The Prognostic Values of PARP-1 Expression in Uveal Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Statistical Analysis
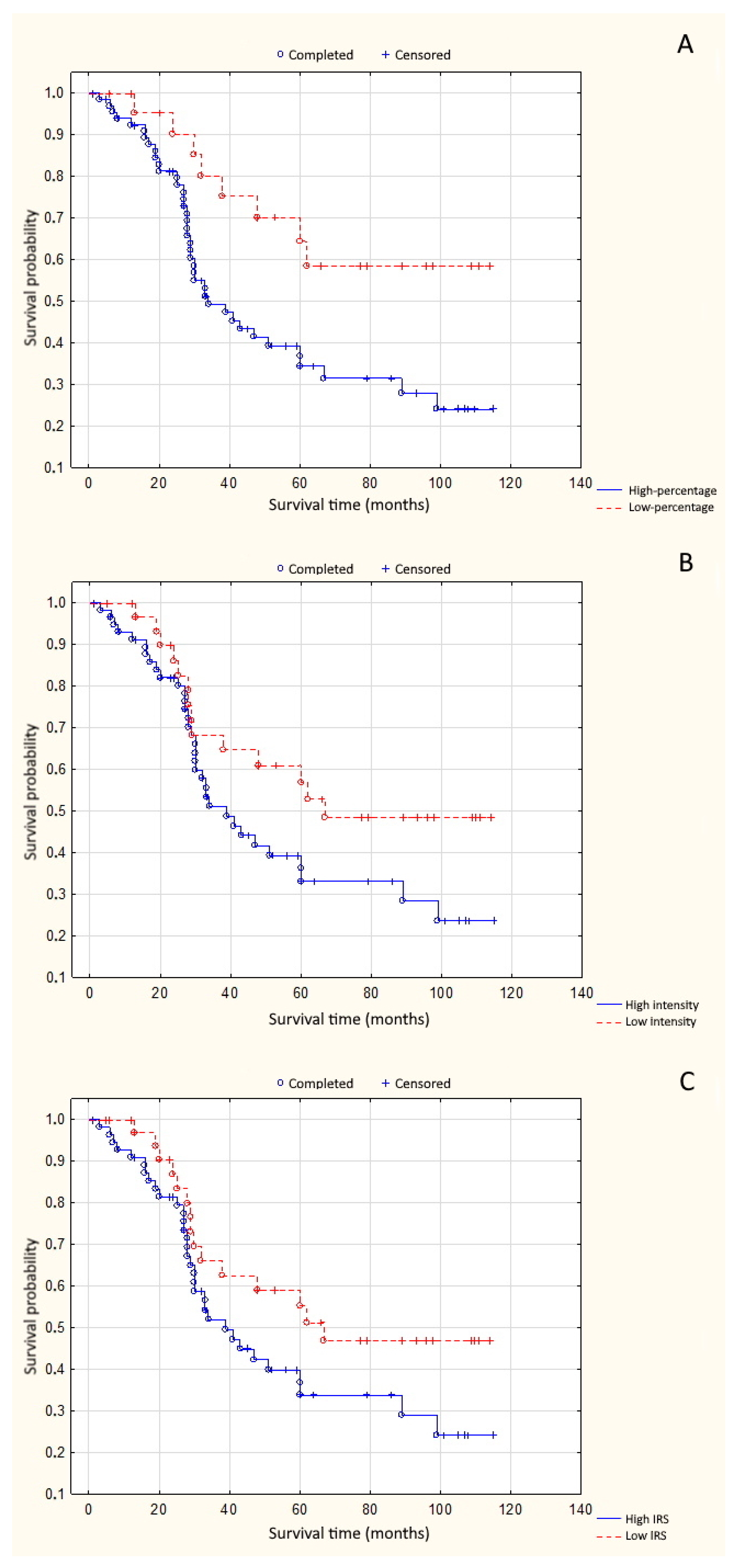
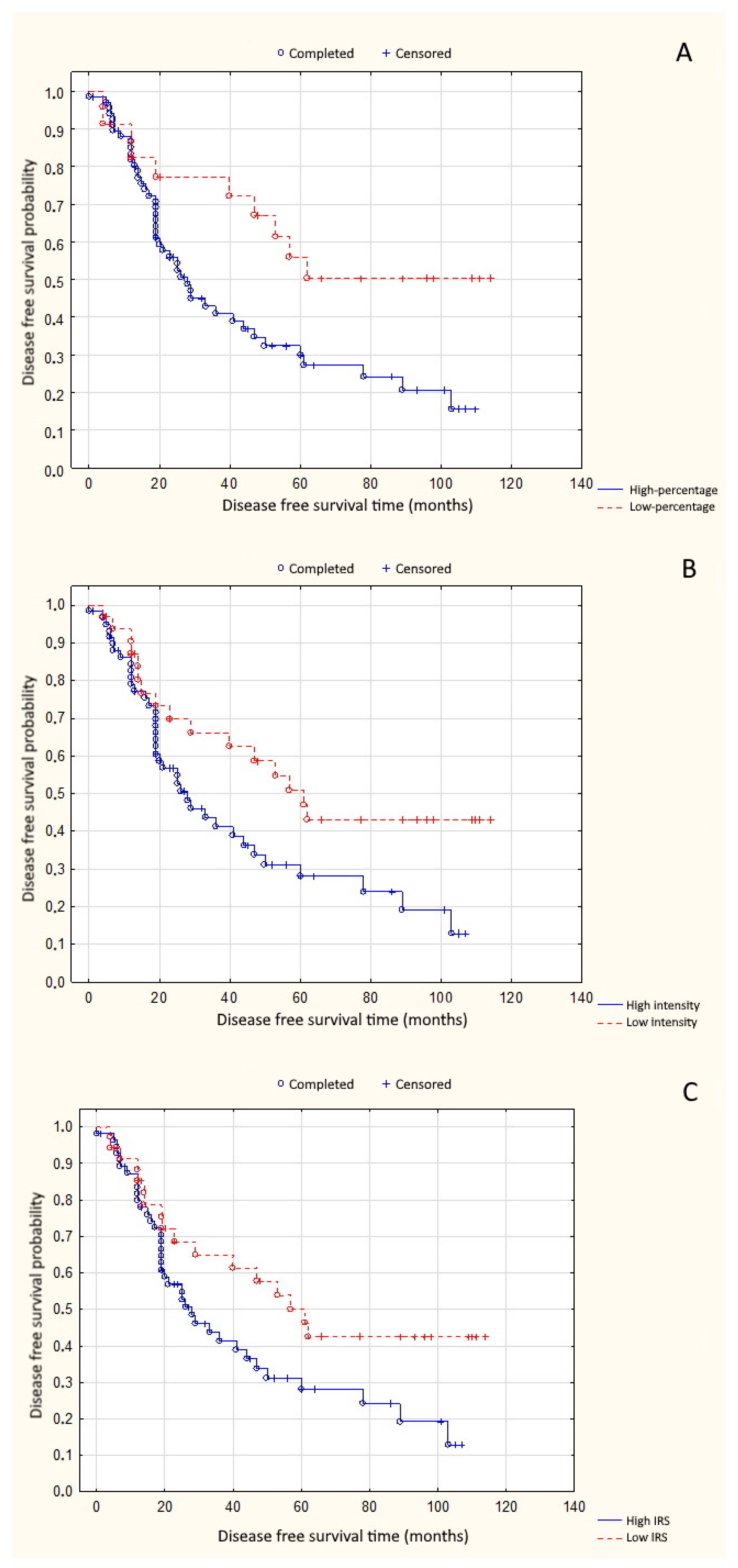
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maheshwari, A.; Finger, P.T. Cancers of the eye. Cancer Metastasis Rev. 2018, 37, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kivela, T.; Simpson, R.E.; Grossniklaus, H.E. Uveal Melanoma. In AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2016; pp. 805–817. [Google Scholar]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jubin, T.; Kadam, A.; Jariwala, M.; Bhatt, S.; Sutariya, S.; Gani, A.R.; Gautam, S.; Begum, R. The PARP family: Insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival. Cell Prolif. 2016, 49, 421–437. [Google Scholar] [CrossRef]
- Izhar, L.; Adamson, B.; Ciccia, A.; Lewis, J.; Pontano-Vaites, L.; Leng, Y.; Liang, A.C.; Westbrook, T.F.; Harper, J.W.; Elledge, S.J. A Systematic Analysis of Factors Localized to Damaged Chromatin Reveals PARP-Dependent Recruitment of Transcription Factors. Cell Rep. 2015, 11, 1486–1500. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, X. The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene 2015, 34, 3349–3356. [Google Scholar] [CrossRef]
- Timinszky, G.; Till, S.; Hassa, P.O.; Hothorn, M.; Kustatscher, G.; Nijmeijer, B.; Colombelli, J.; Altmeyer, M.; Stelzer, E.H.; Scheffzek, K. and Hottiger, M.O. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat. Struct. Mol. Biol. 2009, 16, 923–929. [Google Scholar] [CrossRef]
- Ahel, D.; Hořejší, Z.; Wiechens, N.; Polo, S.E.; Garcia-Wilson, E.; Ahel, I.; Flynn, H.; Skehel, M.; West, S.C.; Jackson, S.P.; et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 2009, 325, 1240–1243. [Google Scholar] [CrossRef]
- Altmeyer, M.; Toledo, L.; Gudjonsson, T.; Grøfte, M.; Rask, M.B.; Lukas, C.; Akimov, V.; Blagoev, B.; Bartek, J.; Lukas, J. The chromatin scaffold protein SAFB1 renders chromatin permissive for DNA damage signaling. Mol. Cell 2013, 52, 206–220. [Google Scholar] [CrossRef]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, 41. [Google Scholar] [CrossRef]
- Bryant, H.E.; Helleday, T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006, 34, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.G.; Sarkaria, J.N.; Kaufmann, S.H. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Soldani, C.; Scovassi, A.I. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis 2002, 7, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Sosna, J.; Voigt, S.; Mathieu, S.; Lange, A.; Thon, L.; Davarnia, P.; Herdegen, T.; Linkermann, A.; Rittger, A.; Chan, F.K.M.; et al. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell. Mol. Life Sci. 2014, 71, 331–348. [Google Scholar] [CrossRef]
- Cohen, M. Interplay between compartmentalized NAD+ synthesis and consumption: A focus on the PARP family. Genes Dev. 2020, 34, 254–262. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sedukhina, A.; Okamoto, N.; Nagasawa, S.; Suzuki, N.; Ohta, T.; Hattori, H.; Roche-Molina, M.; Narváez, A.; Jeyasekharan, D.; et al. NF-κB signaling mediates acquired resistance after PARP inhibition. Oncotarget 2015, 6, 3825–3839. [Google Scholar] [CrossRef]
- Martín-Oliva, D.; O’Valle, F.; Munoz-Gamez, J.A.; Valenzuela, M.T.; Nunez, M.I.; Aguilar, M.; de Almodovar, J.R.; del Moral, R.G.; Oliver, F.J. Crosstalk between PARP-1 and NF-κB modulates the promotion of skin neoplasia. Oncogene 2004, 23, 5275–5283. [Google Scholar] [CrossRef]
- Specht, E.; Kaemmerer, D.; Sänger, J.; Wirtz, R.M.; Schulz, S.; Lupp, A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 2015, 67, 368–377. [Google Scholar] [CrossRef]
- Donizy, P.; Halon, A.; Surowiak, P.; Pietrzyk, G.; Kozyra, C.; Matkowski, R. Correlation between PARP-1 immunoreactivity and cytomorphological features of parthanatos, a specific cellular death in breast cancer cells. Eur. J. Histochem. 2013, 57, 237–240. [Google Scholar] [CrossRef][Green Version]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
- Tanori, M.; Mancuso, M.; Pasquali, E.; Leonardi, S.; Rebessi, S.; Di Majo, V.; Guilly, M.N.; Giangaspero, F.; Covelli, V.; Pazzaglia, S.; et al. PARP-1 cooperates with Ptc1 to suppress medulloblastoma and basal cell carcinoma. Carcinogenesis 2008, 29, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Dörsam, B.; Seiwert, N.; Foersch, S.; Stroh, S.; Nagel, G.; Begaliew, D.; Diehl, E.; Kraus, A.; McKeague, M.; Minneker, V.; et al. PARP-1 protects against colorectal tumor induction, but promotes inflammation-driven colorectal tumor progression. Proc. Natl. Acad. Sci. USA 2018, 115, E4061–E4070. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhao, Y.; Gao, D.; Xing, J.; Liu, H. High PARP-1 expression is associated with tumor invasion and poor prognosis in gastric cancer. Oncol. Lett. 2016, 12, 3825–3835. [Google Scholar] [CrossRef] [PubMed]
- Donizy, P.; Pietrzyk, G.; Halon, A.; Kozyra, C.; Gansukh, T.; Lage, H. Surowiak, P.; Matkowski, R. Nuclear-cytoplasmic PARP-1 expression as an unfavorable prognostic marker in lymph node-negative early breast cancer: 15-year follow-up. Oncol. Rep. 2014, 31, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F.; Garcia-Parra, J.; Zazo, S.; Tusquets, I.; Ferrer-Lozano, J.; Menendez, S.; Eroles, P.; Chamizo, C.; Servitja, S.; Ramirez-Merino, N.; et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 2012, 23, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Müller, B.M.; Loibl, S.; Budczies, J.; Hanusch, C.; Darb-Esfahani, S.; Hilfrich, J.; Weiss, E.; Huober, J.; Blohmer, J.U.; et al. Cytoplasmic poly(adenosine diphosphate-ribose) polymerase expression is predictive and prognostic in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. 2011, 29, 2150–2157. [Google Scholar] [CrossRef]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef]
- Salemi, M.; Galia, A.; Fraggetta, F.; La Corte, C.; Pepe, P.; La Vignera, S.; Improta, G.; Bosco, P.; Calogero, A.E. Poly (ADP-ribose) polymerase 1 protein expression in normal and neoplastic prostatic tissue. Eur. J. Histochem. 2013, 57, 80–82. [Google Scholar] [CrossRef]
- Kim, K.M.; Moon, Y.J.; Park, S.H.; Park, H.J.; Wang, S.I.; Park, H.S.; Lee, H.; Kwon, K.S.; Moon, W.S.; Lee, D.G.; et al. Individual and Combined Expression of DNA Damage Response Molecules PARP1, γH2AX, BRCA1, and BRCA2 Predict Shorter Survival of Soft Tissue Sarcoma Patients. PLoS ONE 2016, 11, e0163193. [Google Scholar] [CrossRef]
- Dziaman, T.; Ludwiczak, H.; Ciesla, J.M.; Banaszkiewicz, Z.; Winczura, A.; Chmielarczyk, M.; Wisniewska, E.; Marszalek, A.; Tudek, B.; Olinski, R. PARP-1 Expression is Increased in Colon Adenoma and Carcinoma and Correlates with OGG1. PLoS ONE 2014, 9, e115558. [Google Scholar] [CrossRef]
- Donizy, P.; Wu, C.L.; Mull, J.; Fujimoto, M.; Chłopik, A.; Peng, Y.; Shalin, S.C.; Selim, M.A.; Puig, S.; Fernandez-Figueras, M.T.; et al. Up-Regulation of PARP1 Expression Significantly Correlated with Poor Survival in Mucosal Melanomas. Cells 2020, 9, 1135. [Google Scholar] [CrossRef] [PubMed]
- Géhl, Z.; Bai, P.; Bodnár, E.; Emri, G.; Remenyik, É.; Németh, J.; Gergely, P.; Virág, L.; Szabó, É. Poly(ADP-ribose) in the skin and in melanomas. Histol. Histopathol. 2012, 27, 651–659. [Google Scholar] [PubMed]
- Molloy-Simard, V.; St-Laurent, J.F.; Vigneault, F.; Gaudreault, M.; Dargis, N.; Guérin, M.C.; Leclerc, S.; Morcos, M.; Black, D.; Molgat, Y.; et al. Altered expression of the Poly(ADP-Ribosyl)ation enzymes in uveal melanoma and regulation of PARG gene expression by the transcription factor ERM. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6219–6231. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Leading Edge Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yuan, B.; Ye, N.; Song, S.-S.; Wang, Y.-T.; Song, Z.; Chen, H.-D. Poly(ADP-ribose)polymerase (PARP) inhibition and anticancer activity of simmiparib, a new inhibitor undergaoing clinical trials. Cancer Lett. 2017, 386, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, C. PARP inhibitor combinations in prostate cancer. Ther. Adv. Med. Oncol. 2012, 12, 1758835919897537. [Google Scholar] [CrossRef]
- Mascolo, M.; Ilardi, G.; Romano, M.F.; Celetti, A.; Siano, M.; Romano, S.; Luise, C.; Merolla, F.; Rocco, A.; Vecchione, M.L.; et al. Overexpression of chromatin assembly factor-1 p60, poly(ADP-ribose) polymerase 1 and nestin predicts metastasizing behaviour of oral cancer. Histopathology 2012, 61, 1089–1105. [Google Scholar] [CrossRef]
- Berger, N.; Bessson, V.; Boulares, H.; Burkle, A.; Chiarugi, A. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br. J. Pharmacol. 2018, 172, 192–222. [Google Scholar] [CrossRef]
- Meira, M.; Sievers, C.; Hoffmann, F.; Bodmer, H.; Derfuss, T.; Kuhle, J.; Haghikia, A.; Kappos, L.; Lindberg, R.L. PARP-1 deregulation in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 1–20. [Google Scholar] [CrossRef]
- De Koning, L.; Decaudin, D.; El Botty, R.; Nicolas, A.; Carita, G.; Schuller, M.; Ouine, B.; Cartier, A.; Naguez, A.; Fleury, J.; et al. Parp inhibition increases the response to chemotherapy in uveal melanoma. Cancers 2019, 11, 751. [Google Scholar] [CrossRef]
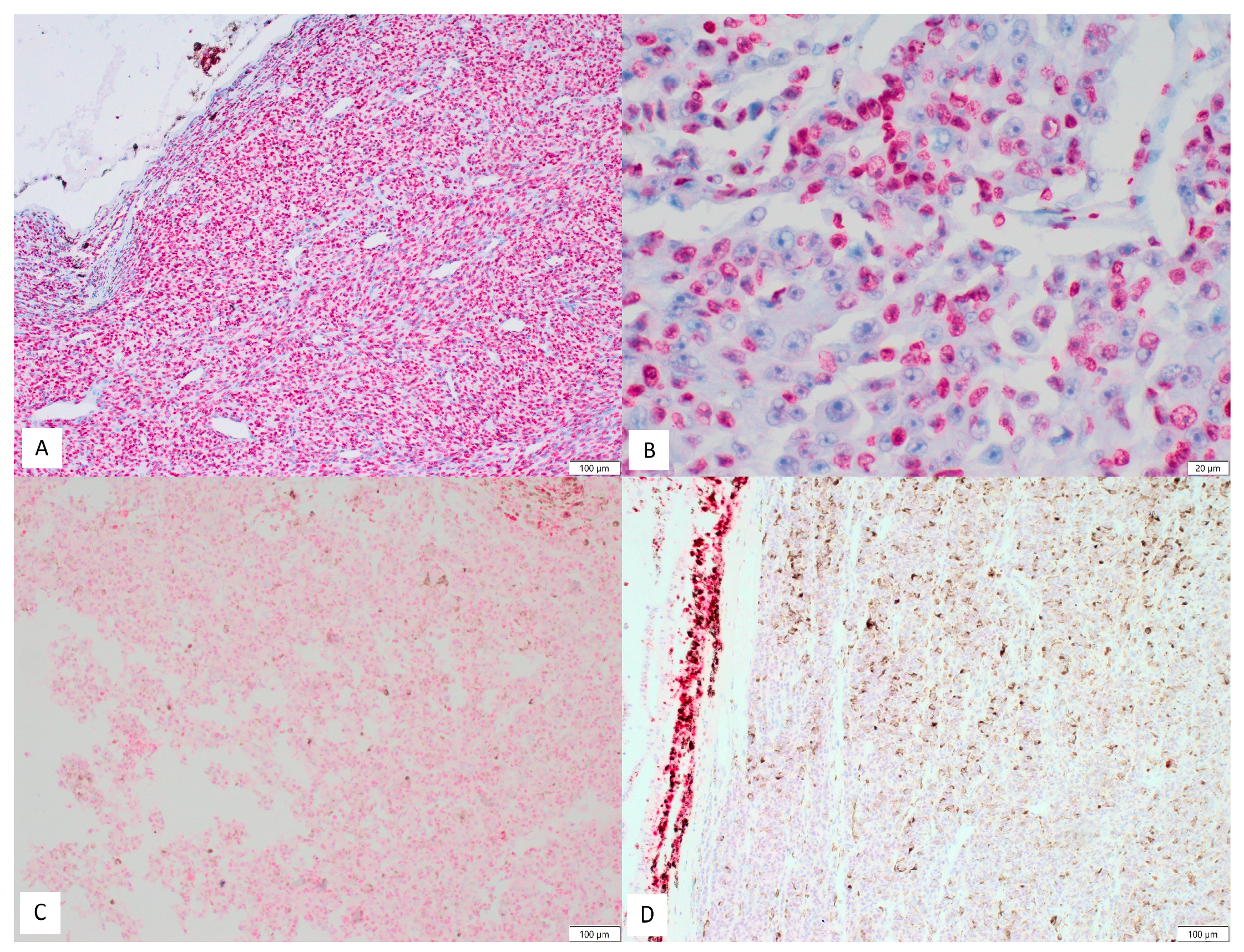
| Clinical Factors | No (%) |
|---|---|
| Gender | |
| Male | 36 (39%) |
| Female | 55 (61%) |
| Age | |
| ≤ 63 | 43 (47%) |
| > 63 | 48 (53%) |
| iliary body involvement | |
| No | 54 (59%) |
| Yes | 37 (41%) |
| Iris involvement | |
| No | 84 (92%) |
| Yes | 7 (8%) |
| Irido-corneal angle involvement | |
| No | 84 (92%) |
| Yes | 7 (8%) |
| Intrascleral extension | |
| No | 13 (14%) |
| Yes | 78 (86%) |
| Extrascleral extension | |
| No | 81 (89%) |
| Yes | 10 (11%) |
| Tumor size | |
| ≤15 mm | 55 (60%) |
| >15 mm | 36 (40%) |
| Mitoses * | |
| ≤4 | 56 (63%) |
| >4 | 33 (37%) |
| Grade | |
| G1+G2 | 72 (79%) |
| G3 | 19 (21%) |
| Chromosome 3 loss ** | |
| No | 16 (24%) |
| Yes | 51 (76%) |
| Metastases | |
| No | 47 (52%) |
| Yes | 44 (48%) |
| Clinical Factors | PARP-1 Low Expression (0–3) | PARP-1 High Expression (4–12) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 9 | 27 | 0.033 |
| Female | 26 | 29 | |
| Age | |||
| ≤ 63 | 20 | 23 | 0.135 |
| > 63 | 15 | 33 | |
| Ciliary body involvement | |||
| No | 24 | 30 | 0.156 |
| Yes | 11 | 26 | |
| Iris involvement | |||
| No | 33 | 51 | 0.576 |
| Yes | 2 | 5 | |
| Irido-corneal angle involvement | |||
| No | 1 | 6 | 0.171 |
| Yes | 34 | 50 | |
| Intrascleral extension | |||
| No | 1 | 12 | 0.014 |
| Yes | 34 | 44 | |
| Extrascleral extension | |||
| No | 32 | 49 | 0.560 |
| Yes | 3 | 7 | |
| Tumor size | |||
| ≤15 mm | 25 | 30 | 0.079 |
| >15 mm | 10 | 26 | |
| Mitoses | |||
| ≤4 | 25 | 31 | 0.126 |
| >4 | 10 | 23 | |
| Grade | |||
| G1 + G2 | 32 | 40 | 0.022 |
| G3 | 3 | 16 | |
| Chromosome 3 loss | |||
| No | 11 | 5 | 0.008 |
| Yes | 16 | 35 | |
| Metastases | |||
| No | 19 | 28 | 0.691 |
| Yes | 16 | 28 |
| Clinical Factors | PARP-1 Low Intensity (0–1) | PARP-1 High Intensity (2–3) | p-value |
|---|---|---|---|
| Gender | |||
| Male | 8 | 28 | 0.036 |
| Female | 24 | 31 | |
| Intrascleral extension | |||
| No | 1 | 12 | 0.025 |
| Yes | 31 | 47 | |
| Tumor size | |||
| ≤ 15 mm | 23 | 32 | 0.039 |
| > 15 mm | 9 | 27 | |
| Chromosome 3 loss | |||
| No | 10 | 6 | 0.017 |
| Yes | 15 | 36 |
| Clinical Factors | PARP-1 low percentage (0–2) | PARP-1 high percentage (3–4) | p-value |
|---|---|---|---|
| Tumor size | |||
| ≤ 15 mm | 33 | 22 | 0.036 |
| > 15 mm | 14 | 22 | |
| Grade | |||
| G1 + G2 | 40 | 32 | 0.024 |
| G3 | 7 | 12 | |
| Chromosome 3 loss | |||
| No | 11 | 5 | 0.033 |
| Yes | 24 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajdzis, M.; Theocharis, S.; Klijanienko, J.; Cassoux, N.; Gardrat, S.; Donizy, P.; Kaczmarek, R.; Gajdzis, P. The Prognostic Values of PARP-1 Expression in Uveal Melanoma. Cells 2021, 10, 285. https://doi.org/10.3390/cells10020285
Gajdzis M, Theocharis S, Klijanienko J, Cassoux N, Gardrat S, Donizy P, Kaczmarek R, Gajdzis P. The Prognostic Values of PARP-1 Expression in Uveal Melanoma. Cells. 2021; 10(2):285. https://doi.org/10.3390/cells10020285
Chicago/Turabian StyleGajdzis, Malgorzata, Stamatios Theocharis, Jerzy Klijanienko, Nathalie Cassoux, Sophie Gardrat, Piotr Donizy, Radoslaw Kaczmarek, and Pawel Gajdzis. 2021. "The Prognostic Values of PARP-1 Expression in Uveal Melanoma" Cells 10, no. 2: 285. https://doi.org/10.3390/cells10020285
APA StyleGajdzis, M., Theocharis, S., Klijanienko, J., Cassoux, N., Gardrat, S., Donizy, P., Kaczmarek, R., & Gajdzis, P. (2021). The Prognostic Values of PARP-1 Expression in Uveal Melanoma. Cells, 10(2), 285. https://doi.org/10.3390/cells10020285






