Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds
Abstract
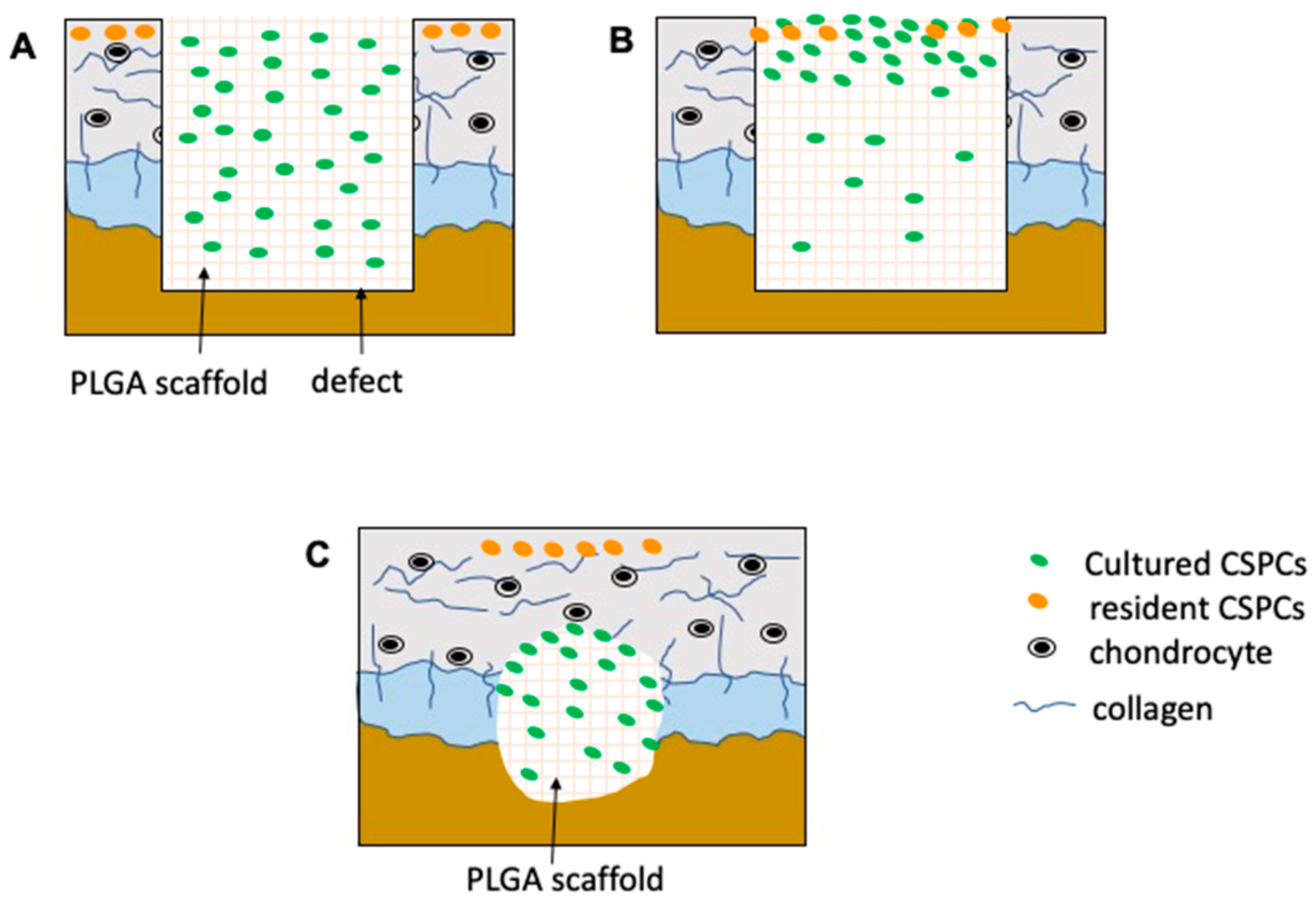
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation
2.2. Colony Formation Analysis
2.3. Multilineage Differentiation
2.4. Immunophenotype
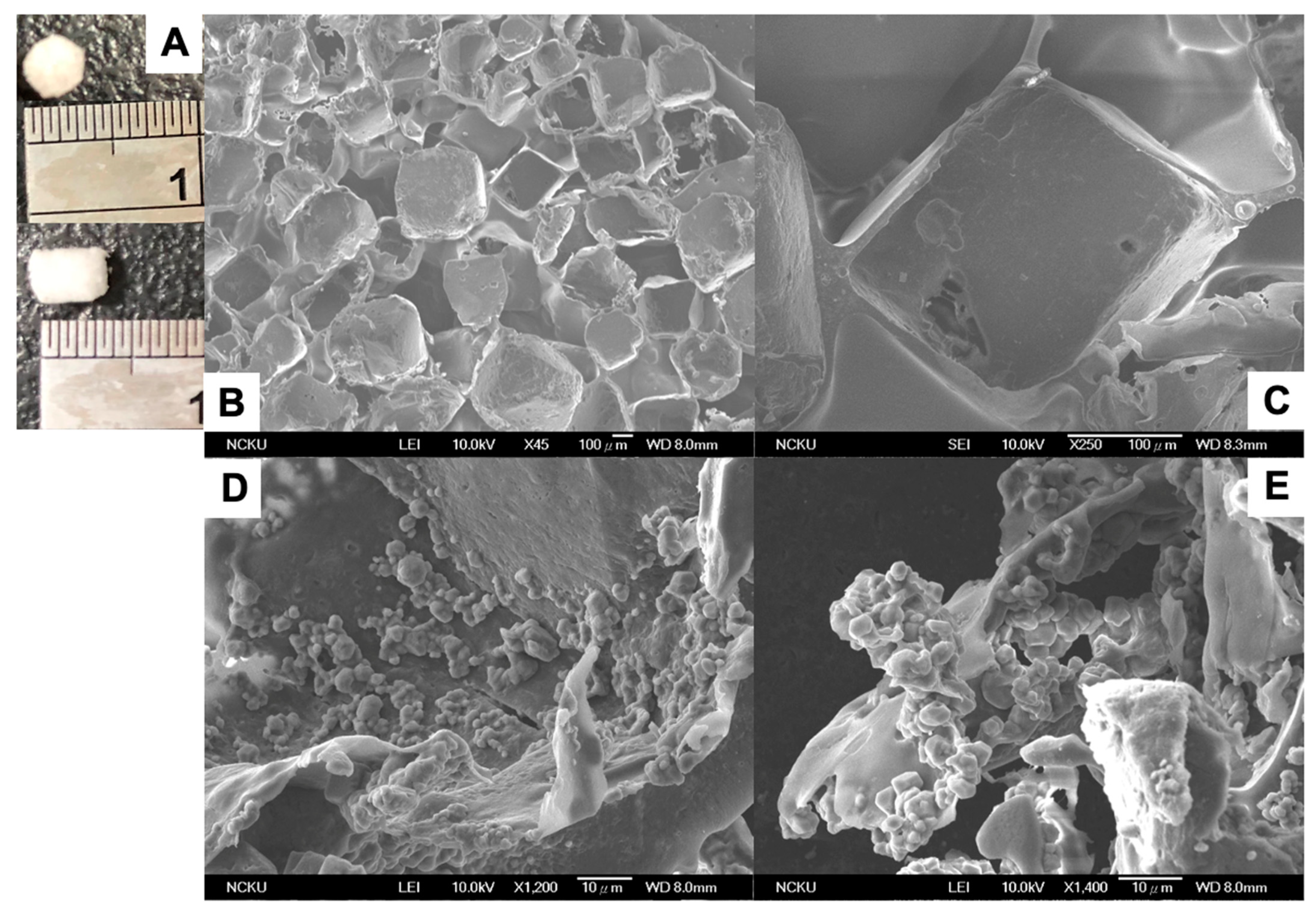
2.5. Fabrication of Porous PLGA and CSPC/PLGA Scaffolds
2.6. Cell Tracking
2.7. Animal Procedures
2.8. Macroscopic Evaluation
2.9. Micro-CT Evaluation
2.10. Histological and Immunohistochemical Processing
2.11. Statistical Analysis
3. Results
3.1. Characterization of CSPCs after Isolation and Cultivation In Vitro
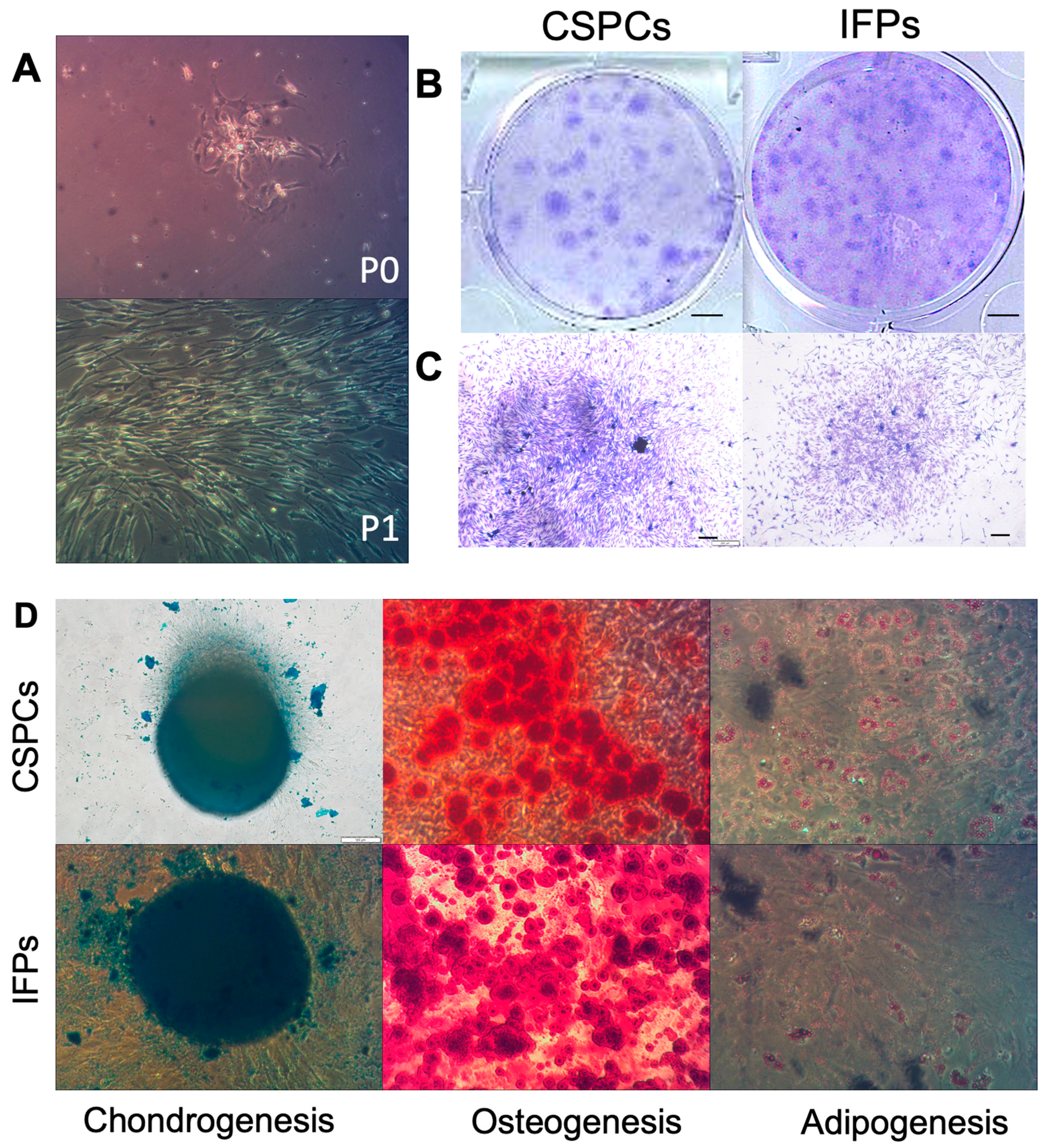
3.1.1. Assessment of CSPC Attachment and Spreading
3.1.2. Colony Formation Analysis
3.1.3. Multilineage Differentiation
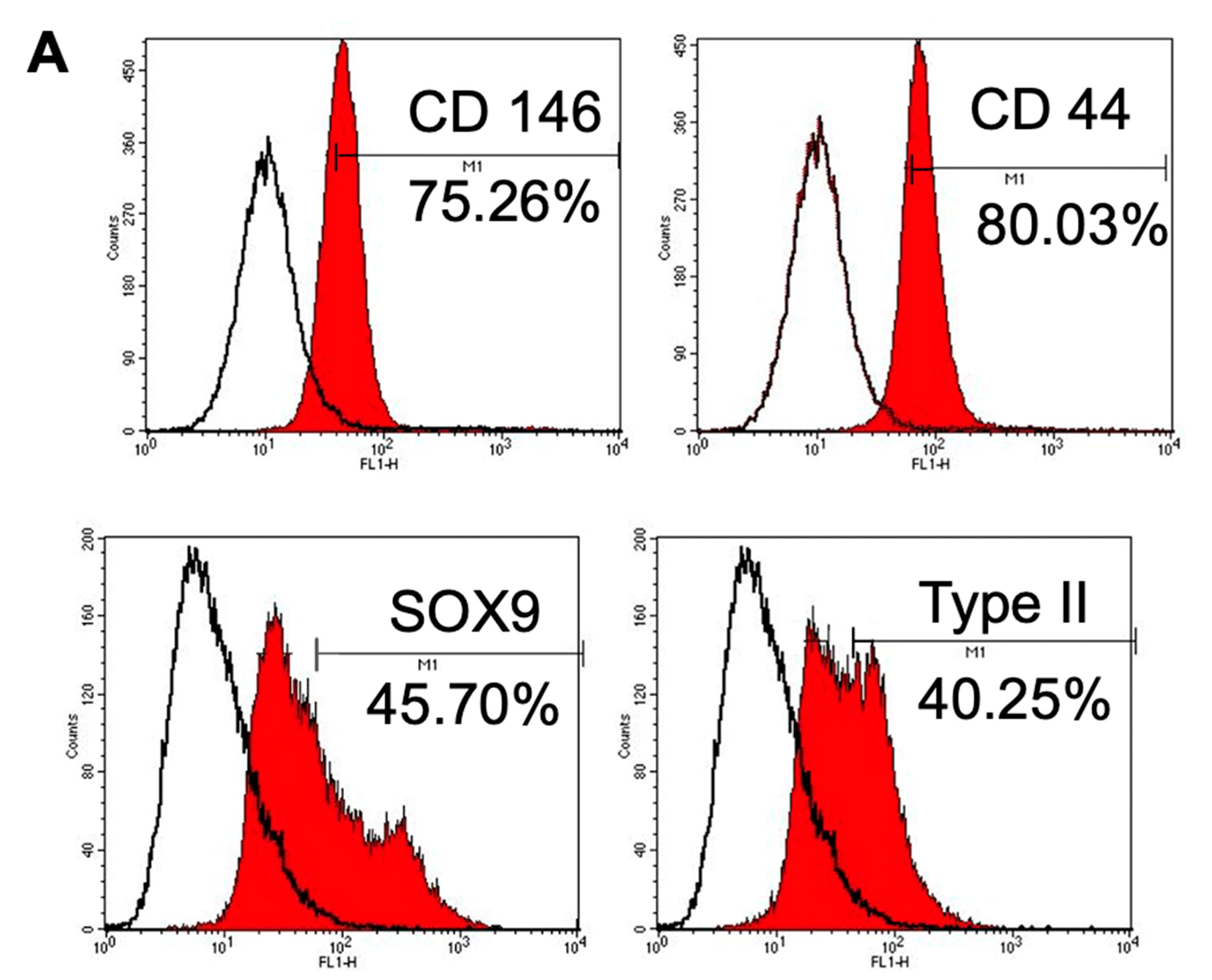
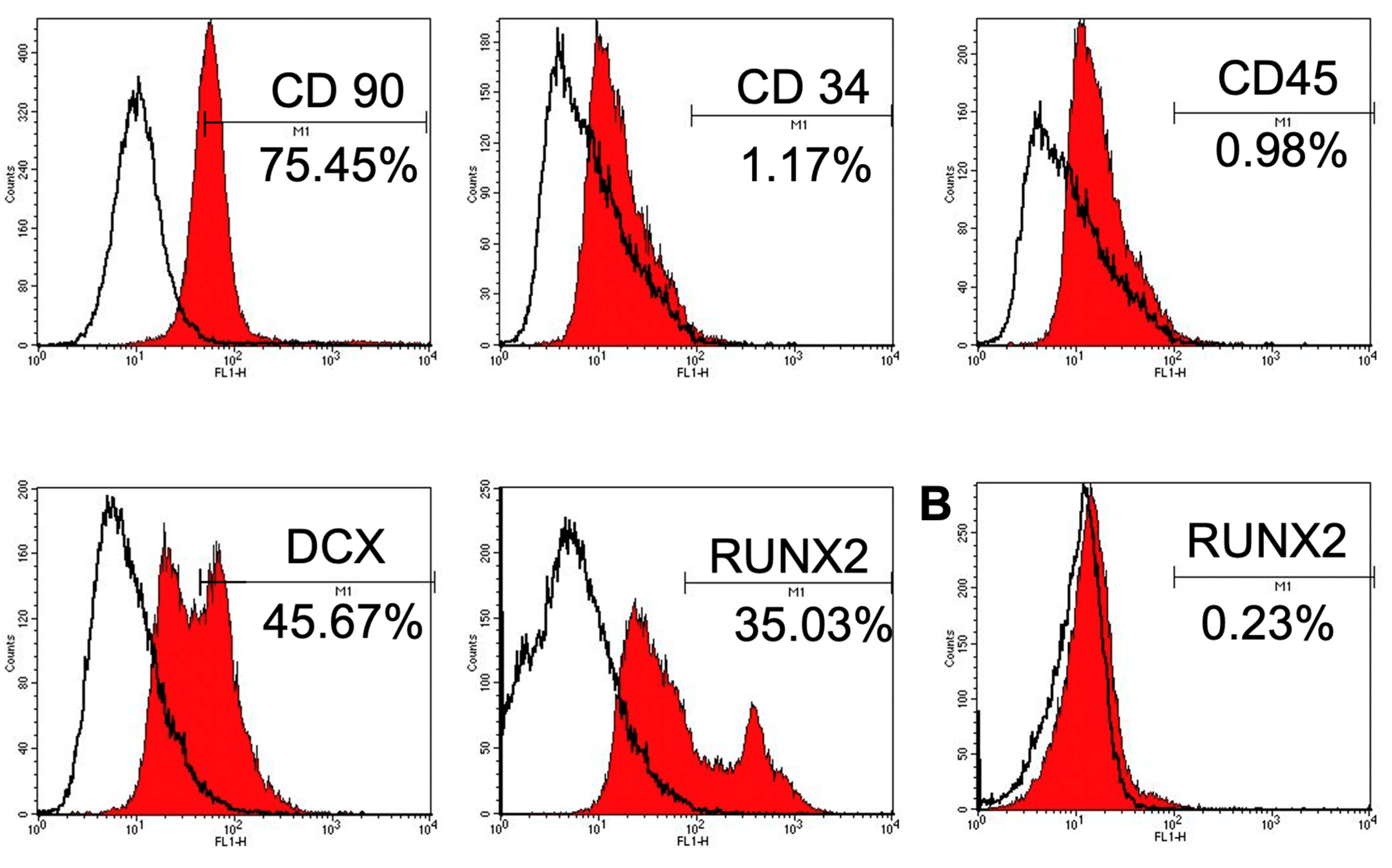
3.1.4. Immunophenotype Assay of CSPCs
3.2. Morphology of CSPCs on PLGA Scaffolds
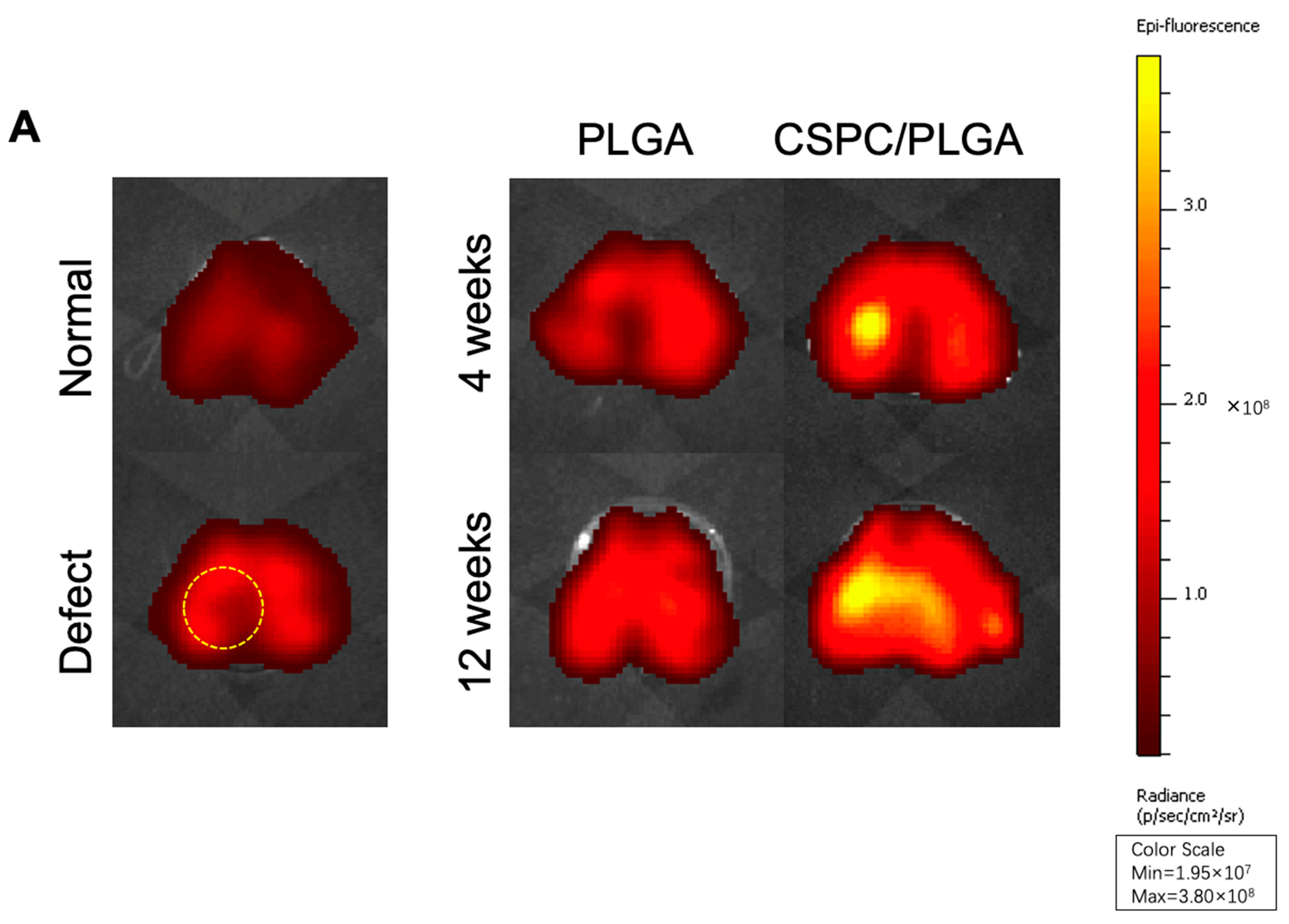
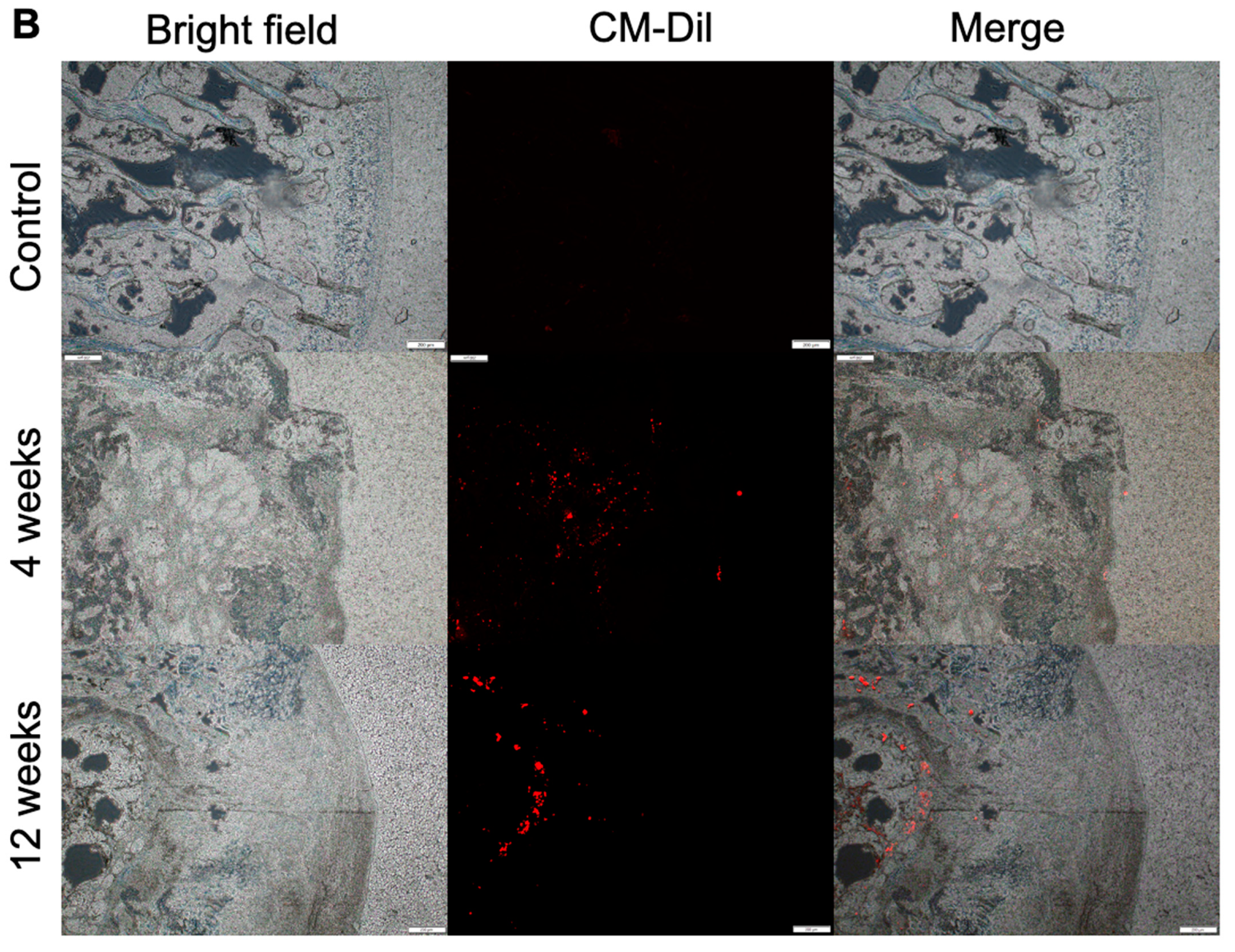
3.3. Location and Biological Activity of CSPCs Evaluated by In Vivo Imaging System (IVIS) and Spectrum CT Analyses In Vivo
3.4. Macroscopic Observations and Quantitative Scores
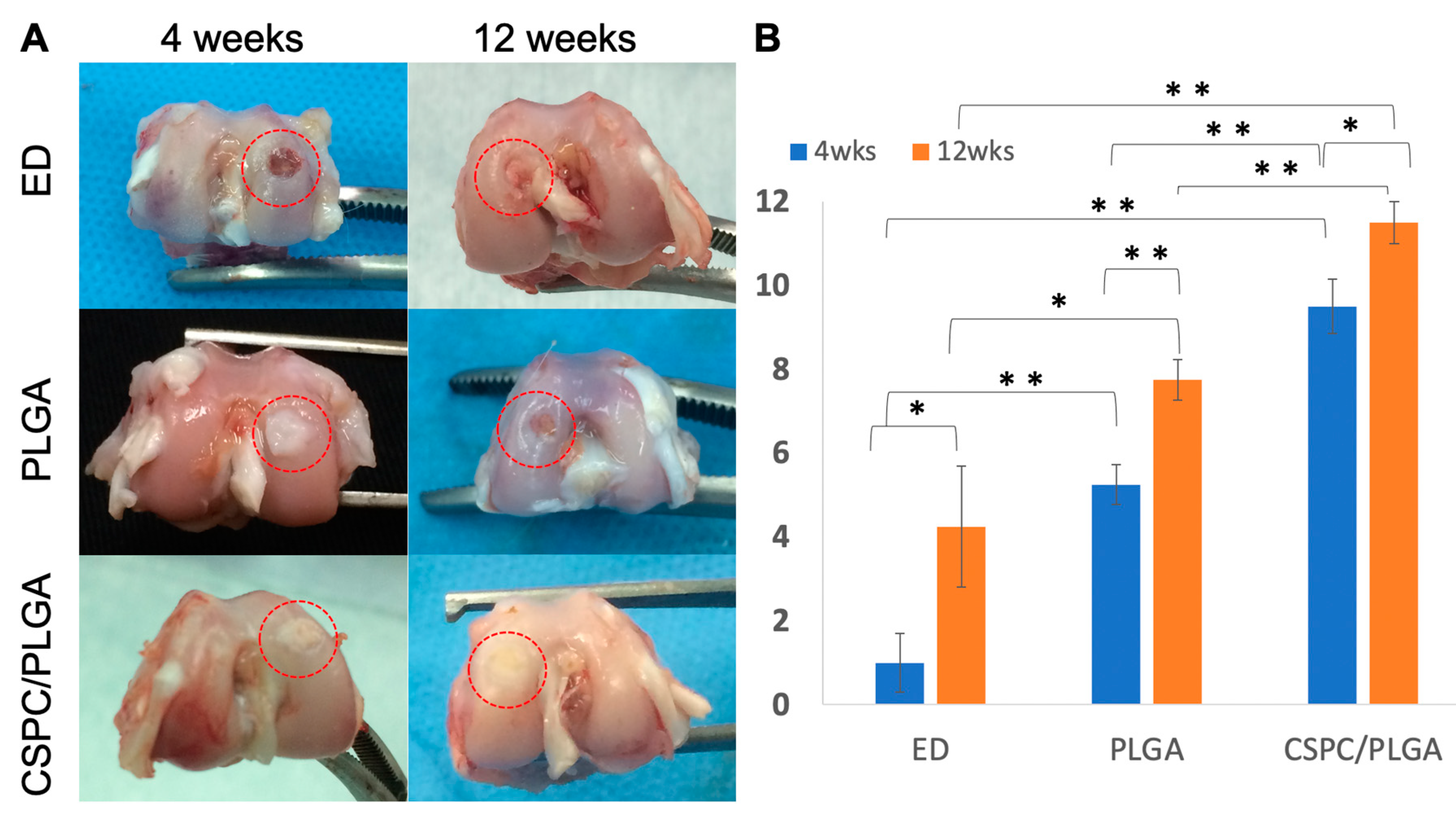
3.4.1. Gross Appearance
3.4.2. Quantitative Scores
3.5. Micro-CT Analysis
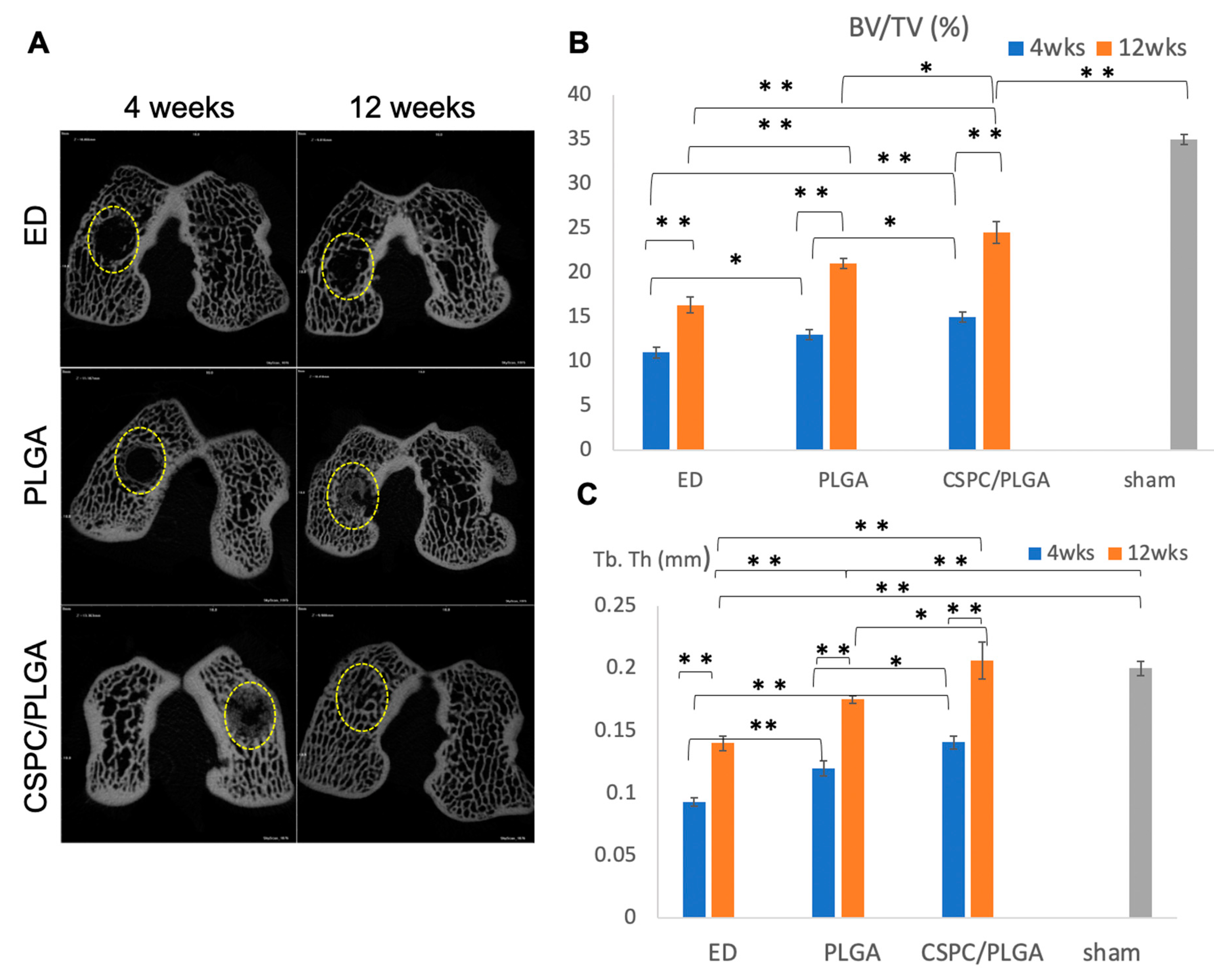
3.5.1. Findings after 4 Weeks
3.5.2. Findings at 12 Weeks
3.5.3. Comparison by Micro-CT Analysis at 4 and 12 Weeks
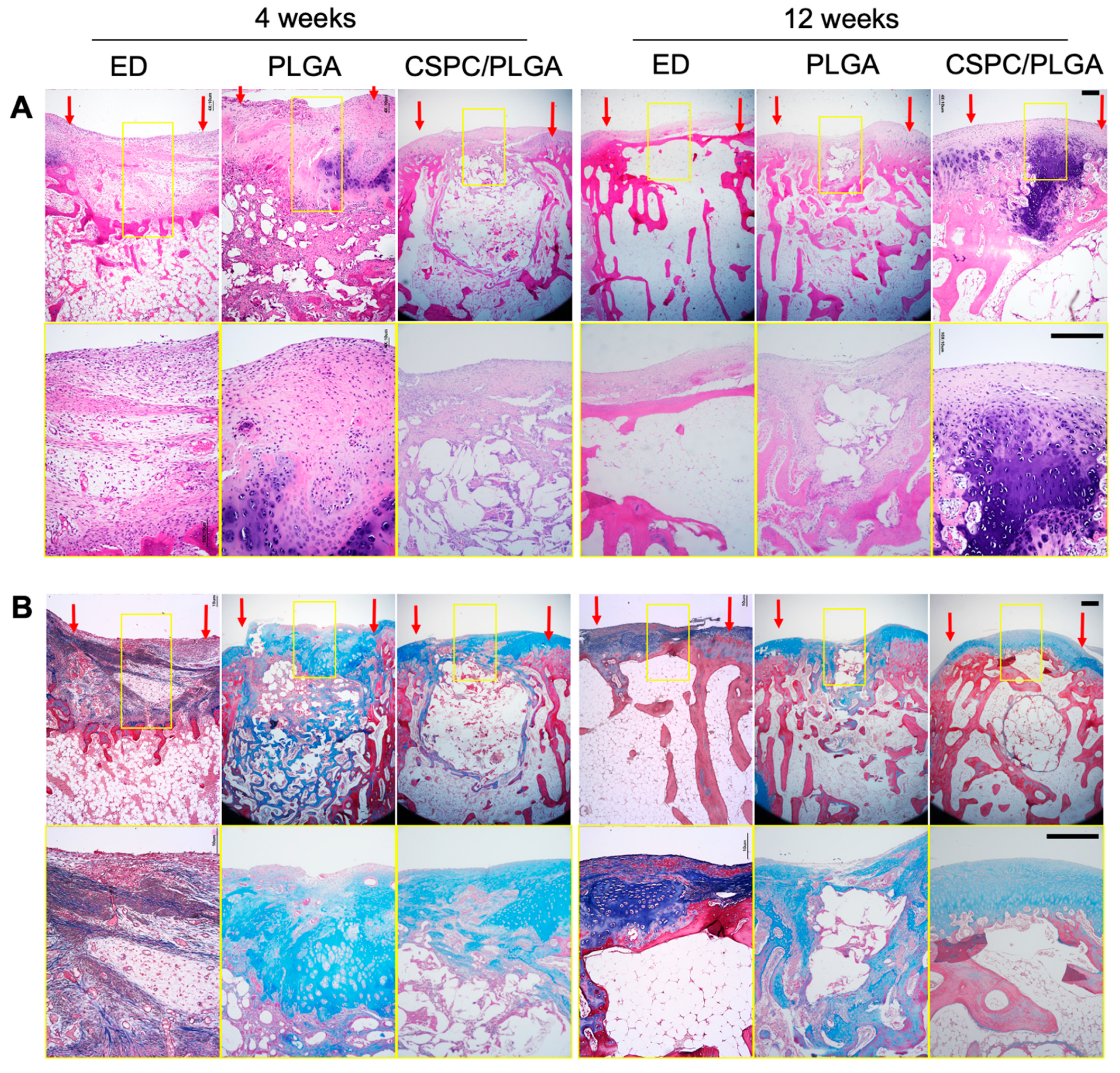
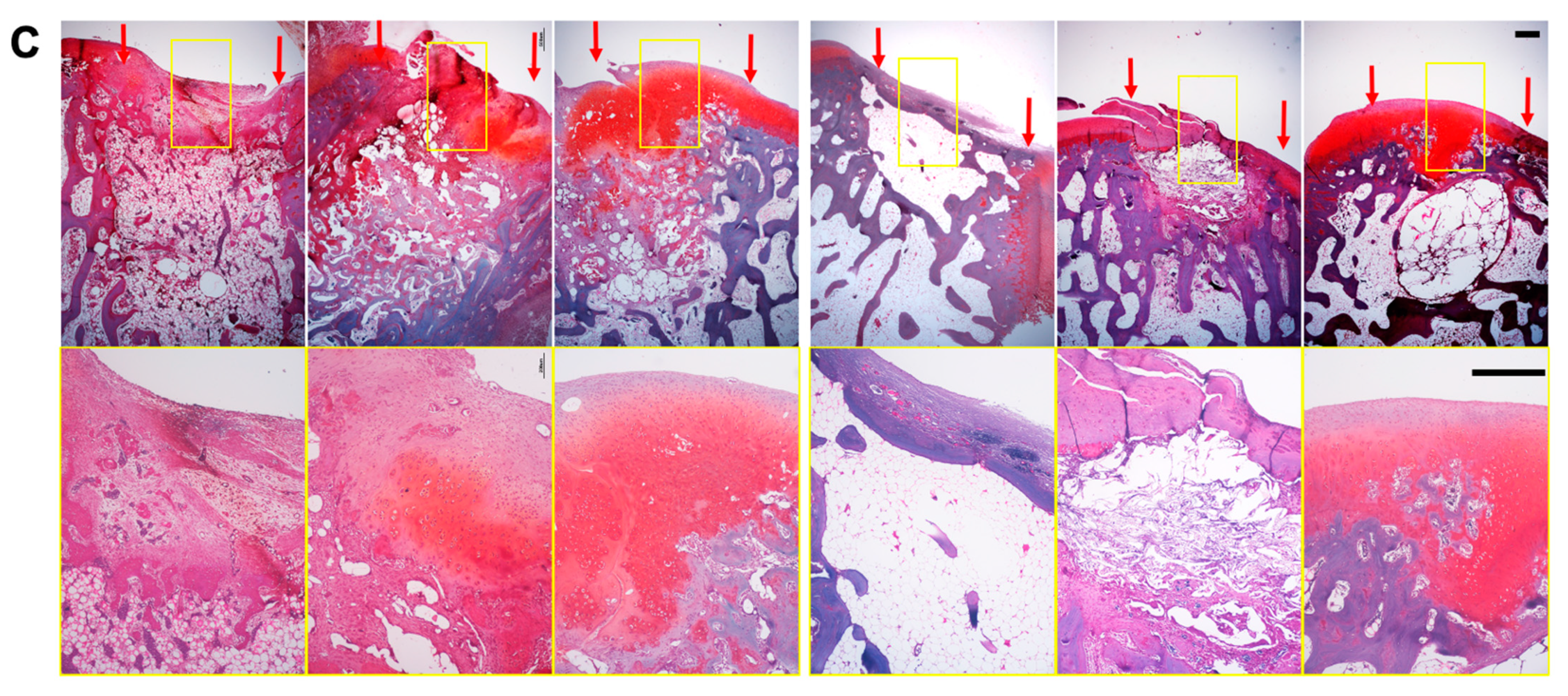
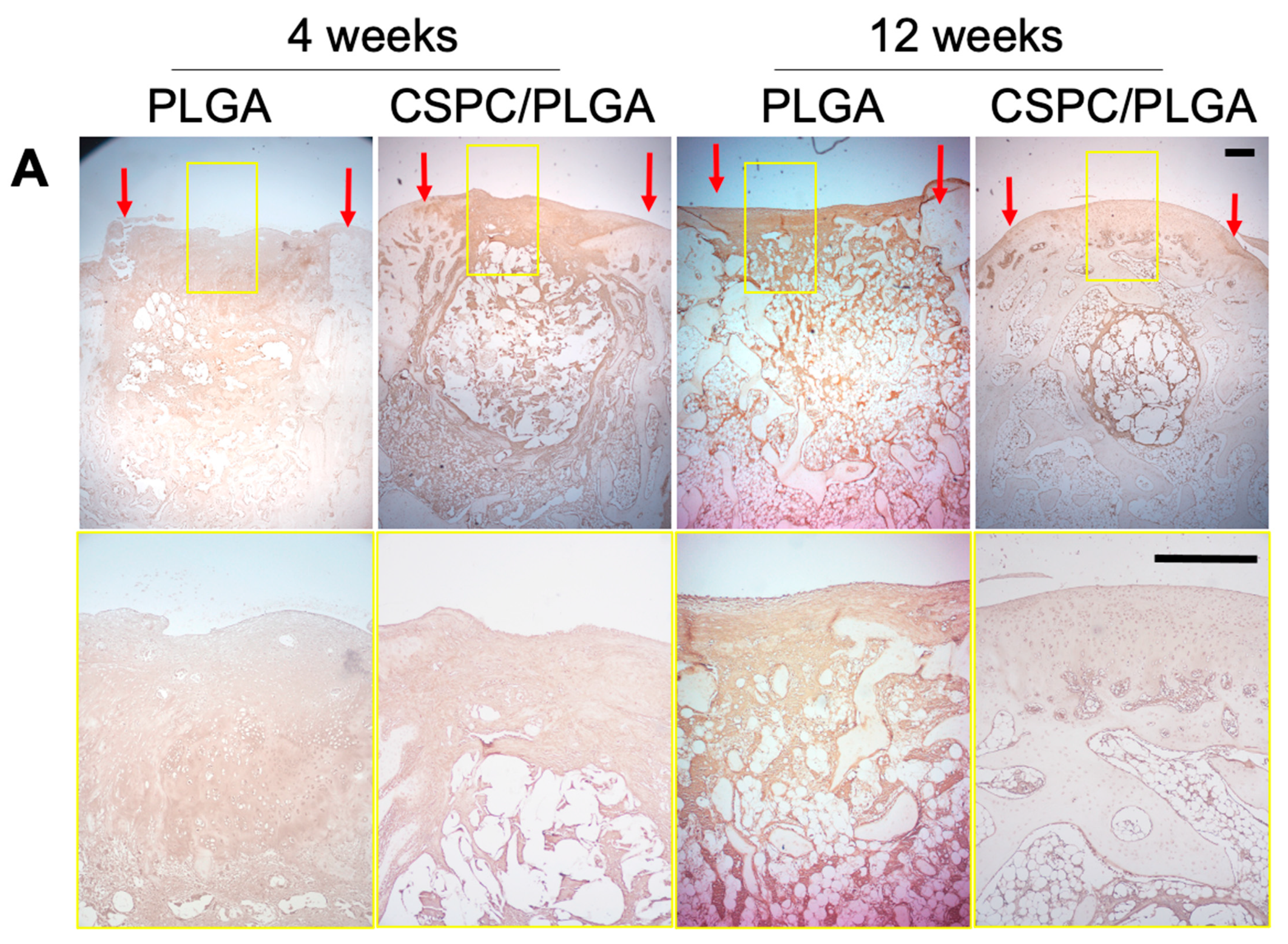
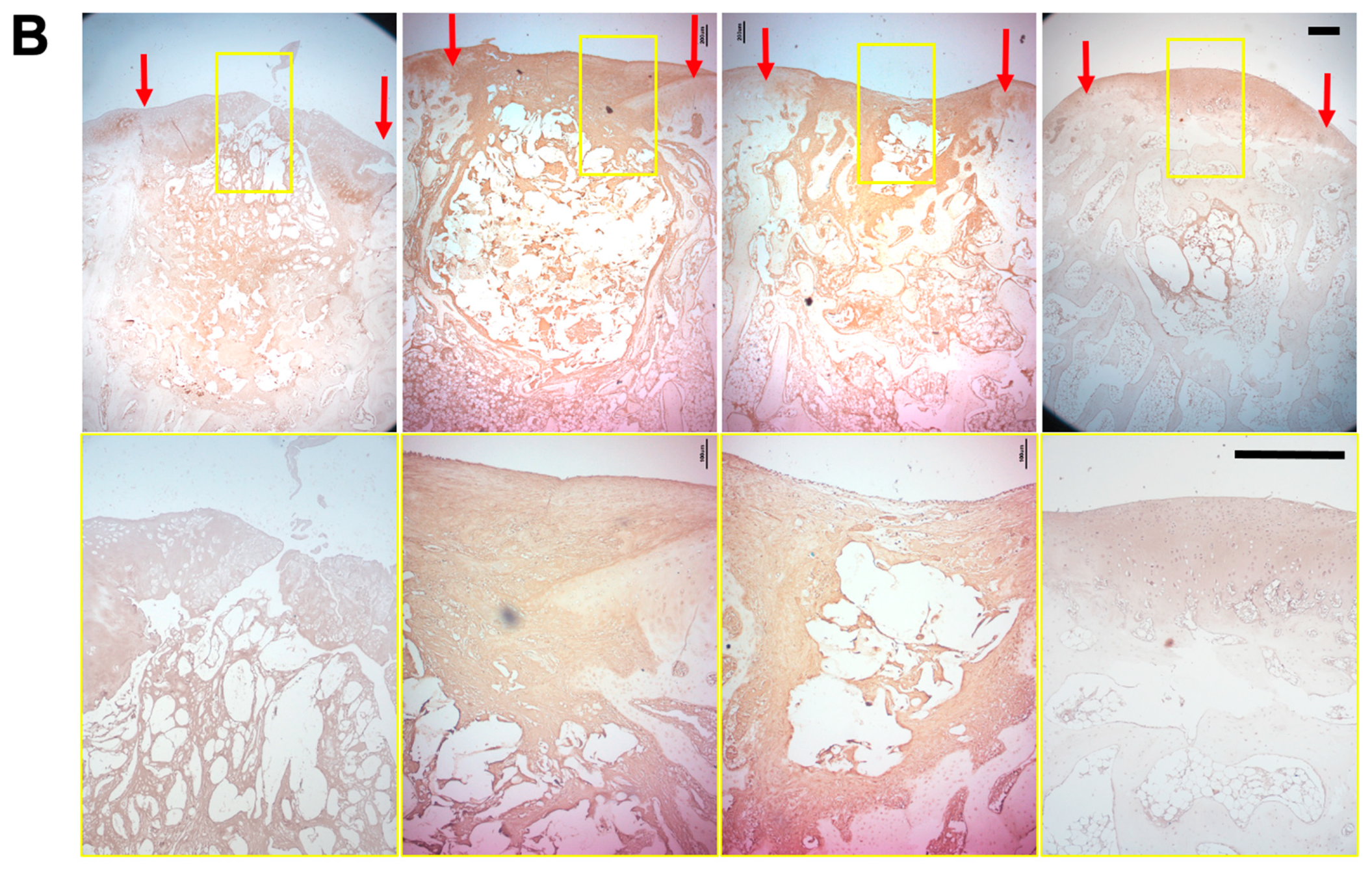
3.6. Histology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, D.; Learmonth, D. Natural progression of osteo-chondral defect in the femoral condyle. Knee 2002, 9, 7–10. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Jt. Surg. Am. Vol. 2010, 92, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Ollat, D.; Lebel, B.; Thaunat, M.; Jones, D.; Mainard, L.; Dubrana, F.; Versier, G. Mosaic osteochondral transplantations in the knee joint, midterm results of the SFA multicenter study. Orthop. Traumatol. Surg. Res. OTSR 2011, 97, S160–S166. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Tang, A.; Ateshian, G.A.; Guo, X.E.; Hung, C.T.; Lu, H.H. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann. Biomed. Eng. 2010, 38, 2183–2196. [Google Scholar] [CrossRef]
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Graziano, A.C.; Lo Furno, D.; Avola, R.; Mangano, S.; Giuffrida, R.; Cardile, V. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014, 116, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jin, C.; Du, X.; Yan, C.; Min, B.H.; Xu, Y.; Wang, L. An Autologous Bone Marrow Mesenchymal Stem Cell–Derived Extracellular Matrix Scaffold Applied with Bone Marrow Stimulation for Cartilage Repair. Tissue Eng. Part A 2014, 20, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Gauss, V.; Von Der Mark, H.; MÜLler, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef]
- in’t Anker, P.S.; Noort, W.A.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Kruisselbrink, A.B.; van Bezooijen, R.L.; Beekhuizen, W.; Willemze, R.; Kanhai, H.H.; Fibbe, W.E. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 2003, 88, 845–852. [Google Scholar]
- Csaki, C.; Schneider, P.R.; Shakibaei, M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann. Anat. Anat. Anz. 2008, 190, 395–412. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Visser, J.; Gawlitta, D.; Benders, K.E.M.; Toma, S.M.H.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.A.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, T.; Wright, K.T.; Kuiper, N.J.; Roberts, S.; Jermin, P.; Gallacher, P.; Kuiper, J.H. An In Vitro System to Study the Effect of Subchondral Bone Health on Articular Cartilage Repair in Humans. Cells 2021, 10, 1903. [Google Scholar] [CrossRef]
- Monaco, G.; Ladner, Y.D.; El Haj, A.J.; Forsyth, N.R.; Alini, M.; Stoddart, M.J. Mesenchymal Stromal Cell Differentiation for Generating Cartilage and Bone-Like Tissues In Vitro. Cells 2021, 10, 2165. [Google Scholar] [CrossRef] [PubMed]
- Caminal, M.; Peris, D.; Fonseca, C.; Barrachina, J.; Codina, D.; Rabanal, R.M.; Moll, X.; Morist, A.; Garcia, F.; Cairo, J.J.; et al. Cartilage resurfacing potential of PLGA scaffolds loaded with autologous cells from cartilage, fat, and bone marrow in an ovine model of osteochondral focal defect. Cytotechnology 2016, 68, 907–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, Y.A.; Nam, Y.; Park, N.; Jung, H.; Lee, K.; Lee, J.; Ju, J.H. Chondrogenic Differentiation from Induced Pluripotent Stem Cells Using Non-Viral Minicircle Vectors. Cells 2020, 9, 582. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells from Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Sun, X.; Deng, C.; Atala, A.; Zhang, Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 2013, 34, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthr. Cartil. 2014, 22, 1318–1326. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef] [Green Version]
- Seol, D.; Yu, Y.; Choe, H.; Jang, K.; Brouillette, M.J.; Zheng, H.; Lim, T.-H.; Buckwalter, J.A.; Martin, J.A. Effect of Short-Term Enzymatic Treatment on Cell Migration and Cartilage Regeneration: In Vitro Organ Culture of Bovine Articular Cartilage. Tissue Eng. Part A 2014, 20, 1807–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seol, D.; McCabe, D.J.; Choe, H.; Zheng, H.; Yu, Y.; Jang, K.; Walter, M.W.; Lehman, A.D.; Ding, L.; Buckwalter, J.A.; et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012, 64, 3626–3637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisbie, D.D.; McCarthy, H.E.; Archer, C.W.; Barrett, M.F.; McIlwraith, C.W. Evaluation of articular cartilage progenitor cells for the repair of articular defects in an equine model. J. Bone Jt. Surg. Am. Vol. 2015, 97, 484–493. [Google Scholar] [CrossRef]
- De Luca, P.; Kouroupis, D.; Vigano, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Contains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerter, R.; Kruegel, J.; Miosge, N. New insights into cartilage repair—The role of migratory progenitor cells in osteoarthritis. Matrix Biol. J. Int. Soc. Matrix Biol. 2012, 31, 206–213. [Google Scholar] [CrossRef]
- Jiang, Y.; Tuan, R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schek, R.M.; Taboas, J.M.; Segvich, S.J.; Hollister, S.J.; Krebsbach, P.H. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004, 10, 1376–1385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, F.; Liu, K.; Shen, H.; Zhu, Y.; Zhang, W.; Liu, W.; Wang, S.; Cao, Y.; Zhou, G. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 2012, 33, 2926–2935. [Google Scholar] [CrossRef]
- Lin, T.-M.; Tsai, J.-L.; Lin, S.-D.; Lai, C.-S.; Chang, C.-C. Accelerated Growth and Prolonged Lifespan of Adipose Tissue-derived Human Mesenchymal Stem Cells in a Medium Using Reduced Calcium and Antioxidants. Stem Cells Dev. 2005, 14, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.J.; Lam, C.F.; Lin, C.C.; Chen, W.L.; Li, C.F.; Lin, Y.T.; Yeh, M.L. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthr. Cartil. 2013, 21, 1613–1622. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Lin, T.H.; Chang, N.J.; Hsu, H.C.; Yeh, M.L. Continuous Passive Motion Promotes and Maintains Chondrogenesis in Autologous Endothelial Progenitor Cell-Loaded Porous PLGA Scaffolds during Osteochondral Defect Repair in a Rabbit Model. Int. J. Mol. Sci. 2019, 20, 259. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.J.; Lin, C.C.; Li, C.F.; Wang, D.A.; Issariyaku, N.; Yeh, M.L. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials 2012, 33, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Wayne, J.S.; McDowell, C.L.; Shields, K.J.; Tuan, R.S. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005, 11, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; Negassa, A.; Edwardes, M.D.; Forrester, J.E. Statistical analysis of correlated data using generalized estimating equations: An orientation. Am. J. Epidemiol. 2003, 157, 364–375. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Yuan, Z.; Fu, L.; Jiang, S.; Gao, C.; Wang, F.; Zha, K.; Tian, G.; Sun, Z.; et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020, 114, 31–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Ryan, J.A.; Di Cesare, P.E.; Liu, J.; Walsh, C.A.; You, Z. Doublecortin is expressed in articular chondrocytes. Biochem. Biophys. Res. Commun. 2007, 363, 694–700. [Google Scholar] [CrossRef]
- Zhang, Q.; Cigan, A.D.; Marrero, L.; Lopreore, C.; Liu, S.; Ge, D.; Savoie, F.H.; You, Z. Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis 2011, 49, 75–82. [Google Scholar] [CrossRef]
- Yamane, S.; Cheng, E.; You, Z.; Reddi, A.H. Gene expression profiling of mouse articular and growth plate cartilage. Tissue Eng. 2007, 13, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, J. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2005, 75, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Koelling, S.; Kruegel, J.; Irmer, M.; Path, J.R.; Sadowski, B.; Miro, X.; Miosge, N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009, 4, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Xu, Y.; Yin, Z.; Yang, X.; Jiang, Y.; Gui, J. Chondrocyte migration affects tissue-engineered cartilage integration by activating the signal transduction pathways involving Src, PLCgamma1, and ERK1/2. Tissue Eng. Part A 2013, 19, 2506–2516. [Google Scholar] [CrossRef]
- Prasadam, I.; van Gennip, S.; Friis, T.; Shi, W.; Crawford, R.; Xiao, Y. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010, 62, 1349–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-C.; Lin, T.-H.; Hsu, C.-C.; Yeh, M.-L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells 2021, 10, 3536. https://doi.org/10.3390/cells10123536
Wang H-C, Lin T-H, Hsu C-C, Yeh M-L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells. 2021; 10(12):3536. https://doi.org/10.3390/cells10123536
Chicago/Turabian StyleWang, Hsueh-Chun, Tzu-Hsiang Lin, Che-Chia Hsu, and Ming-Long Yeh. 2021. "Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds" Cells 10, no. 12: 3536. https://doi.org/10.3390/cells10123536
APA StyleWang, H.-C., Lin, T.-H., Hsu, C.-C., & Yeh, M.-L. (2021). Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells, 10(12), 3536. https://doi.org/10.3390/cells10123536





