Adipokines and Autoimmunity in Inflammatory Arthritis
Abstract
1. Introduction
2. Adipose Tissue and Adipokines
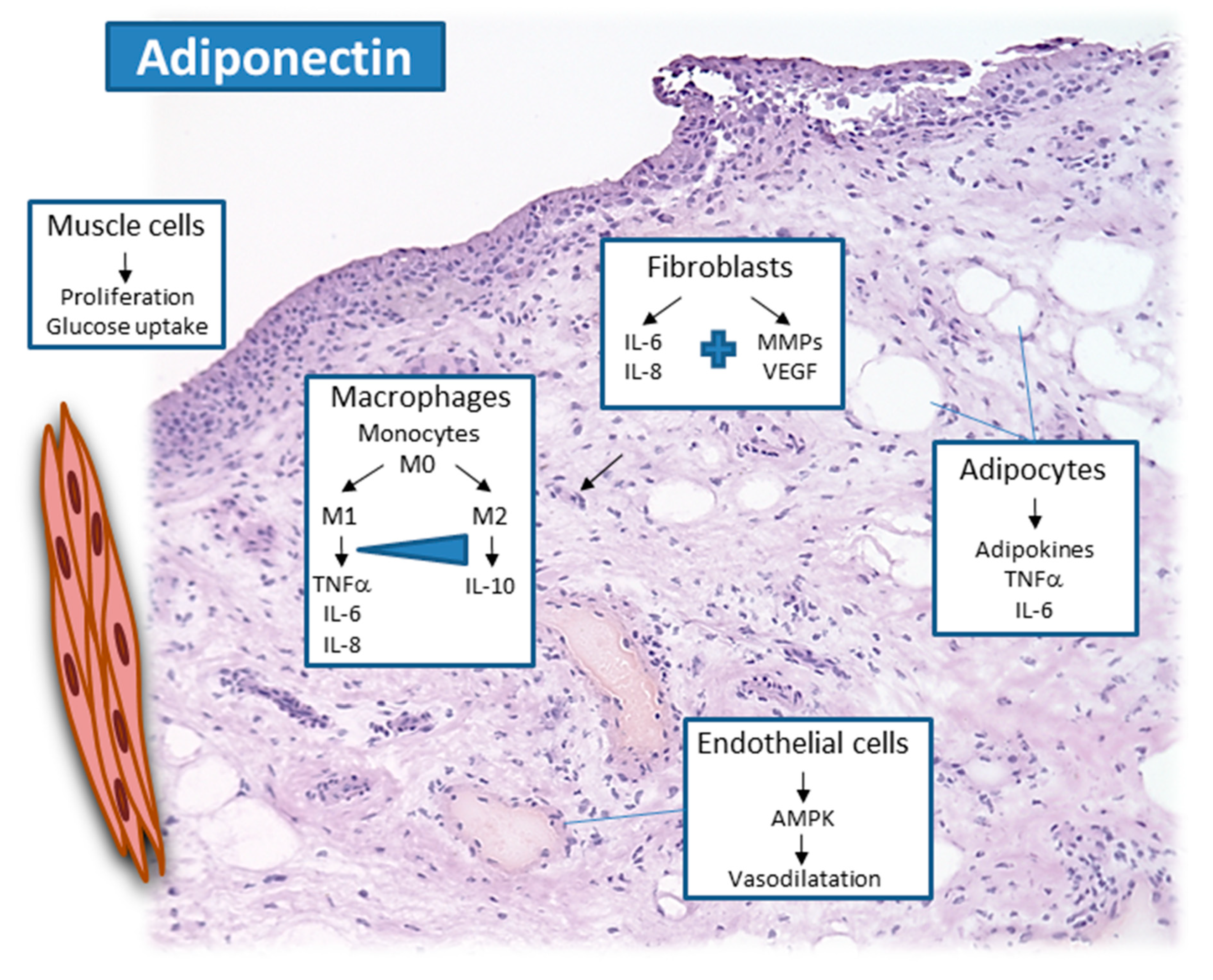
2.1. Adiponectin
2.2. Leptin
2.3. Visfatin
2.4. Resistin
2.5. Chemerin, Vaspin, and Omentin
2.6. Progranulin, Lipocalin-2, and Nesfatin
3. Adipokines in Autoimmune Rheumatoid Arthritis
3.1. Rheumatoid Arthritis
3.1.1. Adiponectin in Rheumatoid Arthritis
3.1.2. Leptin in Rheumatoid Arthritis
3.1.3. Visfatin in Rheumatoid Arthritis
3.1.4. Resistin in Rheumatoid Arthritis
3.1.5. Chemerin, Vaspin, and Omentin in Rheumatoid Arthritis
3.1.6. Progranulin, Nesfatin, and Lipocalin-2 in Rheumatoid Arthritis
3.1.7. Adipokine Network in Rheumatoid Arthritis
3.1.8. Summary and Conclusions
Funding
Conflicts of Interest
References
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Neumann, E.; Lefevre, S.; Zimmermann, B.; Geyer, M.; Lehr, A.; Umscheid, T.; Schonburg, M.; Rehart, S.; Muller-Ladner, U. Migratory potential of rheumatoid arthritis synovial fibroblasts: Additional perspectives. Cell Cycle 2010, 9, 2286–2291. [Google Scholar] [CrossRef]
- Reece, R.J.; Canete, J.D.; Parsons, W.J.; Emery, P.; Veale, D.J. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 1999, 42, 1481–1484. [Google Scholar] [CrossRef]
- Kruithof, E.; Baeten, D.; De Rycke, L.; Vandooren, B.; Foell, D.; Roth, J.; Canete, J.D.; Boots, A.M.; Veys, E.M.; De Keyser, F. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R569–R580. [Google Scholar] [CrossRef]
- McGonagle, D.; Lories, R.J.; Tan, A.L.; Benjamin, M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007, 56, 2482–2491. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Alexiou, I.; Simopoulou, T.; Vlychou, M. Enthesitis in psoriatic arthritis. Semin. Arthritis Rheum. 2013, 43, 325–334. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Junker, S.; Schett, G.; Frommer, K.; Muller-Ladner, U. Adipokines in bone disease. Nat. Rev. Rheumatol. 2016, 12, 296–302. [Google Scholar] [CrossRef]
- Szumilas, K.; Szumilas, P.; Sluczanowska-Glabowska, S.; Zgutka, K.; Pawlik, A. Role of Adiponectin in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 8265. [Google Scholar] [CrossRef] [PubMed]
- Carrion, M.; Frommer, K.W.; Perez-Garcia, S.; Muller-Ladner, U.; Gomariz, R.P.; Neumann, E. The Adipokine Network in Rheumatic Joint Diseases. Int. J. Mol. Sci. 2019, 20, 4091. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Frommer, K.W.; Vasile, M.; Muller-Ladner, U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 2011, 63, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 2018, 3, e99680. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kita, S.; Nishizawa, H.; Fukuda, S.; Fujishima, Y.; Obata, Y.; Nagao, H.; Masuda, S.; Nakamura, Y.; Shimizu, Y.; et al. Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci. Rep. 2019, 9, 16. [Google Scholar] [CrossRef]
- Cesari, M.; Pessina, A.C.; Zanchetta, M.; De Toni, R.; Avogaro, A.; Pedon, L.; Dorigatti, F.; Maiolino, G.; Rossi, G.P. Low plasma adiponectin is associated with coronary artery disease but not with hypertension in high-risk nondiabetic patients. J. Intern. Med. 2006, 260, 474–483. [Google Scholar] [CrossRef]
- Dzielinska, Z.; Januszewicz, A.; Wiecek, A.; Demkow, M.; Makowiecka-Ciesla, M.; Prejbisz, A.; Kadziela, J.; Mielniczuk, R.; Florczak, E.; Janas, J.; et al. Decreased plasma concentration of a novel anti-inflammatory protein--adiponectin--in hypertensive men with coronary artery disease. Thromb. Res. 2003, 110, 365–369. [Google Scholar] [CrossRef]
- Ouchi, N.; Shibata, R.; Walsh, K. Cardioprotection by adiponectin. Trends Cardiovasc. Med. 2006, 16, 141–146. [Google Scholar] [CrossRef]
- Galler, A.; Gelbrich, G.; Kratzsch, J.; Noack, N.; Kapellen, T.; Kiess, W. Elevated serum levels of adiponectin in children, adolescents and young adults with type 1 diabetes and the impact of age, gender, body mass index and metabolic control: A longitudinal study. Eur. J. Endocrinol. 2007, 157, 481–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kato, K.; Osawa, H.; Ochi, M.; Kusunoki, Y.; Ebisui, O.; Ohno, K.; Ohashi, J.; Shimizu, I.; Fujii, Y.; Tanimoto, M.; et al. Serum total and high molecular weight adiponectin levels are correlated with the severity of diabetic retinopathy and nephropathy. Clin. Endocrinol. 2008, 68, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Leth, H.; Andersen, K.; Frystyk, J.; Tarnow, L.; Rossing, P.; Parving, H.; Flyvbjerg, A. Elevated levels of high-molecular-weight adiponectin in type 1 diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 3186–3191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fatel, E.C.S.; Rosa, F.T.; Simao, A.N.C.; Dichi, I. Adipokines in rheumatoid arthritis. Adv. Rheumatol. 2018, 58, 25. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gomez-Reino, J.J.; Mera, A.; Lago, F.; Gomez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Lago, F.; Gomez, R.; Gomez-Reino, J.J.; Dieguez, C.; Gualillo, O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 2009, 34, 500–510. [Google Scholar] [CrossRef]
- Hasenkrug, K.J. The leptin connection: Regulatory T cells and autoimmunity. Immunity 2007, 26, 143–145. [Google Scholar] [CrossRef]
- Feijoo-Bandin, S.; Portoles, M.; Rosello-Lleti, E.; Rivera, M.; Gonzalez-Juanatey, J.R.; Lago, F. 20 years of leptin: Role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 2015, 140, 10–18. [Google Scholar] [CrossRef]
- Busso, N.; Karababa, M.; Nobile, M.; Rolaz, A.; Van Gool, F.; Galli, M.; Leo, O.; So, A.; De Smedt, T. Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS ONE 2008, 3, e2267. [Google Scholar] [CrossRef]
- Cheleschi, S.; Giordano, N.; Volpi, N.; Tenti, S.; Gallo, I.; Di Meglio, M.; Giannotti, S.; Fioravanti, A. A Complex Relationship between Visfatin and Resistin and microRNA: An In Vitro Study on Human Chondrocyte Cultures. Int. J. Mol. Sci. 2018, 19, 3909. [Google Scholar] [CrossRef]
- Zhao, C.W.; Gao, Y.H.; Song, W.X.; Liu, B.; Ding, L.; Dong, N.; Qi, X. An Update on the Emerging Role of Resistin on the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2019, 2019, 1532164. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 2005, 174, 5789–5795. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; He, J.; Wu, B.; Wang, W.; Li, Z. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine 2019, 113, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yamaguchi, Y.; Ge, X.; Robinson, W.H.; Morser, J.; Leung, L.L.K. Chemerin 156F, generated by chymase cleavage of prochemerin, is elevated in joint fluids of arthritis patients. Arthritis Res. Ther. 2018, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Luangsay, S.; Wittamer, V.; Bondue, B.; De Henau, O.; Rouger, L.; Brait, M.; Franssen, J.D.; de Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Berg, V.; Sveinbjornsson, B.; Bendiksen, S.; Brox, J.; Meknas, K.; Figenschau, Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157). Arthritis Res. Ther. 2010, 12, R228. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Tankova, T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed Res. Int. 2015, 2015, 823481. [Google Scholar] [CrossRef]
- Nicholson, T.; Church, C.; Baker, D.J.; Jones, S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. (Lond) 2018, 15, 9. [Google Scholar] [CrossRef]
- Schaffler, A.; Neumeier, M.; Herfarth, H.; Furst, A.; Scholmerich, J.; Buchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- Wei, J.; Hettinghouse, A.; Liu, C. The role of progranulin in arthritis. Ann. N. Y. Acad. Sci. 2016, 1383, 5–20. [Google Scholar] [CrossRef]
- Tang, W.; Lu, Y.; Tian, Q.Y.; Zhang, Y.; Guo, F.J.; Liu, G.Y.; Syed, N.M.; Lai, Y.; Lin, E.A.; Kong, L.; et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 2011, 332, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Pino, J.; Scotece, M.; Conde, J.; Lago, F.; Gonzalez-Gay, M.A.; Mera, A.; Gomez, R.; Mobasheri, A.; Gualillo, O. Progranulin as a biomarker and potential therapeutic agent. Drug Discov. Today 2017, 22, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Ayada, C.; Toru, U.; Korkut, Y. Nesfatin-1 and its effects on different systems. Hippokratia 2015, 19, 4–10. [Google Scholar] [PubMed]
- Kaneko, K.; Miyabe, Y.; Takayasu, A.; Fukuda, S.; Miyabe, C.; Ebisawa, M.; Yokoyama, W.; Watanabe, K.; Imai, T.; Muramoto, K.; et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R158. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Gomez, R.; Bianco, G.; Scotece, M.; Lear, P.; Dieguez, C.; Gomez-Reino, J.; Lago, F.; Gualillo, O. Expanding the adipokine network in cartilage: Identification and regulation of novel factors in human and murine chondrocytes. Ann. Rheum. Dis. 2011, 70, 551–559. [Google Scholar] [CrossRef]
- Chihara, K.; Hattori, N.; Ichikawa, N.; Matsuda, T.; Saito, T. Re-evaluation of serum leptin and adiponectin concentrations normalized by body fat mass in patients with rheumatoid arthritis. Sci. Rep. 2020, 10, 15932. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, X.; Gao, Z.; Liu, Y.; Zhang, B.; Xia, L.; Lu, J.; Shen, H. Association Between Adiponectin and Clinical Manifestations in Rheumatoid Arthritis. J. Interferon Cytokine Res. 2020, 40, 501–508. [Google Scholar] [CrossRef]
- Minamino, H.; Katsushima, M.; Yoshida, T.; Hashimoto, M.; Fujita, Y.; Shirakashi, M.; Yamamoto, W.; Murakami, K.; Murata, K.; Nishitani, K.; et al. Increased circulating adiponectin is an independent disease activity marker in patients with rheumatoid arthritis: A cross-sectional study using the KURAMA database. PLoS ONE 2020, 15, e0229998. [Google Scholar] [CrossRef]
- Kontny, E.; Zielinska, A.; Ksiezopolska-Orlowska, K.; Gluszko, P. Secretory activity of subcutaneous abdominal adipose tissue in male patients with rheumatoid arthritis and osteoarthritis—association with clinical and laboratory data. Reumatologia 2016, 54, 227–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Peltonen, M.; Andersson-Assarsson, J.C.; Svensson, P.A.; Herder, C.; Rudin, A.; Carlsson, L.; Maglio, C. Elevated adiponectin predicts the development of rheumatoid arthritis in subjects with obesity. Scand. J. Rheumatol. 2020, 49, 452–460. [Google Scholar] [CrossRef]
- Fioravanti, A.; Tenti, S.; Bacarelli, M.R.; Damiani, A.; Li Gobbi, F.; Bandinelli, F.; Cheleschi, S.; Galeazzi, M.; Benucci, M. Tocilizumab modulates serum levels of adiponectin and chemerin in patients with rheumatoid arthritis: Potential cardiovascular protective role of IL-6 inhibition. Clin. Exp. Rheumatol. 2019, 37, 293–300. [Google Scholar] [PubMed]
- Toussirot, E.; Marotte, H.; Mulleman, D.; Cormier, G.; Coury, F.; Gaudin, P.; Dernis, E.; Bonnet, C.; Damade, R.; Grauer, J.L.; et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: A 12-month multicentre study. Arthritis Res. Ther. 2020, 22, 224. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M.; Perillo, F.; Metella Refini, R.; Bergantini, L.; Bellisai, F.; Selvi, E.; Cameli, P.; Manganelli, S.; Conticini, E.; Cantarini, L.; et al. Efficacy of baricitinib in treating rheumatoid arthritis: Modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int. Immunopharmacol. 2020, 86, 106748. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Hahm, D.H.; Kim, J.Y.; Sur, B.; Lee, H.M.; Ryu, C.J.; Yang, H.I.; Kim, K.S. Potential therapeutic antibodies targeting specific adiponectin isoforms in rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 245. [Google Scholar] [CrossRef]
- Neumeier, M.; Weigert, J.; Schaffler, A.; Wehrwein, G.; Muller-Ladner, U.; Scholmerich, J.; Wrede, C.; Buechler, C. Different effects of adiponectin isoforms in human monocytic cells. J. Leukoc. Biol. 2006, 79, 803–808. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Jiang, J.; Lin, S.; Luo, A.; Zhao, P.; Tan, W.; Zhang, M. Critical Role of AdipoR1 in Regulating Th17 Cell Differentiation Through Modulation of HIF-1alpha-Dependent Glycolysis. Front. Immunol. 2020, 11, 2040. [Google Scholar] [CrossRef]
- Hasseli, R.; Frommer, K.W.; Schwarz, M.; Hulser, M.L.; Schreiyack, C.; Arnold, M.; Diller, M.; Tarner, I.H.; Lange, U.; Pons-Kuhnemann, J.; et al. Adipokines and Inflammation Alter the Interaction Between Rheumatoid Arthritis Synovial Fibroblasts and Endothelial Cells. Front. Immunol. 2020, 11, 925. [Google Scholar] [CrossRef]
- Yoshino, T.; Kusunoki, N.; Tanaka, N.; Kaneko, K.; Kusunoki, Y.; Endo, H.; Hasunuma, T.; Kawai, S. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern. Med. 2011, 50, 269–275. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Circulating leptin level in rheumatoid arthritis and its correlation with disease activity: A meta-analysis. Z. Rheumatol. 2016, 75, 1021–1027. [Google Scholar] [CrossRef]
- Guimaraes, M.; de Andrade, M.V.M.; Machado, C.J.; Vieira, E.L.M.; Pinto, M.; Junior, A.L.T.; Kakehasi, A.M. Leptin as an obesity marker in rheumatoid arthritis. Rheumatol. Int. 2018, 38, 1671–1677. [Google Scholar] [CrossRef]
- Batun-Garrido, J.A.J.; Salas-Magana, M.; Juarez-Rojop, I.E. Association between leptin and IL-6 concentrations with cardiovascular risk in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Lafaurie, G.I.; Bautista-Molano, W.; Chila-Moreno, L.; Bello-Gualtero, J.M.; Romero-Sanchez, C. Adipokines and periodontal markers as risk indicators of early rheumatoid arthritis: A cross-sectional study. Clin. Oral Investig. 2020. [Online ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Kononoff, A.; Vuolteenaho, K.; Hamalainen, M.; Kautiainen, H.; Elfving, P.; Savolainen, E.; Arstila, L.; Niinisalo, H.; Rutanen, J.; Marjoniemi, O.; et al. Metabolic Syndrome, Disease Activity, and Adipokines in Patients With Newly Diagnosed Inflammatory Joint Diseases. J. Clin. Rheumatol. 2020. [Online ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Guin, A.; Sinhamahapatra, P.; Misra, S.; Choudhury Mazumder, S.R.; Chatterjee, S.; Ghosh, A. Incidence and effect of insulin resistance on progression of atherosclerosis in rheumatoid arthritis patients of long disease duration. Biomed. J. 2019, 42, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martinez, S.; Genre, F.; Calvo-Alen, J.; Aurrecoechea, E.; Llorente, I.; Triguero-Martinez, A.; Blanco, R.; Llorca, J.; Ruiz-Lucea, E.; et al. Anti-IL-6 therapy reduces leptin serum levels in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 1201–1205. [Google Scholar]
- Choi, I.A.; Sagawa, A.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Tocilizumab Increases Body Weight and Serum Adipokine Levels in Patients with Rheumatoid Arthritis Independently of Their Treatment Response: A Retrospective Cohort Study. J. Korean Med. Sci. 2020, 35, e155. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Z.; Bin, Z. The Association Between Serum Leptin Levels and Cardiovascular Events in Patients with Rheumatoid Arthritis. Lab. Med. 2020, 52, 86–92. [Google Scholar] [CrossRef]
- Ma, M.H.Y.; Defranoux, N.; Li, W.; Sasso, E.H.; Ibrahim, F.; Scott, D.L.; Cope, A.P. A multi-biomarker disease activity score can predict sustained remission in rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 158. [Google Scholar] [CrossRef]
- Robinson, C.; Tsang, L.; Solomon, A.; Woodiwiss, A.J.; Gunter, S.; Mer, M.; Hsu, H.C.; Gomes, M.; Norton, G.R.; Millen, A.M.E.; et al. Nesfatin-1 and visfatin expression is associated with reduced atherosclerotic disease risk in patients with rheumatoid arthritis. Peptides 2018, 102, 31–37. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Alonso-Perez, A.; Guillan-Fresco, M.; Jorge-Mora, A.; Gualillo, O.; Gomez-Reino, J.J.; Gomez Bahamonde, R. Visfatin as a therapeutic target for rheumatoid arthritis. Expert Opin. Ther. Targets 2019, 23, 607–618. [Google Scholar] [CrossRef]
- Polyakova, Y.V.; Zavodovsky, B.V.; Sivordova, L.E.; Akhverdyan, Y.R.; Zborovskaya, I.A. Visfatin and Rheumatoid Arthritis: Pathogenetic Implications and Clinical Utility. Curr. Rheumatol. Rev. 2020, 16, 224–239. [Google Scholar] [CrossRef]
- Li, X.; Islam, S.; Xiong, M.; Nsumu, N.N.; Lee, M.W., Jr.; Zhang, L.Q.; Ueki, Y.; Heruth, D.P.; Lei, G.; Ye, S.Q. Epigenetic regulation of NfatC1 transcription and osteoclastogenesis by nicotinamide phosphoribosyl transferase in the pathogenesis of arthritis. Cell Death Discov. 2019, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Senolt, L.; Housa, D.; Vernerova, Z.; Jirasek, T.; Svobodova, R.; Veigl, D.; Anderlova, K.; Muller-Ladner, U.; Pavelka, K.; Haluzik, M. Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann. Rheum. Dis. 2007, 66, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Bustos Rivera-Bahena, C.; Xibille-Friedmann, D.X.; Gonzalez-Christen, J.; Carrillo-Vazquez, S.M.; Montiel-Hernandez, J.L. Peripheral blood leptin and resistin levels as clinical activity biomarkers in Mexican Rheumatoid Arthritis patients. Reumatol. Clin. 2016, 12, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Muraoka, S.; Kusunoki, N.; Masuoka, S.; Yamada, S.; Ogasawara, H.; Imai, T.; Akasaka, Y.; Tochigi, N.; Takahashi, H.; et al. Resistin upregulates chemokine production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, R.; Herenius, M.M.; Wijbrandts, C.A.; de Jager, W.; van Tuyl, L.H.; Nurmohamed, M.T.; Prakken, B.J.; Gerlag, D.M.; Tak, P.P. Treatment-specific changes in circulating adipocytokines: A comparison between tumour necrosis factor blockade and glucocorticoid treatment for rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Nagaev, I.; Andersen, M.; Olesen, M.K.; Nagaeva, O.; Wikberg, J.; Mincheva-Nilsson, L.; Andersen, G.N. Resistin Gene Expression is Downregulated in CD4(+) T Helper Lymphocytes and CD14(+) Monocytes in Rheumatoid Arthritis Responding to TNF-alpha Inhibition. Scand. J. Immunol. 2016, 84, 229–236. [Google Scholar] [CrossRef]
- Hoffman, E.; Rahat, M.A.; Feld, J.; Elias, M.; Rosner, I.; Kaly, L.; Lavie, I.; Gazitt, T.; Zisman, D. Effects of Tocilizumab, an Anti-Interleukin-6 Receptor Antibody, on Serum Lipid and Adipokine Levels in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 4633. [Google Scholar] [CrossRef]
- Huang, H.; Tong, T.T.; Yau, L.F.; Wang, J.R.; Lai, M.H.; Zhang, C.R.; Wen, X.H.; Li, S.N.; Li, K.Y.; Liu, J.Q.; et al. Chemerin isoform analysis in human biofluids using an LC/MRM-MS-based targeted proteomics approach with stable isotope-labeled standard. Anal. Chim. Acta 2020, 1139, 79–87. [Google Scholar] [CrossRef]
- Tolusso, B.; Gigante, M.R.; Alivernini, S.; Petricca, L.; Fedele, A.L.; Di Mario, C.; Aquilanti, B.; Magurano, M.R.; Ferraccioli, G.; Gremese, E. Chemerin and PEDF Are Metaflammation-Related Biomarkers of Disease Activity and Obesity in Rheumatoid Arthritis. Front. Med. 2018, 5, 207. [Google Scholar] [CrossRef]
- Mohammed Ali, D.M.; Al-Fadhel, S.Z.; Al-Ghuraibawi, N.H.A.; Al-Hakeim, H.K. Serum chemerin and visfatin levels and their ratio as possible diagnostic parameters of rheumatoid arthritis. Reumatologia 2020, 58, 67–75. [Google Scholar] [PubMed]
- Senolt, L.; Polanska, M.; Filkova, M.; Cerezo, L.A.; Pavelka, K.; Gay, S.; Haluzik, M.; Vencovsky, J. Vaspin and omentin: New adipokines differentially regulated at the site of inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1410–1411. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Omma, A.; Sandikci, S.C.; Yucel, C.; Omma, T.; Turhan, T. Vaspin, neutrophil gelatinase-associated lipocalin and apolipoprotein levels in patients with psoriatic arthritis. Bratisl. Lekárske Listy 2019, 120, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Wang, Q.F. Low vaspin levels are related to endothelial dysfunction in patients with ankylosing spondylitis. Braz. J. Med. Biol. Res. 2016, 49, e5231. [Google Scholar] [CrossRef]
- Maijer, K.I.; Neumann, E.; Muller-Ladner, U.; Drop, D.A.; Ramwadhdoebe, T.H.; Choi, I.Y.; Gerlag, D.M.; de Hair, M.J.; Tak, P.P. Serum Vaspin Levels Are Associated with the Development of Clinically Manifest Arthritis in Autoantibody-Positive Individuals. PLoS ONE 2015, 10, e0144932. [Google Scholar] [CrossRef]
- Frommer, K.W.; Vasile, M.; Muller-Ladner, U.; Neumann, E. The Adipokine Omentin in Late-stage Rheumatoid Arthritis and Endstage Osteoarthritis. J. Rheumatol. 2017, 44, 539–541. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, L.; Cheng, Q.; Chen, H.; Yu, Y.; Lin, Y.; Yang, X.; Kong, N.; Zhu, X.; Xu, X.; et al. Adipokines in psoriatic arthritis patients: The correlations with osteoclast precursors and bone erosions. PLoS ONE 2012, 7, e46740. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Shi, J.; Zhang, L.; Li, J.; Chen, S.; Wu, C.; Shen, B. Serum progranulin irrelated with Breg cell levels, but elevated in RA patients, reflecting high disease activity. Rheumatol. Int. 2016, 36, 359–364. [Google Scholar] [CrossRef]
- Assmann, G.; Zinke, S.; Gerling, M.; Bittenbring, J.T.; Preuss, K.D.; Thurner, L. Progranulin-autoantibodies in sera of rheumatoid arthritis patients negative for rheumatoid factor and anti-citrullinated peptide antibodies. Clin. Exp. Rheumatol. 2020, 38, 94–98. [Google Scholar]
- Wang, N.; Zhang, J.; Yang, J.X. Growth factor progranulin blocks tumor necrosis factor-alpha-mediated inhibition of osteoblast differentiation. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Liu, L.; Qu, Y.; Liu, Y.; Zhao, H.; Ma, H.C.; Noor, A.F.; Ji, C.J.; Nie, L.; Si, M.; Cheng, L. Atsttrin reduces lipopolysaccharide-induced neuroinflammation by inhibiting the nuclear factor kappa B signaling pathway. Neural Regen. Res. 2019, 14, 1994–2002. [Google Scholar] [PubMed]
- Naghashian, F.; Hosseinzadeh-Attar, M.J.; Akhlaghi, M.; Yekaninejad, M.S.; Aryaeian, N.; Derakhshanian, H. The relationship between anthropometric status and rheumatoid arthritis. Exploring the role of nesfatin and asymmetric dimethylarginine. Acta Reumatol. Port. 2019, 44, 126–131. [Google Scholar] [PubMed]
- Gupta, K.; Shukla, M.; Cowland, J.B.; Malemud, C.J.; Haqqi, T.M. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007, 56, 3326–3335. [Google Scholar] [CrossRef] [PubMed]
- Katano, M.; Okamoto, K.; Arito, M.; Kawakami, Y.; Kurokawa, M.S.; Suematsu, N.; Shimada, S.; Nakamura, H.; Xiang, Y.; Masuko, K.; et al. Implication of granulocyte-macrophage colony-stimulating factor induced neutrophil gelatinase-associated lipocalin in pathogenesis of rheumatoid arthritis revealed by proteome analysis. Arthritis Res. Ther. 2009, 11, R3. [Google Scholar] [CrossRef]
- Gulkesen, A.; Akgol, G.; Poyraz, A.K.; Aydin, S.; Denk, A.; Yildirim, T.; Kaya, A. Lipocalin 2 as a clinical significance in rheumatoid arthritis. Cent. Eur. J. Immunol. 2017, 42, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Inman, R.D.; Streutker, C.J.; Zhang, Z.; Pritzker, K.P.H.; Tsui, H.W.; Tsui, F.W.L. Lipocalin 2 links inflammation and ankylosis in the clinical overlap of inflammatory bowel disease (IBD) and ankylosing spondylitis (AS). Arthritis Res. Ther. 2020, 22, 51. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumann, E.; Hasseli, R.; Ohl, S.; Lange, U.; Frommer, K.W.; Müller-Ladner, U. Adipokines and Autoimmunity in Inflammatory Arthritis. Cells 2021, 10, 216. https://doi.org/10.3390/cells10020216
Neumann E, Hasseli R, Ohl S, Lange U, Frommer KW, Müller-Ladner U. Adipokines and Autoimmunity in Inflammatory Arthritis. Cells. 2021; 10(2):216. https://doi.org/10.3390/cells10020216
Chicago/Turabian StyleNeumann, Elena, Rebecca Hasseli, Selina Ohl, Uwe Lange, Klaus W. Frommer, and Ulf Müller-Ladner. 2021. "Adipokines and Autoimmunity in Inflammatory Arthritis" Cells 10, no. 2: 216. https://doi.org/10.3390/cells10020216
APA StyleNeumann, E., Hasseli, R., Ohl, S., Lange, U., Frommer, K. W., & Müller-Ladner, U. (2021). Adipokines and Autoimmunity in Inflammatory Arthritis. Cells, 10(2), 216. https://doi.org/10.3390/cells10020216





