1. Introduction
The corneal endothelium (CEn) is a monolayer of cells on the posterior surface of the cornea that transports water out of the stroma to maintain the cornea transparent [
1,
2]. Human corneal endothelial cells (hCEnC) are highly polarized flat cells with tightly hexagonal apical surfaces. However, their basal surface is irregular, as they lie on an amorphous collagenous membrane called Descemet’s membrane (DM) [
2]. To sustain relatively hydrated cornea, the CEn constantly keeps up the “Pump-Leak” mechanism, where active transport properties such as the sodium-potassium pump (Na
+/K
+ ATPase) represents the “Pump” and the corneal swelling pressure presents the “Leak”. All of this is regulated by the barrier properties of tight junction proteins such as Zona Occludens-1 (ZO-1) [
3,
4]. Mature CEn does not normally replicate in vivo enough to replace dead or damaged cells, since the cell cycle of the hCEnCs is arrested in the G1 phase [
4]. The number of hCEnCs gradually decreases with age but, it can also decline dramatically due to endothelial damage caused by trauma or disease, such as Fuchs’ dystrophy, leading to corneal swelling and, eventually, to blindness [
5]. Furthermore, damage can be caused by surgical complications, e.g., after intraocular lens implantation [
6,
7]. Despite that the majority (~80%) of all corneal blindness cases are avoidable and reversible, corneal transplantation is still often needed. To date, keratoplasty is the most performed allogenic transplant worldwide, with a high success rate (90–95%) [
8]. Many corneal transplants (185,000) were performed worldwide in 2012, and most of them (39%) were for patients suffering from Fuchs’ dystrophy. Currently, cadaveric donor cornea transplantation using Descemet membrane endothelial keratoplasty (DMEK) or Descemet stripping with automated endothelial keratoplasty (DSAEK) are the preferred therapies for treating the loss of hCEnCs [
9]. Unfortunately, the demand of global corneal transplantation was estimated at 12.7 million [
10]. Despite the increase in cornea donations in recent years (+1.9% change from 2018 to 2019), there is still huge need for corneal transplants worldwide [
8,
11]. Extensive methodological development has been made with the in vitro expansion methods for primary cells [
12]. However, the quality of cultured CEnCs is largely dependent on donor age; thus, other cell sources totally independent from donor cornea are more desirable. Renewable, expandable and bankable human pluripotent stem cells (hPSC), including embryonic stem cells (hESC) and induced pluripotent stem cells (hiPSC), are becoming attractive sources for the stem cell-based therapy of diseased or wounded corneal layers, including CEnCs [
13].
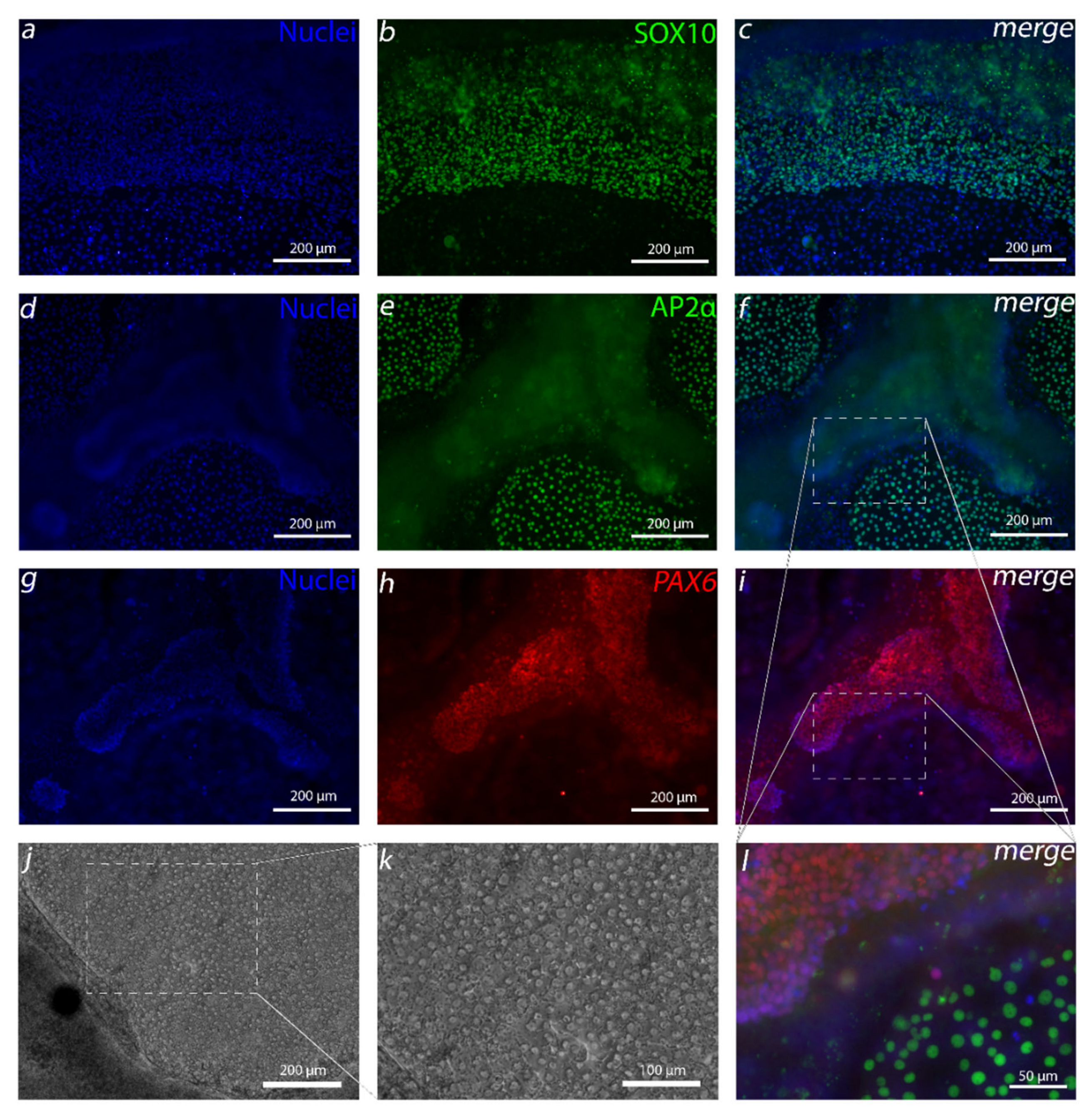
The embryonic development pathway for the human Cen is still not fully known. Previous research has shown that, during eye development, hCEnCs are derived from neural crest cells (NCC)—specifically, from periocular mesenchyme (POM) [
14]. Several molecular markers have been used to analyze NCC and POM. For example, transcription factors such as activating enhancer binding protein 2 alpha (AP2α) and transcription factor SOX10 have been used to identify NCCs [
15,
16] and paired-like homeodomain transcription factor 2 (PITX2) and Forkhead box C1 (FOXC1) to identify POM cells [
17]. It is widely known that transforming growth factor beta (TGF-β), WNT and bone morphogenetic (BMP) signaling pathways play crucial roles in NCC and CEnC induction, and thus, many research groups have capitalized on this knowledge in their differentiation protocols [
14,
16,
18,
19,
20,
21]. Thus, over the years, protocols to differentiate hCEnCs from hPSCs have improved significantly, but to our best knowledge, none of the developed protocols have reached clinical grade cell production. Previously, hCEnC-like cells have been successfully differentiated from hPSCs, e.g., by using the bovine CEnC conditional medium [
22] or a coculture with human corneal stroma cells and lens epithelial cell-conditioned medium [
23]. Chen et al. first used all-trans retinoic acid (RA) to derive NCCs out from mouse embryoid bodies (EB), followed by lens epithelial cell-conditioned medium to differentiate mCEnC-like cells [
24]. Steps towards more defined protocols were taken by McCabe et al., who introduced the hCEnC protocol by using Dual Smad inhibition. In their study, NCCs were first derived from hESCs by a TGF beta inhibitor (SB431542) and Noggin, and then, cells were switched to a medium supplemented with a hCEnC induction cocktail, including platelet-derived growth factor B (PDGF-BB), WNT inhibitor/activator Dickopf-related protein 2 (DKK-2) and basic fibroblast growth factor (bFGF) [
19]. More recently, Wagoner et al. produced similar hCEnC-like cells, but instead of noggin, they used CHIR99021, a GSK-3-specific inhibitor that leads to WNT activation during NCC induction [
20].
Despite these achievements, many of the protocols still include undefined components (e.g., conditioned medium) and animal-derived materials (e.g., Matrigel) that possibly increase the undesired variability in differentiation and are problematic considering the clinical applications. In this study, our goal was to use a more directed protocol with defined components for the differentiation of hCEnC from both hESCs and hiPSCs, facilitating the method translation to clinical use. As an outcome, we introduced a novel, defined and fast method to produce hCEnC-like cells from hPSCs.
2. Materials and Methods
2.1. Human PSC Establishment and Characterization
Tampere University, Faculty of Medicine and Health Technology, has the approval of the National Authority Fimea (Dnro FIMEA/2020/003758) to conduct research on human embryos. The research group also had supportive statements from the Ethical Committee of the Pirkanmaa Hospital District to derive, culture and differentiate hESC lines (R05116); to establish and use hiPSC lines in ophthalmic research (R16116) and to use human corneas that are not suitable for transplantation in the research (R11134). No new hESC lines were derived for the research conducted here. The previously established hESC line Regea08/017 and hiPSC line WT001.TAU.bB2 from a healthy donor was used in this study. The derivation and culture of the hESC line Regea08/017 has been previously described [
25], and here, the establishment of the hiPSC line WT001.TAU.bB2 was shortly described. Peripheral blood monocytes (PBMC) were isolated from 20 mL of healthy donor blood sample, and cells were reprogrammed into human iPSCs using an integration-free CytoTune-iPS Sendai Reprogramming kit (Thermo Fisher Scientific, Waltham, MA, USA) in feeder independent culture conditions according to the protocols provided by the manufacturer. After the manual selection of several clones, the removal of the reprogramming vectors was confirmed, and the further expansion of the hiPSCs (from passage 4 onwards) and cryopreservation were conducted to establish adequate master and working cell banks for research purposes. For that, the feeder-free culture was done as previously described in Hongisto et al. [
26] using Corning
® CellBIND
® 24-well culture plates (Corning, Corning, New York, NY, USA) coated with human recombinant laminin-521 (LN521™, Biolamina, Sundbyberg, Sweden) and Essential 8™ Flex Medium (E8, Thermo Fisher Scientific) supplemented with Essential 8 Flex supplement (50×) and 50-U/mL Penicillin-Streptomycin (Gibco, Thermo Fisher Scientific). The hPSC pluripotency was verified by observing their spontaneous differentiation as embryoid bodies, followed by immunofluorescence labeling for derivative cells of the three embryonic germ layers, as described previously [
26]. For the experiments, the quality of hPSCs were continuously monitored for attachment, growth and morphology with a Nikon Eclipse TE2000-S phase contrast microscope (Nikon Instruments Europe B.V. Amstelveen, The Netherlands), and prior to differentiations, the hPSCs were thoroughly characterized (e.g., OCT-3/4, SSEA-3, SSEA-4, TRA-1-81 and LIN28); the normal karyotype was conformed (G-banding service by Fimlab Laboratoriot Oy Ltd., Tampere, Finland) and cells were tested for mycoplasma negativity with Venor
®GeM Classic (Minerva biolabs, Berlin, Germany) (not shown).
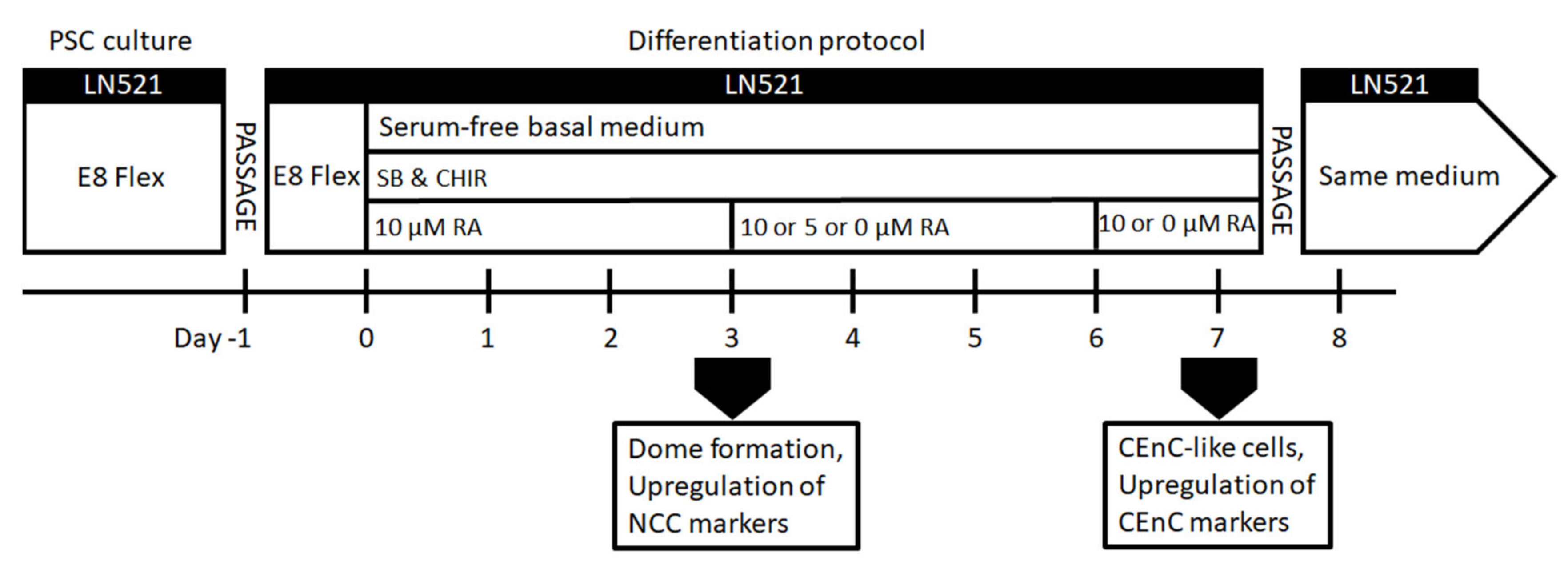
2.2. Differentiation of hPSCs into NCCs and Further to CEnC-Like Cells
As described above, the hPSC maintenance was performed on LN521, which was in addition to LN511, a major laminin form present in adult human Descemet’s membranes [
27]. Therefore, also, hPSC differentiation was carried out on LN521 to minimize the number of different components in the protocols. First, NCCs were differentiated from hPSCs based on modified protocol from Tchieu et al., as summarized in
Supplementary Figure S1a [
16]. Briefly, hPSCs were seeded on LN521™-coated CellBind 12 or 24-well plates (Corning, Corning, New York, NY, USA) at 75,000–200,000 cells/cm
2. The cells were cultured in E8 medium for 24 h. For initiation of the differentiation, a serum=free basal medium consisting of KnockOut Dulbecco’s Modified Eagle Medium (KO-DMEM), 15% KnockOut serum replacement, 2-mM GlutaMax-I, 0.1-mM 2-mercaptoethanol, 50-U/mL penicillin/streptomycin (all from Thermo Fisher Scientific, Waltham, MA, USA), 1% nonessential amino acids (Thermo Fisher Scientific, Sigma-Aldrich, St. Louis, MO, USA) were used. On day 0 of differentiation, E8 medium was replaced with induction medium consisting of the above-mentioned basal medium supplemented with 500-nM bone morphogenetic (BMP) pathway inhibitor LDN193189 (LDN; Sigma-Aldrich) and 10-µM TGF-β inhibitor SB431542 (SB; Stemcell Technologies, Vancouver, BC, Canada). On day 1, 3-µM GSK3 inhibitor/WNT pathway activator CHIR99021 (CHIR; Stemcell Technologies) was added with 10-µM SB and 500-nM LDN. From day 2 onwards, the medium was supplemented with 10-µM SB and 3-µM CHIR. For the N2 medium experiments, the cells were differentiated either with N2 (control,
Figure S1a) or without N2 (
Figure S1b). In the control culture (
Figure S1a), the basal medium was gradually changed to N2 medium during days 4–10 of the differentiation. The N2 content of the whole medium at day 4 was changed to 25%, day 6 to 50%, day 8 to 75% and day 10 to 100%. In the experiment culture (
Figure S1b), the N2 medium was discarded completely, and the differentiation was carried out in the basal medium throughout, supplemented as described above. For experiments without LDN and N2 medium (
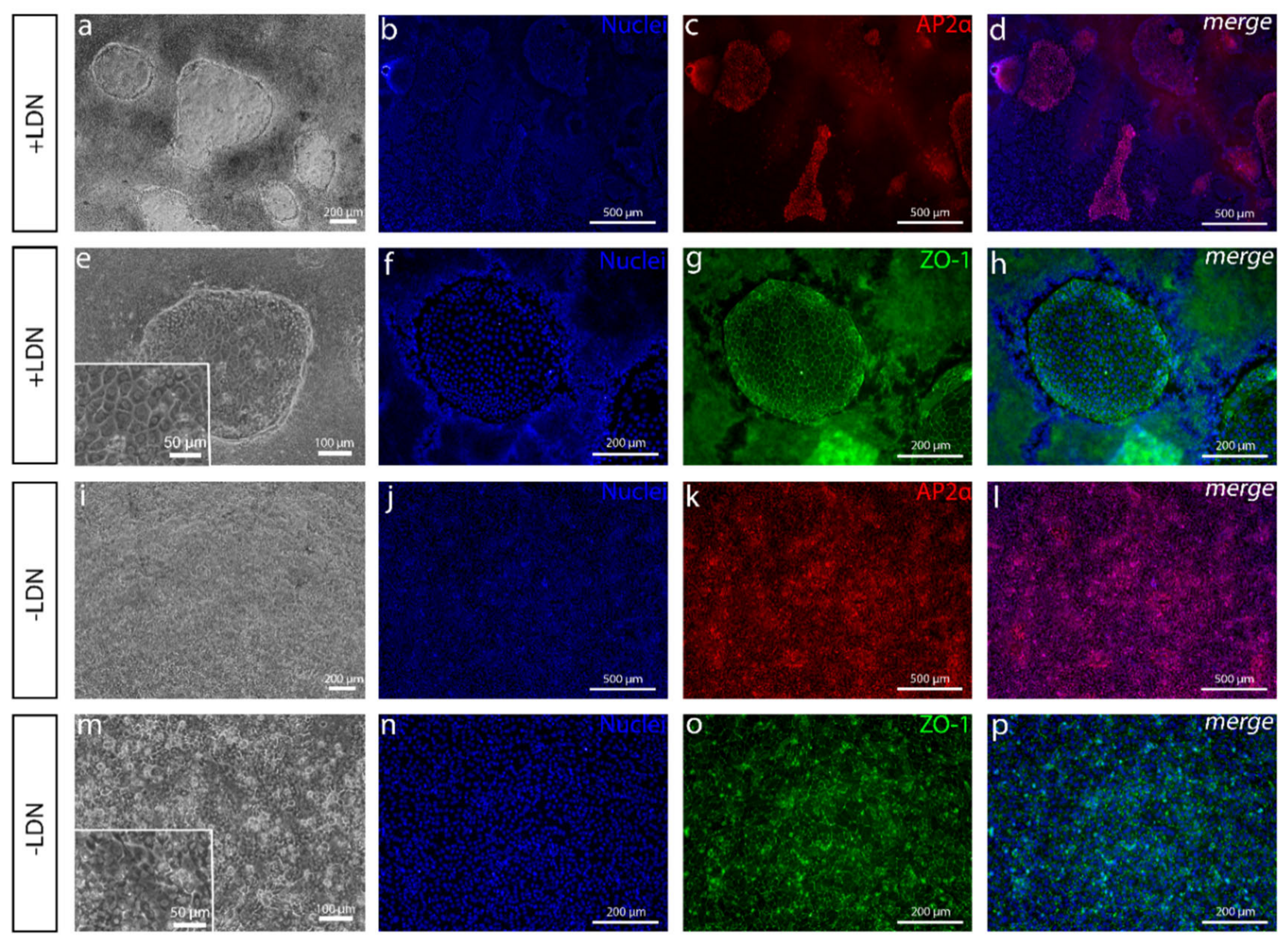
Figure S1c), hPSCs were seeded at 30,000–60,000 cells/cm2 on LN521™, and induction was started on day 0 in the basal medium supplemented with 4-µM CHIR and 10-µM SB throughout.
For enhancing the differentiation towards POM and CEnCs (
Figure 1), hPSCs were seeded on LN521™-coated CellBind 6 or 12-well plates (Corning, Corning, New York, NY, USA) at 10,000–60,000 cells/cm
2. The cells were cultured in E8 medium for 24 h. On day 0 of the differentiation, E8 medium was replaced with the basal medium supplemented with 10-µM SB, 4-µM CHIR and 10-µM retinoic acid (RA; Sigma-Aldrich). RA was either kept throughout the differentiation, or the concentration was lowered to 5 µM during days 3–6 and then removed or RA was removed completely already after day 3 (
Figure 1). After day 7, CEnC-like cells formed, and they were harvested for analyses or further passage into LN521-coated well plates or chamber slides (Thermo Fisher Scientific, Waltham, MA, USA) in the same medium. CEnC-like cells were passaged using the TrypLE Select (Thermo Fisher Scientific) dissociation enzyme and incubated for 3–6 min at 37 °C. Subsequently, the CEnC-like cells were detached gently by trituration, filtered through a 40-µm cell strainer (Thermo Fisher Scientific, Waltham, MA, USA) and centrifuged for 5 min at 300×
g. Phase contrast light microscope Nikon Eclipse TE2000-S with a DS-Fi1 camera (Nikon Corp., Tokyo, Japan) was used to capture images of the cell morphology.
2.3. Harvesting of Primary hCEnCs for Cell Culturing
A donor human cornea of a young adult, which was not suitable for transplantation due to hepatitis infection of the donor, was used for harvesting CenCs. Cornea was inserted CenC side-up on a cutting block of Barron Marking Corneal Donor Punch (Katena, Parsippany, NJ, USA). To form a well, the seating ring was placed on the top of cornea, which was filled with TrypLE Select and incubated for 15 min at 37 °C. Subsequently, the cells were detached by trituration or scratching and seeded on collagen IV (Sigma-Aldrich) and LN521™-coated CellBind 24-well plates (Corning, Corning, New York, NY, USA). The primary CenCs were cultured until confluency (3 weeks) in a medium consisting of DMEM/F12, 10% fetal bovine serum, 2-mM GlutaMax-I, 50-U/mL penicillin/streptomycin (all from Thermo Fisher Scientific, Waltham, MA, USA), 2-ng/mL bFGF (Peprotech, Rocky Hill, NJ, USA) and 10-µM ROCK inhibitor Y27632 (Stemcell Technologies). Medium was changed twice a week.
2.4. Immunocytochemistry
The cells were fixed with 4% paraformaldehyde (PFA, Sigma-Aldrich) for 15 min. Next, the cells were permeabilized for 10 min with 0.1% Triton X-100 (Sigma-Aldrich), followed by blocking with 3% bovine serum albumin (BSA) for 1 h. Then, the cells were first incubated with 1:400 SOX10 (Santa-Cruz, Santa Cruz, CA, USA, sc-365692) 1:400 activating enhancer binding protein 2 alpha (AP2α; Thermo Fisher Scientific, MA1-872), 1:400 paired box protein 6 (PAX6; Sigma-Aldrich, HPA030775), 1:400 zona occludens-1 (ZO-1; Thermo Fisher Scientific, 61-7300), 1:200 anti-alpha 1 sodium potassium ATPase (Na+/K+-ATPase, Abcam, Cambridge, UK, ab7671), 1:400 CD166 (BD Biosciences, 559260 and Abcam, ab109215), 1:400 N-cadherin (NCAD; Abcam, ab18203 and Sigma-Aldrich, C2542), 1:400 paired-like homeodomain transcription factor 2 (PITX2; Thermo Fisher Scientific, PA5-11479), 1:400 Forkhead box C1 (FOXC1; Abcam, ab227977) and 1:400 octamer binding transcription factor (OCT) ¾ (R&D Systems, Minneapolis, MN, USA, AF1759) primary antibodies overnight at 4 °C. The cells were next treated with 1:800 donkey anti-rabbit immunoglobulin G (IgG) secondary antibody, Alexa Fluor 488, 1:800 donkey anti-rabbit IgG secondary antibody, Alexa Fluor 568, 1:800 donkey anti-mouse IgG secondary antibody, Alexa Fluor 488, 1:800 donkey anti-mouse IgG secondary antibody, Alexa Fluor 568, 1:800 donkey anti-goat IgG secondary antibody, Alexa Fluor 488, 1:800 donkey anti-goat IgG secondary antibody or Alexa Fluor 568 (all from Thermo Fisher Scientific, Waltham, MA, USA), according to the host of the primary antibody for 1 h at room temperature. The nuclei were counterstained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Vector Laboratories, Peterborough, UK) or Hoechst 33342 (Thermo Fisher Scientific, Waltham, MA, USA). The images of mounted cells were captured using a fluorescence microscope (Olympus IX51; Olympus, Tokyo, Japan) or confocal microscope (Zeiss LSM 700, Zeiss LSM 800, Carl Zeiss AG, Oberkochen, Germany) and prepared using image editing software (Adobe Photoshop CC 2018; Adobe Inc., San Jose, CA, USA)
2.5. Immunohistochemistry
A human donor cornea was fixed with 4% PFA for 4 h at room temperature (RT) and embedded into a paraffin block. The block was sectioned with microtome. Sections were attached on glass slides at 60 °C for 90 min. Then, they were deparaffinated with xylene for 5, 4, 3 and 1 min, then 2 × 4 min 99.9% ethanol, 2 × 3 min 96% ethanol and 3 min 70% ethanol. Finally, sections were hydrated in water. Antigen retrieval was concluded with boiling 10-mM sodium citrate and 0.05% Tween 20 in pH 6 for 12 min. Samples were treated with blocking buffer consisting of 10% donkey serum in TBS and 5% bovine serum albumin (BSA) for 1 h at 37 °C in a moisture chamber. Then, the sections were incubated with 1:50 NCAD (Sigma-Aldrich, C2542), 1:50 Na+/K+-ATPase (Abcam, ab7671) and 1:50 CD166 (Abcam, ab109215) primary antibodies in blocking buffer overnight at +4 °C in a moisture chamber. Then, they were incubated in 1:200 donkey anti-rabbit IgG secondary antibody, Alexa Fluor 488 for CD166 and 1:200 donkey anti-mouse IgG secondary antibody, Alexa Fluor 568 for NCAD and Na+/K+-ATPase for 1 h in a RT moisture chamber. Sections were mounted, and nuclei were counterstained with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, Waltham, MA, USA). The images of mounted cells were captured using a confocal microscope (Zeiss LSM 700, Carl Zeiss AG, Oberkochen, Germany) and prepared using image editing software (Adobe Photoshop CC 2018; Adobe Inc.).
2.6. RNA Extraction and RT-qPCR
Total RNA was extracted from undifferentiated hPSCs (d0) and from 3 time points during hCEnC induction (d3, d6 and d9) with a Rneasy Mini Kit Plus (Qiagen, Hilden, Germany) or using TRI reagent (Sigma-Aldrich). RNA concentration of each sample was determined using NanoDrop-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA was purified from endogenous DNA using Dnase I (Thermo Fisher Scientific, Waltham, MA, USA). From each RNA sample, 400 ng were used to synthesize complementary DNA (cDNA) using the High-Capacity cDNA RT kit (Applied Biosystems, Foster City, CA, USA). The resulting cDNA samples were analyzed with qPCR using sequence-specific TaqMan Gene Expression Assays (Thermo Fisher Scientific, Waltham, MA, USA) for OCT4 (Hs00999632_g1), PITX2 (Hs01553179_m1), aquaporin 1 (AQP1) (Hs01028916_m1), ATPase subunit alpha 1 (ATP1A1) (Hs00167556_m1), Cadherin 2 (Hs00983056_m1), SLC4A4 (Hs00186798_m1) and FOXC1 (Hs00559473_s1). All samples (
n = 1/condition) were run as triplicate reactions with the 7300 Real-Time PCR system (Applied Biosystems). Results were analyzed with the 7300 System SDS Software (Applied Biosystems) and Microsoft Excel. Based on the cycle threshold (C
T) values given by the software, the relative quantification of each gene was calculated by applying the −2
ΔΔCt method [
28]. Results were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1), with the undifferentiated hPSCs as the calibrator to determine the relative quantities of gene expression in each sample.
2.7. Cell Density Calculation
The density of the hPSC-CenC-like cells was calculated manually from ZO-1 immunofluorescence images using image editing software (Adobe Photoshop CC 2018; Adobe Inc.).
4. Discussion
In the complex development of the human cornea, it has been found that several regions of the human eye, including CEn, are derived from NCC-derived POM cells [
14,
30]. Our first goal was to generate these cells before aiming to differentiate CEnCs. The first attempt to produce NCCs was greatly inspired by Tchieu et al. [
16]. The resulting small AP2α-positive colonies of cells surrounded by a thick cell mass that had clear similarities with CEnCs caught our attention. Due to the importance of the AP2 family to CEn development [
31], we pursued to find a method to increase the amount of these cells. Surprisingly, discarding BMP inhibition from the protocol had the highest impact on the yield of AP2α-positive cells. BMP activity is known to have effects on NCC differentiation, but the dynamics are poorly understood, and the optimal level of BMP activity is difficult to adjust [
32]. Furthermore, in more undefined culture conditions, BMP ligands can be present in used media components, or those can be secreted endogenously by the differentiating side populations, which makes the controlling the in vitro concentration difficult [
32,
33]. In our case, discarding the exogenous BMP inhibition from the defined differentiation protocol removed the unwanted thick PAX6 positive cell mass, leaving, primarily, the AP2α-positive cells. In our hands, in addition to PAX6 and AP2α, the Tchieu et al. [
16]-based protocol produced colonies of SOX10-positive cells that eradicated upon the removal of BMP inhibition and increase of CHIR concentration. SOX10 is a commonly used NCC marker that is regulated by WNT signaling, and it has been shown in
Xenopus to be expressed more in trunk neural crest (NC) and lesser in cranial NC [
34], from which POM and CEn are derived [
30]. Interestingly, the findings of Gomez et al. showed that, when differentiating NC from hESCs, CHIR concentrations below 6.5 µM and above 3.5 µM yield no SOX10-positive cells [
35]. Thus, the increase of the CHIR concentration from 3 µM to 4 µM in our study might give an explanation why SOX10 cells disappeared from our differentiation cultures as well. This may also explain why our cell cultures without RA were more confluent, containing intact round cells instead of SOX10-positive scattered spindle like cells that Wagoner et al. differentiated with 3-µM CHIR [
20].
The capability of producing NCC with TGF-β inhibition and WNT activation and with the further evidence that a relatively high RA concentration guides NCC to form POM [
29] convinced us to hypothesize that mixing these agents together would induce the differentiation of CEnCs. RA is an essential morphogen signaling molecule, as well as an important regulator of embryonic development, and the dysregulation of RA levels during embryogenesis has been associated with ocular defects [
29]. It has been demonstrated that, during NCC migration, RA activity is the highest within the prosencephalon and developing eye [
29,
36]. Previous studies have shown that the absence of RA signaling in the eye development downregulates the expression of POM markers
PITX2 and
FOXC1 [
37]. Similar results were gained in our study where the addition of RA clearly increased
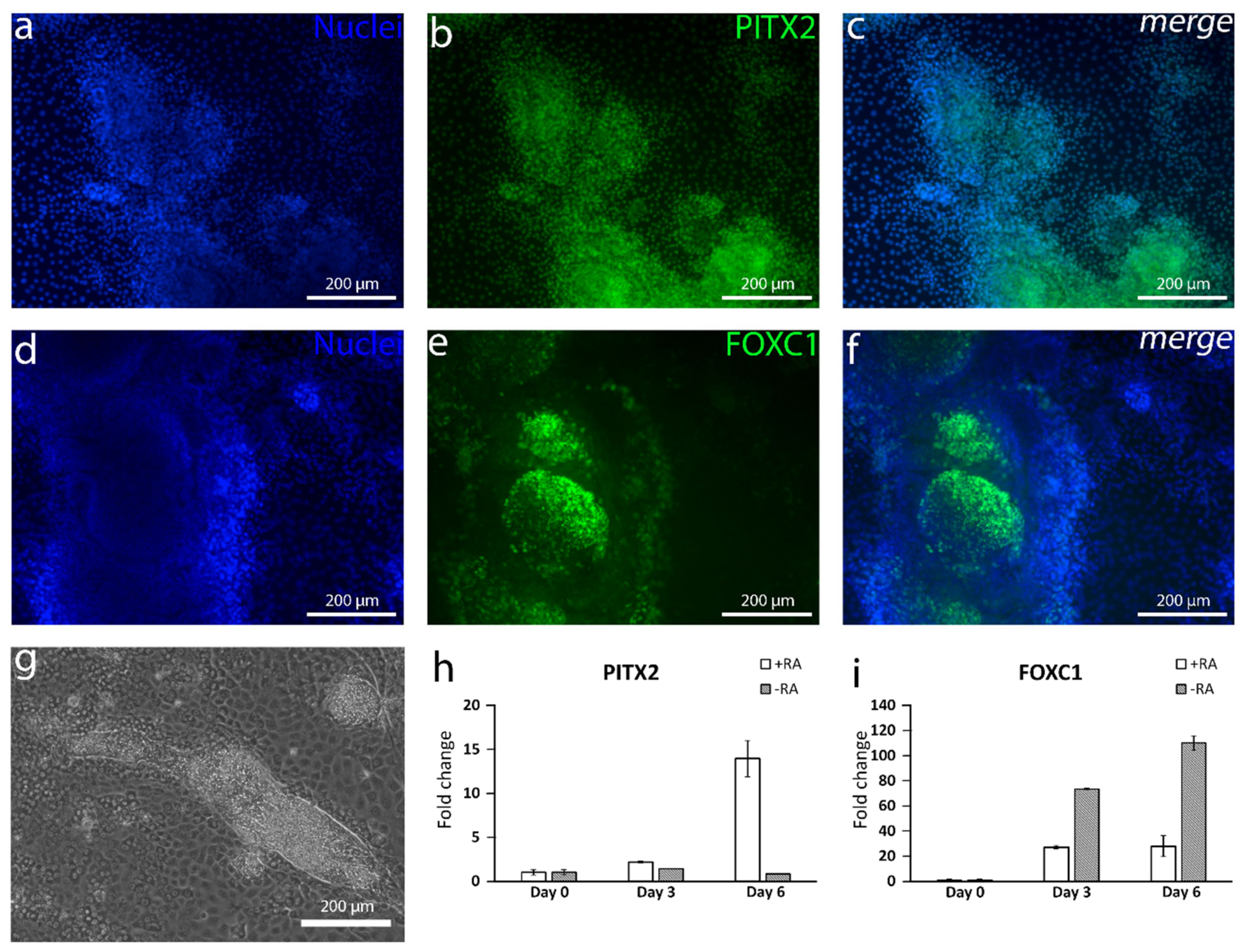
PITX2 expression during the first days of differentiation. Interestingly, the
FOXC1 expression was higher at the mRNA level without RA supplementation, potentially due to the presence of other NC derivatives than cornea cells [
38]. At the protein level, the +RA supplementation did induce the formation of mesenchymal-like structures positive for both PITX2 and FOXC1.
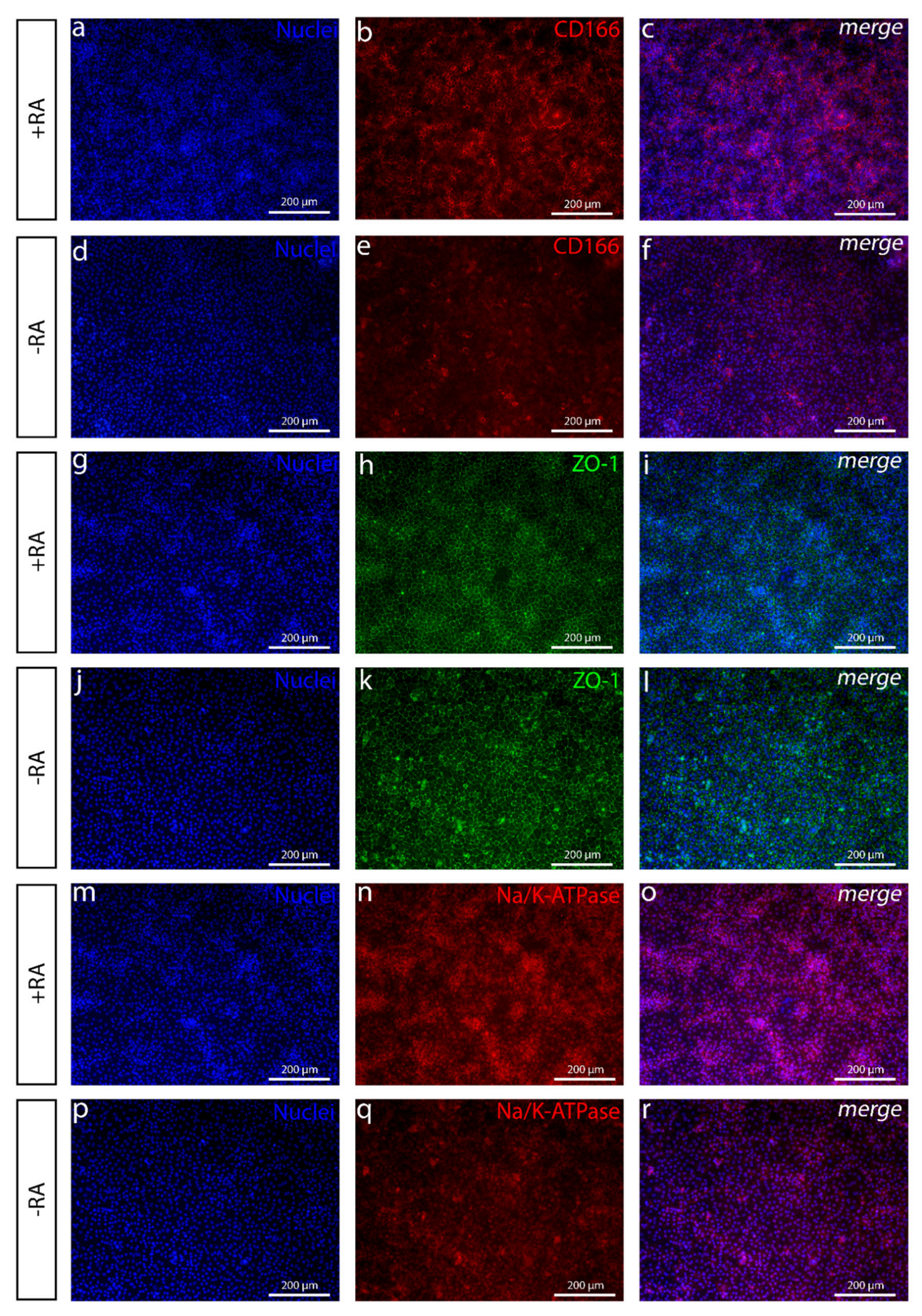
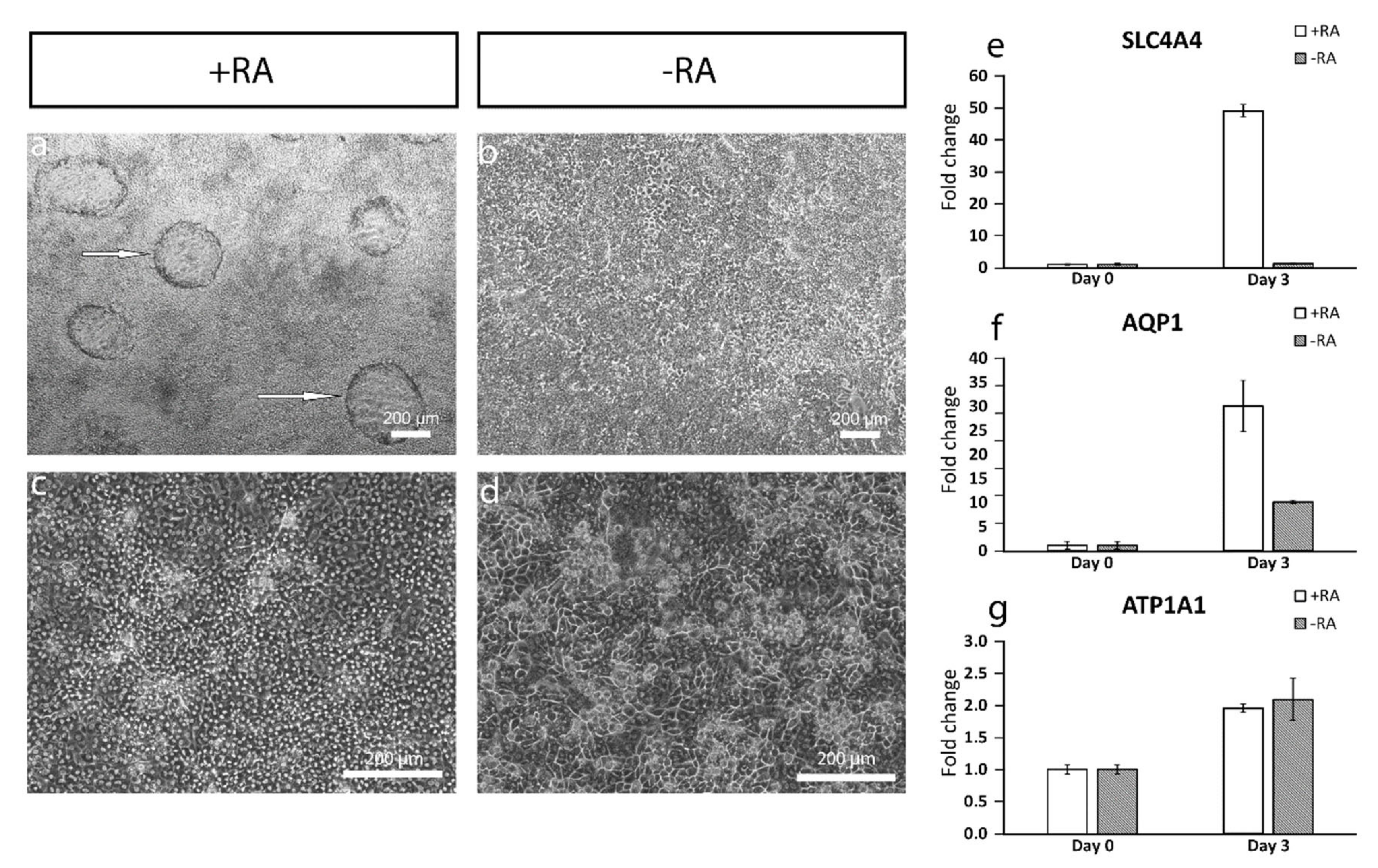
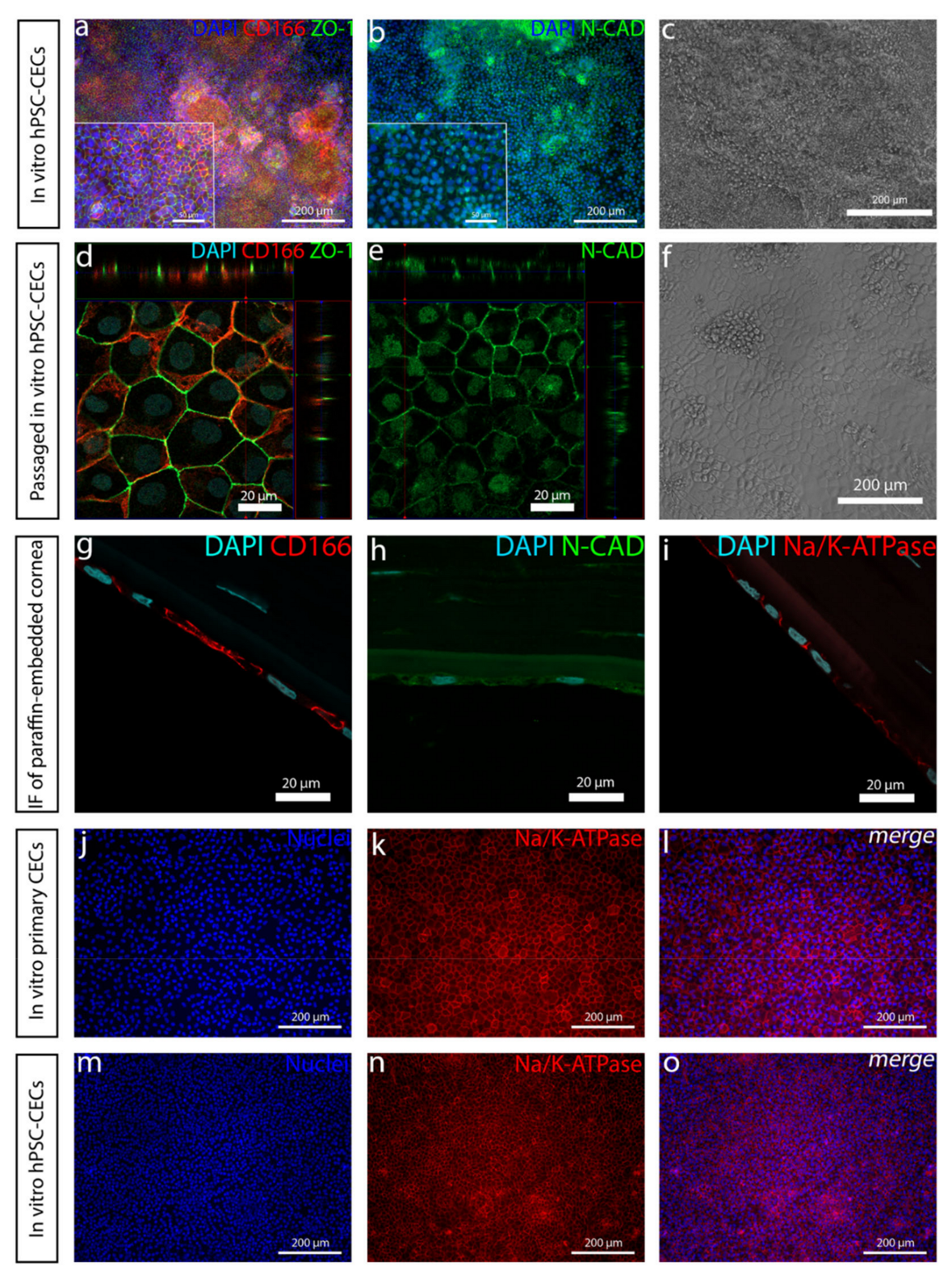
The addition of RA increased the polygonal morphology of the cells, as well as the expression of important hCEnC makers CD166, Na
+/K
+-ATPase and ZO-1, with correct intracellular localization and the formation of dome structures created by the detachment of the cell layer from the culture surface due to the fluid accumulation in between. This suggested fluid transport by the cells that could potentially reflect the pump activity, which was further supported by the increased expression of
AQP1,
ATP1A1 and
SLC4A4. Especially, the difference between +RA and −RA conditions regarding the expression of the sodium bicarbonate cotransporter
SLC4A4 was clear, and the RA supplementation had a clear upregulating impact on its expression. The SLC4A4 transporter is crucial for hCEnC, having an important role in maintaining the solute gradients essential for the regulation of water transport from the stroma to the anterior chamber [
39]. In the native cornea, the basolateral membrane of CEnCs pump fluids out of the stroma through the Descemet membrane [
3], whereas, in the dome structures formed in our in vitro cultures, the potentially active fluid transport appears to be more apical to basal and, thus, directed towards the cell culture plastic. We assumed this was due to the early stage of the maturation, and the cells were not yet fully polarized. Confocal immunofluorescence images of Na
+/K
+-ATPase of the cells in the dome structure could confirm this hypothesis, but further methodological development is needed to harvest a proper intact dome structure into immunocytochemistry analyses from cell culture plates. Further functional studies are also needed to address this aspect in more detail to confirm the appropriate pump properties in order to ensure clinical efficacy. During the differentiation method development, we also noticed that prolonged use of RA in the differentiation protocol caused some of the cells to accumulate big intracellular vacuoles, potentially indicating cellular stress (
Figure S8). This could be a consequence from too-intense RA induction, which can disrupt the Golgi apparatus [
40]. We also noticed that the total removal of RA from the induction phase (during days 3–6) prevented vacuolization, but the amount of dome structures decreased, and the cells lost some of their polygonality. Therefore, finding the balance for the timing and concentration with the RA supplementation is crucial for optimal differentiation.
In comparison to previous publications, the clear difference in our protocol compared to the others is that we only used RA in addition to SB and CHIR, with relatively fast differentiation of the CEnC-like cells. Several other groups used the B27, PDGF-BB, DKK-2 cocktail discovered by McCabe et al. [
19] to further differentiate CEnCs from NCCs [
19,
20,
41]. Replacing this cocktail with early RA induction seemed to produce CEnC-like cells with very short induction times needed. Nevertheless, the optimal RA concentration in later stages of the CEn maturation is still to be discovered, together with the further development and in vivo functional assessment of hPSC-CEnCs.
To conclude, in this study we developed a simple and directed differentiation protocol to derive CEnC-like cells from hPSCs with a small molecule inhibition of TGF-β and induction of WNT combined with RA. Our results demonstrated successfully differentiated hPSC-CEnC-like cells expressing proteins important for CEnCs [
2] and with a polygonal, tightly packed morphology. The developed method is fast, feeder-free and uses only defined components. In addition, xeno-free components are available for the final production development for clinical applications, including xeno-free SR (Thermo Fisher Scientific, Waltham, MA, USA).