis, E.; Burra, P.; De la Mata, M.; Lerut, J. Biopsy-proven acute cellular rejection as an efficacy endpoint of randomized trials in liver transplantation: A systematic review and critical appraisal. Transpl. Int. 2016, 29, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Paya, C.; Humar, A.; Dominguez, E.; Washburn, K.; Blumberg, E.; Alexander, B.; Freeman, R.; Heaton, N.; Pescovitz, M.D.; Valganciclovir Solid Organ Transplant Study Group. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am. J. Transplant. 2004, 4, 611–620. [Google Scholar] [CrossRef]
- Humar, A.; Lebranchu, Y.; Vincenti, F.; Blumberg, E.A.; Punch, J.D.; Limaye, A.P.; Abramowicz, D.; Jardine, A.G.; Voulgari, A.T.; Ives, J.; et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am. J. Transplant. 2010, 10, 1228–1237. [Google Scholar] [CrossRef]
- Harvala, H.; Stewart, C.; Muller, K.; Burns, S.; Marson, L.; MacGilchrist, A.; Johannessen, I. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J. Med. Virol. 2013, 85, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, S.K.; Eid, A.J.; Pedersen, R.A.; Kremers, W.K.; Cosio, F.G.; Patel, R.; Razonable, R.R. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin. Infect. Dis. 2008, 46, 840–846. [Google Scholar] [CrossRef]
- DeMeritt, I.B.; Podduturi, J.P.; Tilley, A.M.; Nogalski, M.T.; Yurochko, A.D. Prolonged activation of NF-kappaB by human cytomegalovirus promotes efficient viral replication and late gene expression. Virology 2006, 346, 15–31. [Google Scholar] [CrossRef]
- DeMeritt, I.B.; Milford, L.E.; Yurochko, A.D. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J. Virol. 2004, 78, 4498–4507. [Google Scholar] [CrossRef]
- Kowalik, T.F.; Wing, B.; Haskill, J.S.; Azizkhan, J.C.; Baldwin, A.S., Jr.; Huang, E.S. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 1993, 90, 1107–1111. [Google Scholar] [CrossRef]
- Montag, C.; Wagner, J.; Gruska, I.; Hagemeier, C. Human cytomegalovirus blocks tumor necrosis factor alpha- and interleukin-1beta-mediated NF-kappaB signaling. J. Virol. 2006, 80, 11686–11698. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.A.; Borton, J.A.; Keech, A.M.; Wong, J.; Britt, W.J.; Magun, B.E.; Nelson, J.A. Human cytomegalovirus attenuates interleukin-1beta and tumor necrosis factor alpha proinflammatory signaling by inhibition of NF-kappaB activation. J. Virol. 2006, 80, 5588–5598. [Google Scholar] [CrossRef] [PubMed]
- Adamzik, M.; Schafer, S.; Frey, U.H.; Becker, A.; Kreuzer, M.; Winning, S.; Frede, S.; Steinmann, J.; Fandrey, J.; Zacharowski, K.; et al. The NFKB1 promoter polymorphism (-94ins/delATTG) alters nuclear translocation of NF-kappaB1 in monocytes after lipopolysaccharide stimulation and is associated with increased mortality in sepsis. Anesthesiology 2013, 118, 123–133. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | ins 1/ins (n = 88) | ins/del 2 (n = 130) | del/del (n = 38) | p |
|---|---|---|---|---|
| Recipient age (y), mean ± SD | 52.48 ± 11.51 | 53.09 ± 12.91 | 55.53 ± 12.16 | 0.452 |
| Male sex, n (%) | 59 (67.0) | 80 (61.5) | 26 (68.4) | 0.606 |
| Body mass index (kg/m2), mean ± SD | 25.53 ± 3.72 | 25.87 ± 5.09 | 26.66 ± 3.78 | 0.466 |
| Type of transplantation, n (%) | ||||
| Kidney | 58 (65.9) | 99 (76.2) | 25 (65.8) | 0.193 |
| Combined kidney and pancreas | 30 (34.1) | 31 (23.8) | 13 (34.2) | |
| Donor age (y), mean ± SD | 48.69 ± 16.24 | 51.95 ± 16.76 | 53.76 ± 18.95 | 0.220 |
| Cold ischemia time for kidney (h), mean ± SD | 11.35 ± 4.31 | 11.42 ± 5.40 | 11.16 ± 5.44 | 0.961 |
| Delayed graft function, n (%) | 24 (27.3) | 37 (28.5) | 9 (23.7) | 0.887 |
| HLA 3 mismatch, n (%) | ||||
| 0–1 | 12 (13.6) | 19 (14.6) | 3 (7.9) | 0.345 |
| 2–4 | 51 (58.0) | 73 (56.2) | 17 (44.7) | |
| ≥ 5 | 21 (23.9) | 31 (23.8) | 13 (34.2) | |
| Unknown | 4 (4.5) | 7 (5.4) | 5 (13.2) | |
| Induction with ATG 4, n (%) | 77 (87.50) | 107 (82.31) | 32 (84.21) | 0.584 |
| Immunosuppressive regimen, n (%) | ||||
| MMF 5, prednisone and tacrolimus | 70 (79.5) | 94 (72.3) | 32 (84.2) | 0.194 |
| MMF, prednisone and cyclosporine | 8 (9.1) | 11 (8.5) | 0 (0.00) | |
| Other | 10 (11.4) | 25 (19.2) | 6 (15.8) | |
| Pretransplant CMV 6 donor (D)/recipient (R) serostatus, n (%) | ||||
| High risk (D+/R–) 7 | 18 (20.5) | 29 (22.3) | 9 (23.7) | 0.294 |
| Medium risk (D+/–/R+) 8 | 61 (69.3) | 72 (55.4) | 21 (55.3) | |
| Low risk (D–/R–) 9 | 9 (10.2) | 26 (20.0) | 7 (18.4) | |
| Unknown | 0 (0.00) | 3 (2.3) | 1 (2.6) | |
| Prophylactic anti-CMV therapy, n (%) | ||||
| Perioperative | 5 (5.7) | 21 (16.2) | 3 (7.9) | 0.251 |
| 3 months | 55 (62.5) | 77 (59.2) | 24 (63.2) | |
| 6 months | 24 (27.3) | 25 (19.2) | 10 (26.3) | |
| Unknown | 4 (4.5) | 7 (5.4) | 1 (2.6) |
| Outcome | ins 1/ins (n = 88) | ins/del 2 (n = 130) | del/del (n = 38) | p |
|---|---|---|---|---|
| CMV 3 infection, n (%) | 33 (37.5) | 28 (21.5) | 9 (23.7) | 0.023 |
| Donor/Recipient CMV serostatus | ||||
| D+/R– 4 (n = 18/56; 32.1%) | 7 (21.2) | 9 (32.1) | 2 (22.2) | 0.610 |
| D+/–/R+ 5 (n = 46/154; 29.9%) | 24 (72.7) | 17 (60.7) | 5 (55.6) | 0.108 |
| D–/R– 6 (n = 5/42; 11.9%) | 2 (6.1) | 2 (7.1) | 1 (11.1) | 0.418 |
| Unknown (n = 1/4; 25.0%) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 0.665 |
| Duration of CMV chemoprophylaxis | ||||
| Perioperative (n = 5/29; 17.2%) | 1 (3.0) | 3 (10.7) | 1 (11.1) | 0.705 |
| 3 months (n = 38/156; 24.4%) | 18 (54.5) | 16 (57.1) | 4 (44.4) | 0.183 |
| 6 months (n = 25/59; 42.4%) | 13 (39.4) | 8 (28.6) | 4 (44.4) | 0.288 |
| Unknown (n = 2/12; 16.7%) | 1 (3.0) | 1 (3.6) | 0 (0.0) | 0.269 |
| Time of transplantation to CMV infection (d), median (IQR) | 194 (117–267) | 158 (82–195) | 95 (84–123) | 0.025 |
| Donor/Recipient CMV serostatus | ||||
| D+/R– (127; 98–194) | 129 (122–290) | 126 (106–173) | 62 (51–73) | 0.098 |
| D+/–/R+ (166; 97–218) | 200 (123–240) | 165 (72–195) | 106 (95–123) | 0.114 |
| D–/R– (87; 45–184) | 196 (121–272) | 110 (72–147) | 87 (87–87) | 0.819 |
| Unknown (195; 195–195) | – | – | 195 (195–195) | 1.000 |
| Duration of CMV chemoprophylaxis | ||||
| Perioperative (64; 35–87) | 139 (139–139) | 35 (34–50) | 87 (87–87) | 0.202 |
| 3 months (126; 95–172) | 128 (114–222) | 138 (95–168) | 90 (73–102) | 0.093 |
| 6 months (209; 186–284) | 231 (206–293) | 202 (161–282) | 150 (95–196) | 0.115 |
| Unknown (172; 108–236) | 45 (45–45) | 87 (87–87) | – | 0.317 |
| CMV disease, n (%) | 8 (9.1) | 9 (6.9) | 3 (7.9) | 0.829 |
| BPAR 7, n (%) | 34 (38.6) | 38 (29.2) | 11 (28.9) | 0.307 |
| HR 1 | 95% CI 2 | p | |
|---|---|---|---|
| NFKB1 promotor polymorphism (-94ins/delATTG) | |||
| ins/ins 3 | 1 | – | – |
| ins/del–del/del 4 | 0.568 | 0.355–0.908 | 0.018 |
| Recipient age (per year) | 0.999 | 0.980–1.018 | 0.905 |
| Recipient sex male (vs. female) | 1.002 | 0.614–1.634 | 0.994 |
| Recipient body mass index (per 1) | 1.021 | 0.968–1.077 | 0.448 |
| Type of transplantation | |||
| Kidney | 1 | – | – |
| Kidney + pancreas | 1.065 | 0.639–1.775 | 0.810 |
| Donor age (per year) | 1.005 | 0.990–1.109 | 0.521 |
| Cold ischemia time (per hour) | 1.021 | 0.975–1.068 | 0.385 |
| Delayed graft function (vs. none) | 1.364 | 0.818–2.276 | 0.235 |
| BPAR 5 (vs. none) | 1.115 | 0.681–1.827 | 0.666 |
| Induction with ATG 6 (vs. other) | 1.127 | 0.577–2.202 | 0.726 |
| Immunosuppressive regimen | |||
| MMF 7, prednisone and tacrolimus | 1 | – | – |
| MMF, prednisone and cyclosporine | 1.311 | 0.563–3.053 | 0.530 |
| Other | 1.138 | 0.608–2.132 | 0.686 |
| CMV 8 risk status | |||
| Low risk (D–/R–) 9 | 1 | – | – |
| Medium risk (D+/–/R+) 10 | 2.724 | 1.082–6.858 | 0.033 |
| High risk (D+/R– )11 | 3.029 | 1.124–8.161 | 0.028 |
| Prophylactic anti-CMV therapy | |||
| Perioperative | 1 | – | – |
| 3 months | 1.409 | 0.555–3.580 | 0.471 |
| 6 months | 2.484 | 0.951–6.492 | 0.063 |
| HR 1 | 95% CI 2 | p | |
|---|---|---|---|
| NFKB1 promotor polymorphism (-94ins/delATTG) | |||
| ins/ins 3 | 1 | – | – |
| ins/del–del/del 4 | 0.605 | 0.376–0.973 | 0.038 |
| CMV 5 risk status | |||
| Low risk (D–/R–) 6 | 1 | – | – |
| Medium risk (D+/–/R+) 7 | 2.468 | 0.975–6.247 | 0.057 |
| High risk (D+/R–) 8 | 2.837 | 1.051–7.661 | 0.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, H.; Vornweg, S.; Rump, K.; Rahmel, T.; Unterberg, M.; Koos, B.; Schenker, P.; Viebahn, R.; Adamzik, M.; Bergmann, L. The NFKB1 Promoter Polymorphism (-94ins/delATTG) Is Associated with Susceptibility to Cytomegalovirus Infection after Kidney Transplantation and Should Have Implications on CMV Prophylaxis Regimens. Cells 2021, 10, 380. https://doi.org/10.3390/cells10020380
Nowak H, Vornweg S, Rump K, Rahmel T, Unterberg M, Koos B, Schenker P, Viebahn R, Adamzik M, Bergmann L. The NFKB1 Promoter Polymorphism (-94ins/delATTG) Is Associated with Susceptibility to Cytomegalovirus Infection after Kidney Transplantation and Should Have Implications on CMV Prophylaxis Regimens. Cells. 2021; 10(2):380. https://doi.org/10.3390/cells10020380
Chicago/Turabian StyleNowak, Hartmuth, Svenja Vornweg, Katharina Rump, Tim Rahmel, Matthias Unterberg, Björn Koos, Peter Schenker, Richard Viebahn, Michael Adamzik, and Lars Bergmann. 2021. "The NFKB1 Promoter Polymorphism (-94ins/delATTG) Is Associated with Susceptibility to Cytomegalovirus Infection after Kidney Transplantation and Should Have Implications on CMV Prophylaxis Regimens" Cells 10, no. 2: 380. https://doi.org/10.3390/cells10020380
APA StyleNowak, H., Vornweg, S., Rump, K., Rahmel, T., Unterberg, M., Koos, B., Schenker, P., Viebahn, R., Adamzik, M., & Bergmann, L. (2021). The NFKB1 Promoter Polymorphism (-94ins/delATTG) Is Associated with Susceptibility to Cytomegalovirus Infection after Kidney Transplantation and Should Have Implications on CMV Prophylaxis Regimens. Cells, 10(2), 380. https://doi.org/10.3390/cells10020380






