Sulfated Hydrogels in Intervertebral Disc and Cartilage Research
Abstract
:1. Introduction
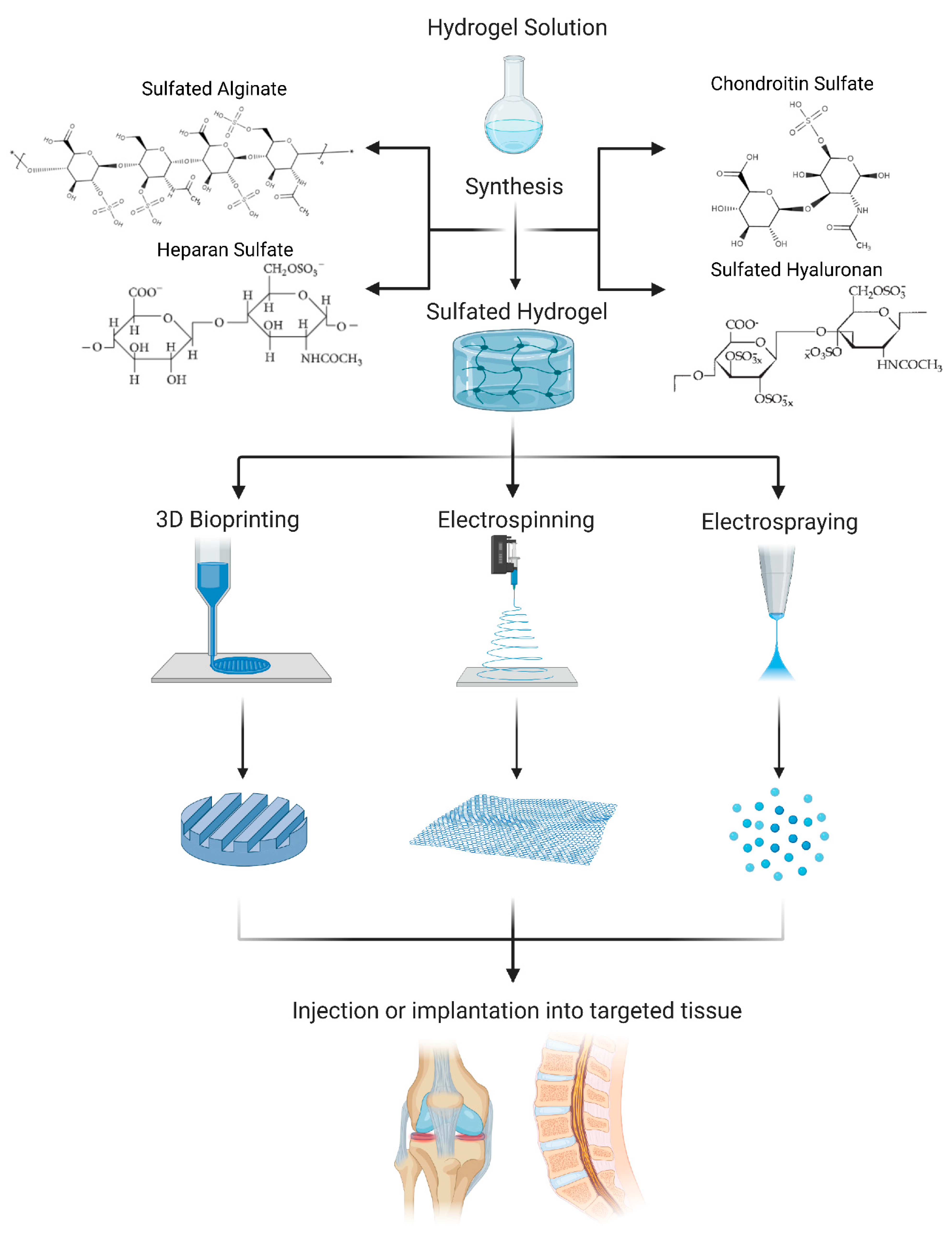
2. Sulfated Hydrogels Used in IVD and Cartilage Research: Overview
3. Chondroitin Sulfate (CS)
4. Heparan Sulfate (HS)
5. Sulfated Alginate (SA)
6. Sulfated Hyaluronan (SH)
7. Biofabrication Techniques
8. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassidy, J.J.; Hiltner, A.; Baer, E. Hierarchical Structure of the Intervertebral Disc. Connect. Tissue Res. 1989, 23, 75–88. [Google Scholar]
- Censi, R.; di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for Protein Delivery in Tissue Engineering. J. Control. Release 2012, 161, 680–692. [Google Scholar]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the Extracellular Matrix of the Nucleus Pulposus and Hyaline Cartilage: A Requisite for Tissue Engineering of Intervertebral Disc. Eur. Cell Mater. 2004, 8, 58–63. [Google Scholar]
- Yang, J.; Xiao, Y.; Tang, Z.; Luo, Z.; Li, D.; Wang, Q.; Zhang, X. The Negatively Charged Microenvironment of Collagen Hydrogels Regulates the Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in vitro and in vivo. J. Mater. Chem. B 2020, 8, 4680–4693. [Google Scholar]
- Jin, G.Z.; Kim, H.W. Efficacy of Collagen and Alginate Hydrogels for the Prevention of Rat Chondrocyte Dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar]
- Lim, J.J.; Temenoff, J.S. The Effect of Desulfation of Chondroitin Sulfate on Interactions with Positively Charged Growth Factors and Upregulation of Cartilaginous Markers in Encapsulated Mscs. Biomaterials 2013, 34, 5007–5018. [Google Scholar]
- Purcell, B.P.; Kim, I.L.; Chuo, V.; Guinen, T.; Dorsey, S.M.; Burdick, J.A. Incorporation of Sulfated Hyaluronic Acid Macromers into Degradable Hydrogel Scaffolds for Sustained Molecule Delivery. Biomater. Sci. 2014, 2, 693–702. [Google Scholar]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering Growth Factors for Regenerative Medicine Applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar]
- Hachim, D.; Whittaker, T.E.; Kim, H.; Stevens, M.M. Glycosaminoglycan-Based Biomaterials for Growth Factor and Cytokine Delivery: Making the Right Choices. J. Control. Release 2019, 313, 131–147. [Google Scholar]
- Ozturk, E.; Arlov, O.; Aksel, S.; Li, L.; Ornitz, D.M.; Skjak-Braek, G.; Zenobi-Wong, M. Sulfated Hydrogel Matrices Direct Mitogenicity and Maintenance of Chondrocyte Phenotype through Activation of Fgf Signaling. Adv. Funct. Mater. 2016, 26, 3649–3662. [Google Scholar]
- Thones, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gomez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/Collagen Hydrogels Containing Sulfated Hyaluronan Improve Wound Healing by Sustained Release of Heparin-Binding Egf-Like Growth Factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated Hyaluronic Acid Hydrogels with Retarded Degradation and Enhanced Growth Factor Retention Promote Hmsc Chondrogenesis and Articular Cartilage Integrity with Reduced Hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar]
- Fan, M.; Ma, Y.; Tan, H.; Jia, Y.; Zou, S.; Guo, S.; Zhao, M.; Huang, H.; Ling, Z.; Chen, Y.; et al. Covalent and Injectable Chitosan-Chondroitin Sulfate Hydrogels Embedded with Chitosan Microspheres for Drug Delivery and Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 67–74. [Google Scholar]
- Shin, J.; Kang, E.H.; Choi, S.; Jeon, E.J.; Cho, J.H.; Kang, D.; Lee, H.; Yun, I.S.; Cho, S.W. Tissue-Adhesive Chondroitin Sulfate Hydrogel for Cartilage Reconstruction. ACS Biomater. Sci. Eng. 2021, 7, 4230–4243. [Google Scholar]
- Wang, T.; Yang, F. A Comparative Study of Chondroitin Sulfate and Heparan Sulfate for Directing Three-Dimensional Chondrogenesis of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 284. [Google Scholar]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar]
- Wiltsey, C.; Kubinski, P.; Christiani, T.; Toomer, K.; Sheehan, J.; Branda, A.; Kadlowec, J.; Iftode, C.; Vernengo, J. Characterization of Injectable Hydrogels Based on Poly(N-Isopropylacrylamide)-G-Chondroitin Sulfate with Adhesive Properties for Nucleus Pulposus Tissue Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 837–847. [Google Scholar]
- Borrelli, C.; Buckley, C.T. Injectable Disc-Derived Ecm Hydrogel Functionalised with Chondroitin Sulfate for Intervertebral Disc Regeneration. Acta Biomater. 2020, 117, 142–155. [Google Scholar]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Chondrogenesis in Injectable Enzymatically Crosslinked Heparin/Dextran Hydrogels. J. Control. Release 2011, 152, 186–195. [Google Scholar]
- Kim, M.; Hong, B.; Lee, J.; Kim, S.E.; Kang, S.S.; Kim, Y.H.; Tae, G. Composite System of Plcl Scaffold and Heparin-Based Hydrogel for Regeneration of Partial-Thickness Cartilage Defects. Biomacromolecules 2012, 13, 2287–2298. [Google Scholar]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q.; Becher, J.; Moller, S.; Schnabelrauch, M.; Zenobi-Wong, M. Chondrocyte Culture in Three Dimensional Alginate Sulfate Hydrogels Promotes Proliferation While Maintaining Expression of Chondrogenic Markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar]
- Ozturk, E.; Stauber, T.; Levinson, C.; Cavalli, E.; Arlov, O.; Zenobi-Wong, M. Tyrosinase-Crosslinked, Tissue Adhesive and Biomimetic Alginate Sulfate Hydrogels for Cartilage Repair. Biomed. Mater. 2020, 15, 045019. [Google Scholar]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Junger, S.; Alini, M.; Eglin, D. Injectable Thermoreversible Hyaluronan-Based Hydrogels for Nucleus Pulposus Cell Encapsulation. Eur. Spine J. 2012, 21, S839–S849. [Google Scholar]
- Arlov, O.; Skjak-Braek, G. Sulfated Alginates as Heparin Analogues: A Review of Chemical and Functional Properties. Molecules 2017, 22, 778. [Google Scholar]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar]
- Uzieliene, I.; Bironaite, D.; Bernotas, P.; Sobolev, A.; Bernotiene, E. Mechanotransducive Biomimetic Systems for Chondrogenic Differentiation in vitro. Int. J. Mol. Sci. 2021, 22, 9690. [Google Scholar]
- Sanchez-Tellez, D.A.; Tellez-Jurado, L.; Rodriguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar]
- Amhare, A.F.; Lei, J.; Deng, H.; Lv, Y.; Han, J.; Zhang, L. Biomedical Application of Chondroitin Sulfate with Nanoparticles in Drug Delivery Systems: Systematic Review. J. Drug Target. 2021, 29, 259–268. [Google Scholar]
- Liang, Y.; Kiick, K.L. Heparin-Functionalized Polymeric Biomaterials in Tissue Engineering and Drug Delivery Applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar]
- Peng, Y.; Tellier, L.E.; Temenoff, J.S. Heparin-Based Hydrogels with Tunable Sulfation & Degradation for Anti-Inflammatory Small Molecule Delivery. Biomater. Sci. 2016, 4, 1371–1380. [Google Scholar]
- Choi, D.H.; Kang, S.N.; Kim, S.M.; Gobaa, S.; Park, B.J.; Kim, I.H.; Joung, Y.K.; Han, D.K. Growth Factors-Loaded Stents Modified with Hyaluronic Acid and Heparin for Induction of Rapid and Tight Re-Endothelialization. Colloids Surf. B Biointerfaces 2016, 141, 602–610. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar]
- Thornton, J.A.; Alsberg, E.; Hill, E.E.; Mooney, D.J. Shape Retaining Injectable Hydrogels for Minimally Invasive Bulking. J. Urol. 2004, 172, 763–768. [Google Scholar]
- Ma, H.L.; Chen, T.H.; Ho, L.L.; Hung, S.C. Neocartilage from Human Mesenchymal Stem Cells in Alginate: Implied Timing of Transplantation. J. Biomed. Mater. Res. A 2005, 74, 439–446. [Google Scholar]
- Lee, C.S.; Moyer, H.R.; Gittens, R.A.; Williams, J.K.; Boskey, A.L.; Boyan, B.D.; Schwartz, Z. Regulating in vivo Calcification of Alginate Microbeads. Biomaterials 2010, 31, 4926–4934. [Google Scholar]
- Wang, B.; Diaz-Payno, P.J.; Browe, D.C.; Freeman, F.E.; Nulty, J.; Burdis, R.; Kelly, D.J. Affinity-Bound Growth Factor within Sulfated Interpenetrating Network Bioinks for Bioprinting Cartilaginous Tissues. Acta Biomater. 2021, 128, 130–142. [Google Scholar]
- Freeman, I.; Kedem, A.; Cohen, S. The Effect of Sulfation of Alginate Hydrogels on the Specific Binding and Controlled Release of Heparin-Binding Proteins. Biomaterials 2008, 29, 3260–3268. [Google Scholar]
- Kshitiz; Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.H. Control of Stem Cell Fate and Function by Engineering Physical Microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar]
- Bachmann, B.; Spitz, S.; Schadl, B.; Teuschl, A.H.; Redl, H.; Nurnberger, S.; Ertl, P. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front. Bioeng. Biotechnol. 2020, 8, 373. [Google Scholar]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar]
- Zare, P.; Pezeshki-Modaress, M.; Davachi, S.M.; Zare, P.; Yazdian, F.; Simorgh, S.; Ghanbari, H.; Rashedi, H.; Bagher, Z. Alginate Sulfate-Based Hydrogel/Nanofiber Composite Scaffold with Controlled Kartogenin Delivery for Tissue Engineering. Carbohydr. Polym. 2021, 266, 118123. [Google Scholar]
- Park, H.; Lee, H.J.; An, H.; Lee, K.Y. Alginate Hydrogels Modified with Low Molecular Weight Hyaluronate for Cartilage Regeneration. Carbohydr. Polym. 2017, 162, 100–107. [Google Scholar]
- Roig-Roig, F.; Solans, C.; Esquena, J.; García-Celma, M.J. Preparation, Characterization, and Release Properties of Hydrogels Based on Hyaluronan for Pharmaceutical and Biomedical Use. J. Appl. Polym. Sci. 2013, 130, 1377–1382. [Google Scholar]
- Qin, L.; Beier, F. Egfr Signaling: Friend or Foe for Cartilage? JBMR Plus 2019, 3, e10177. [Google Scholar]
- Abbadessa, A.; Blokzijl, M.M.; Mouser, V.H.; Marica, P.; Malda, J.; Hennink, W.E.; Vermonden, T. A Thermo-Responsive and Photo-Polymerizable Chondroitin Sulfate-Based Hydrogel for 3d Printing Applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar]
- Muller, M.; Ozturk, E.; Arlov, O.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar]
- Guo, J.; Zhou, H.; Akram, M.Y.; Mu, X.; Nie, J.; Ma, G. Characterization and Application of Chondroitin Sulfate/Polyvinyl Alcohol Nanofibres Prepared by Electrospinning. Carbohydr. Polym. 2016, 143, 239–245. [Google Scholar]
- Daemi, H.; Mashayekhi, M.; Modaress, M.P. Facile Fabrication of Sulfated Alginate Electrospun Nanofibers. Carbohydr. Polym. 2018, 198, 481–485. [Google Scholar]
- Delgado-Rangel, L.H.; Hernández-Vargas, J.; Becerra-González, M.; Martínez-Torres, A.; Prokhorov, E.; Campos, J.B.G. Development of Collagen/Poly(Vinyl Alcohol)/Chondroitin Sulfate and Collagen/Poly(Vinyl Alcohol)/Ha Electrospun Scaffolds for Tissue Engineering. Fibers Polym. 2019, 20, 2470–2484. [Google Scholar]
- Khanal, S.; Adhikari, U.; Rijal, N.; Pai, D.; Sankar, J.; Bhattarai, N. Synthesis and Characterization of Alginate-Based Hydrogel Microbeads for Magnesium Release. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Phoenix, AZ, USA, 11–17 November 2016. [Google Scholar]
- Guo, Q.; Aly, A.; Schein, O.; Trexler, M.M.; Elisseeff, J.H. Moxifloxacin in Situ Gelling Microparticles-Bioadhesive Delivery System. Results Pharma Sci. 2012, 2, 66–71. [Google Scholar]
| Biomaterial | Cell Type | Growth Factor | Release | Ref |
|---|---|---|---|---|
| Sulfated Alginate Hydrogels | ||||
| Alginate sulfate hydrogels with high chlorosulfonic acid (ClSO3H) concentrations | Chondrocytes | FGF2 | • Almost 40% of FGF2 retained after two weeks compared to the almost 20% retained by the controls | [10] |
| Sulfated Hyaluronan Hydrogels | ||||
| Sulfated hyaluronan (sHA) | Keratinocytes and dermal fibroblasts | EGF | • Prolonged release of EGF when studied over three days • Non sulfated hydrogels released a ten fold greater amount of EGF on day one when compared to sulfated hydrogel •Non sulfated hydrogel released a two fold greater amount on day three when compared to the sulfated hydrogel • Sulfated hydrogel had a fairly constant release rate | [11] |
| Sulfated HEMA-HA (HEMA-SHA) | N/A | SDF-1α | • Prolonged release when studied over twelve days • Sulfated hydrogel released the growth factor at ⅓ the rate compared to the non sulfated hydrogel control | [7] |
|
LS-MeHA and HS-MeHA hydrogels | Human MSCs | TGF-β1 | • Has extended release of TGF-β1 • 40–50% lower release amount when compared to non-sulfated hydrogel • Studied for days | [12] |
| Chondroitin Sulfate Hydrogels | ||||
| Chitosan-based microspheres (CMs) into CMC-OCS hydrogels | Chondrocytes | BSA | • Lowest release rate of BSA over two weeks • 30% of the sulfated hydrogel had released compared to 80% and 51% for the controls | [13] |
| Biomaterial | Cell type | Gene Expression(s) | Cell Response(s) | Ref |
|---|---|---|---|---|
| Chondroitin Sulfate Hydrogels | ||||
| Catechol-functionalized chondroitin sulfate | Human ADSCs | • Collagen type II and SOX9 ⬆ (compared to pellet culture) | • Good cell viability • Chondrogenesis ⬆ (compared to a pellet culture) • Significant GAG deposition • Good adhesion to cartilage tissue in vivo rabbit • Minimal loss of tissue in vivo rabbit • No significant pro-inflammatory cytokine secretion | [14] |
| Chondroitin sulfate methacrylate | Human MSCs | • Collagen type II and aggrecan ⬆ (in softer hydrogels) • Collagen type X ⬇ (in softer hydrogels) † • MMP13 ⬆ (in softer hydrogels) † • Collagen type I and MMP13 ⬆ (in stiffer hydrogels) | • GAG and collagen deposition ⬆ • Neocartilage deposition ⬆ (but decreases as stiffness increases) † • Homogeneous distribution of collagen type I & II with minimal collagen type X • Cellular remodeling observed | [15] |
| CMP-TA/CS-TA | Porcine auricular chondrocytes | • Collagen type I ⬇ • Collagen type II ⬆ • Aggrecan ⬆ | • Cell viability and proliferation ⬆ • Highest collagen type II and aggrecan deposition with a 3/1 ratio • Fibrous tissue develop and no macroscopic sign of inflammation of toxicity in a rat model | [16] |
| PNIPAAm-g-CS | Human embryonic kidney 293 cells | • Low cytotoxicity • Good adhesive interphase with surrounding tissue | [17] | |
| Bovine NP disc-derived self-assembled ECM functionalized with chondroitin sulfate | Porcine nasal tissue | • GAG/collagen ratio synthesis ⬆‡ • GAG deposition ⬆ • Collagen deposition ⬆ (predominantly collagen type II) • Rounded cell morphology | [18] | |
| Heparin-based Hydrogels | ||||
| Heparan sulfate- methacrylate | Human MSCs | • Collagen type II and aggrecan ⬆ (in softer hydrogels) • High MMP13 expression (but decrease with increasing heparin sulfate concentration) | • Homogeneous distribution of collagen type I & II deposition • Collagen type X deposition ⬇ • Neocartilage deposition ⬆ | [15] |
| HRP-crosslinked Hep-TA/Dex-TA | Bovine chondrocytes | • Collagen type II ⬆ | • Cell viability ⬆ • Homogeneous distribution of collagen type II and CS deposition ⬆ | [19] |
| Gelatin incorporated PLCL scaffold with Hep-SH | Rabbit articular cartilage chondrocytes | • Collagen type II ⬆ in vitro and in vivo rabbit model • Collagen type I ⬇ in vitro and in vivo rabbit model | • GAG deposition ⬆ • Collagen type II and aggrecan deposition in the scaffold ⬆ | [20] |
| Sulfated Alginate Hydrogels | ||||
| Calcium-crosslinked sulfated alginate | Calf cartilage chondrocytes | • COL1A2/COL2A1 ratio ⬆‡ • SOX9/RUNX2 ratio ⬆‡ | • Cell proliferation ⬆† • RhoA activity ⬆† | [21] |
| Barium-crosslinked sulfated alginate | Bovine articular cartilage chondrocytes | • FGFR2 ⬇‡ • Sef ⬇† • Collagen type II ⬆‡ • Aggrecan ⬇ • Collagen type I ⬇ | • Cellular recognition/adhesion ⬆† • Cell proliferation ⬆† • FGF retention ⬆† • Collagen type II deposition ⬆ • PG deposition ⬆ • Collagen type II ⬆ (in lower sulfation level) • PG deposition ⬆ (in higher sulfation level) | [10] |
| Tyrosinase-crosslinked alginate sulfate tyramine | Human and bovine articular cartilage chondrocytes | • Collagen type II ⬆‡ • Aggrecan ⬆‡ • Sox9 ⬇‡ • Collagen type I ⬇ • ADAMTS5 ⬇ • MMP13 ⬇ | • Good cell viability • Demonstrates chondroprotective effects with FGF signalling • Collagen type I deposition ⬇ • Aggrecan deposition ⬆ • Fibrous capsule formation with cartilage specific matrix after 4 weeks in vivo mice | [22] |
| Sulfated Hyaluronan Hydrogels | ||||
| HA-pNIPAM | Bovine NP | • Collagen type I ⬇ • Aggrecan ⬆ • MMP13 and Has2 ⬆ | • NP phenotype ⬆ • Normal cytocompatibility/viability • Generation of a NP cavity | [23] |
| Sulfated HA | hMSCs | • Collagen type II ⬆ • Aggrecan ⬆ • MMP13 ⬇ • Collagen type X ⬇ | • Sulfation has no effect on cell viability • Uniform GAG deposition | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarus, E.; Bermudez-Lekerika, P.; Farchione, D.; Schofield, T.; Howard, S.; Mambetkadyrov, I.; Lamoca, M.; Rivero, I.V.; Gantenbein, B.; Lewis, C.L.; et al. Sulfated Hydrogels in Intervertebral Disc and Cartilage Research. Cells 2021, 10, 3568. https://doi.org/10.3390/cells10123568
Lazarus E, Bermudez-Lekerika P, Farchione D, Schofield T, Howard S, Mambetkadyrov I, Lamoca M, Rivero IV, Gantenbein B, Lewis CL, et al. Sulfated Hydrogels in Intervertebral Disc and Cartilage Research. Cells. 2021; 10(12):3568. https://doi.org/10.3390/cells10123568
Chicago/Turabian StyleLazarus, Emily, Paola Bermudez-Lekerika, Daniel Farchione, Taylor Schofield, Sloan Howard, Iskender Mambetkadyrov, Mikkael Lamoca, Iris V. Rivero, Benjamin Gantenbein, Christopher L. Lewis, and et al. 2021. "Sulfated Hydrogels in Intervertebral Disc and Cartilage Research" Cells 10, no. 12: 3568. https://doi.org/10.3390/cells10123568
APA StyleLazarus, E., Bermudez-Lekerika, P., Farchione, D., Schofield, T., Howard, S., Mambetkadyrov, I., Lamoca, M., Rivero, I. V., Gantenbein, B., Lewis, C. L., & Wuertz-Kozak, K. (2021). Sulfated Hydrogels in Intervertebral Disc and Cartilage Research. Cells, 10(12), 3568. https://doi.org/10.3390/cells10123568







