Screening Analysis of Platelet miRNA Profile Revealed miR-142-3p as a Potential Biomarker in Modeling the Risk of Acute Coronary Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Clinical Characterization of Study and Control Group
2.3. Blood Platelet Isolation
2.4. RNA Isolation and Synthesis of Complementary DNA (cDNA)
2.5. Microarray Analysis
2.6. Validation of Selected miRNAs with Real-Time PCR
3. Results
3.1. Screening of Platelet miRNome with Microarrays
3.2. Validation of Blood Platelet miRNA by RT-qPCR
3.3. Statistical Analysis and Modeling of Potential Biomarkers
3.4. Bioinformatic Analysis of Potential mRNA Targets and Protein–Protein Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs); Fact Sheets; WHO: Geneva, Switzerland, 2021.
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cannon, C.P. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quévillon Huberdeau, M.; Simard, M.J. A guide to microRNA-mediated gene silencing. FEBS J. 2019, 286, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kowdley, K.V. MicroRNAs in Common Human Diseases. Genom. Proteom. Bioinf. 2012, 10, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA–target interaction database. Nucleic Acids Res. 2020, 48, D148–D154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar] [CrossRef] [Green Version]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2016, 38, 785–791. [Google Scholar] [CrossRef]
- Zimmerman, G.A.; Weyrich, A.S. Signal-dependent protein synthesis by activated platelets: New pathways to altered phenotype and function. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s17–s24. [Google Scholar] [CrossRef] [Green Version]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czajka, P.; Fitas, A.; Jakubik, D.; Eyileten, C.; Gasecka, A.; Wicik, Z.; Siller-Matula, J.M.; Filipiak, K.J.; Postula, M. MicroRNA as Potential Biomarkers of Platelet Function on Antiplatelet Therapy: A Review. Front. Physiol. 2021, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. OMIM.org: Leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019, 47, D1038–D1043. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.-Q. KEGG Pathway Database. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 1068–1069. [Google Scholar] [CrossRef]
- Huang, J.; Swieringa, F.; Solari, F.A.; Provenzale, I.; Grassi, L.; De Simone, I.; Baaten, C.C.F.M.J.; Cavill, R.; Sickmann, A.; Frontini, M.; et al. Assessment of a complete and classified platelet proteome from genome-wide transcripts of human platelets and megakaryocytes covering platelet functions. Sci. Rep. 2021, 11, 12358. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Fälker, K. Characterization of human platelet microRNA by quantitative PCR coupled with an annotation network for predicted target genes. Platelets 2011, 22, 433–441. [Google Scholar] [CrossRef]
- Nagalla, S.; Shaw, C.; Kong, X.; Kondkar, A.A.; Edelstein, L.C.; Ma, L.; Chen, J.; McKnight, G.S.; López, J.A.; Yang, L.; et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood 2011, 117, 5189–5197. [Google Scholar] [CrossRef]
- Plé, H.; Landry, P.; Benham, A.; Coarfa, C.; Gunaratne, P.H.; Provost, P. The repertoire and features of human platelet microRNAs. PLoS ONE 2012, 7, e50746. [Google Scholar] [CrossRef]
- Shi, R.; Ge, L.; Zhou, X.; Ji, W.-J.; Lu, R.-Y.; Zhang, Y.-Y.; Zeng, S.; Liu, X.; Zhao, J.-H.; Zhang, W.-C.; et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb. Res. 2013, 131, 508–513. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhou, X.; Ji, W.J.; Shi, R.; Lu, R.Y.; Li, J.L.; Yang, G.H.; Luo, T.; Zhang, J.Q.; Zhao, J.H.; et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J. Thromb. Thrombolysis 2014, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chapnik, E.; Rivkin, N.; Mildner, A.; Beck, G.; Pasvolsky, R.; Metzl-Raz, E.; Birger, Y.; Amir, G.; Tirosh, I.; Porat, Z.; et al. miR-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. eLife 2014, 3, e01964. [Google Scholar] [CrossRef]
- Shin, E.-K.; Park, H.; Noh, J.-Y.; Lim, K.-M.; Chung, J.-H. Platelet Shape Changes and Cytoskeleton Dynamics as Novel Therapeutic Targets for Anti-Thrombotic Drugs. Biomol. Ther. 2017, 25, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, H.; Yao, Q.P.; Huang, K.; Chen, X.H.; Han, Y.; Jiang, Z.L.; Gao, L.Z.; Qi, Y.X. Platelet-derived miR-142-3p induces apoptosis of endothelial cells in hypertension. Cell. Mol. Biol. 2017, 63, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peckova, M.; Fahrenbruch, C.E.; Cobb, L.A.; Hallstrom, A.P. Circadian variations in the occurrence of cardiac arrests: Initial and repeat episodes. Circulation 1998, 98, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Ohkura, N.; Oishi, K.; Sudo, T.; Hayashi, H.; Shikata, K.; Ishida, N.; Matsuda, J.; Horie, S. CLOCK regulates circadian platelet activity. Thromb. Res. 2009, 123, 523–527. [Google Scholar] [CrossRef]
- Ward, J.A.; Esa, N.; Pidikiti, R.; Freedman, J.E.; Keaney, J.F.; Tanriverdi, K.; Vitseva, O.; Ambros, V.; Lee, R.; McManus, D.D. Circulating Cell and Plasma microRNA Profiles Differ between Non-ST-Segment and ST-Segment-Elevation Myocardial Infarction. Fam. Med. Med. Sci. Res. 2013, 2, 108. [Google Scholar] [CrossRef] [Green Version]
- Garabet, L.; Ghanima, W.; Rangberg, A.; Teruel-Montoya, R.; Martinez, C.; Lozano, M.L.; Nystrand, C.F.; Bussel, J.B.; Sandset, P.M.; Jonassen, C.M. Circulating microRNAs in patients with immune thrombocytopenia before and after treatment with thrombopoietin-receptor agonists. Platelets 2020, 31, 198–205. [Google Scholar] [CrossRef]
- Coskunpinar, E.; Cakmak, H.A.; Kalkan, A.K.; Tiryakioglu, N.O.; Erturk, M.; Ongen, Z. Circulating miR-221-3p as a novel marker for early prediction of acute myocardial infarction. Gene 2016, 591, 90–96. [Google Scholar] [CrossRef]
- Pordzik, J.; Pisarz, K.; De Rosa, S.; Jones, A.D.; Eyileten, C.; Indolfi, C.; Malek, L.; Postula, M. The Potential Role of Platelet-Related microRNAs in the Development of Cardiovascular Events in High-Risk Populations, Including Diabetic Patients: A Review. Front. Endocrinol. 2018, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.J.; Liu, T.; Zhang, H.; Yang, S.J. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 323–329. [Google Scholar]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef]
- Cengiz, M.; Yavuzer, S.; Kılıçkıran Avcı, B.; Yürüyen, M.; Yavuzer, H.; Dikici, S.A.; Karataş, Ö.F.; Özen, M.; Uzun, H.; Öngen, Z. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin. Exp. Hypertens. 2015, 37, 643–649. [Google Scholar] [CrossRef]
- Bergmeier, W.; Hynes, R.O. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb. Perspect. Biol. 2012, 4, a005132. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; De Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef]
- Fullard, J.F. The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr. Pharm. Des. 2004, 10, 1567–1576. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Ding, Z. Role of P2Y(12) Receptor in Thrombosis. Adv. Exp. Med. Biol. 2017, 906, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.F.; Canobbio, I.; Torti, M. PI3K/Akt in platelet integrin signaling and implications in thrombosis. Adv. Biol. Regul. 2015, 59, 36–52. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling During Platelet Adhesion and Activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Qu, J.; He, L.; Zhang, F.; Zhou, Z.; Yang, S.; Zhou, Y. Calcium in Vascular Smooth Muscle Cell Elasticity and Adhesion: Novel Insights Into the Mechanism of Action. Front. Physiol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Pleines, I.; Elvers, M.; Strehl, A.; Pozgajova, M.; Varga-Szabo, D.; May, F.; Chrostek-Grashoff, A.; Brakebusch, C.; Nieswandt, B. Rac1 is essential for phospholipase C-γ2 activation in platelets. Pflüg. Archiv-Eur. J. Physiol. 2009, 457, 1173–1185. [Google Scholar] [CrossRef]
- Jones, C.I.; Bray, S.; Garner, S.F.; Stephens, J.; de Bono, B.; Angenent, W.G.J.; Bentley, D.; Burns, P.; Coffey, A.; Deloukas, P.; et al. A functional genomics approach reveals novel quantitative trait loci associated with platelet signaling pathways. Blood 2009, 114, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.R.; Amisten, S.; Goodall, A.H.; Mahaut-Smith, M.P. Transcriptomic analysis of the ion channelome of human platelets and megakaryocytic cell lines. Thromb. Haemost. 2016, 116, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, L.; Wang, Y.; Draznin, J.; Eslin, D.; Bennett, J.S.; Poncz, M.; Wu, D.; Abrams, C.S. The relative role of PLCbeta and PI3Kgamma in platelet activation. Blood 2005, 106, 110–117. [Google Scholar] [CrossRef]
- Jurkovicova, D.; Sedlakova, B.; Lacinova, L.; Kopacek, J.; Sulova, Z.; Sedlak, J.; Krizanova, O. Hypoxia differently modulates gene expression of inositol 1,4,5-trisphosphate receptors in mouse kidney and HEK 293 cell line. Ann. N. Y. Acad. Sci. 2008, 1148, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Durrant, T.N.; Hers, I. PI3K inhibitors in thrombosis and cardiovascular disease. Clin. Transl. Med. 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Aspartate aminotransferase and cardiovascular disease—A narrative review. J. Lab. Precis. Med. 2021, 6, 8725. [Google Scholar] [CrossRef]
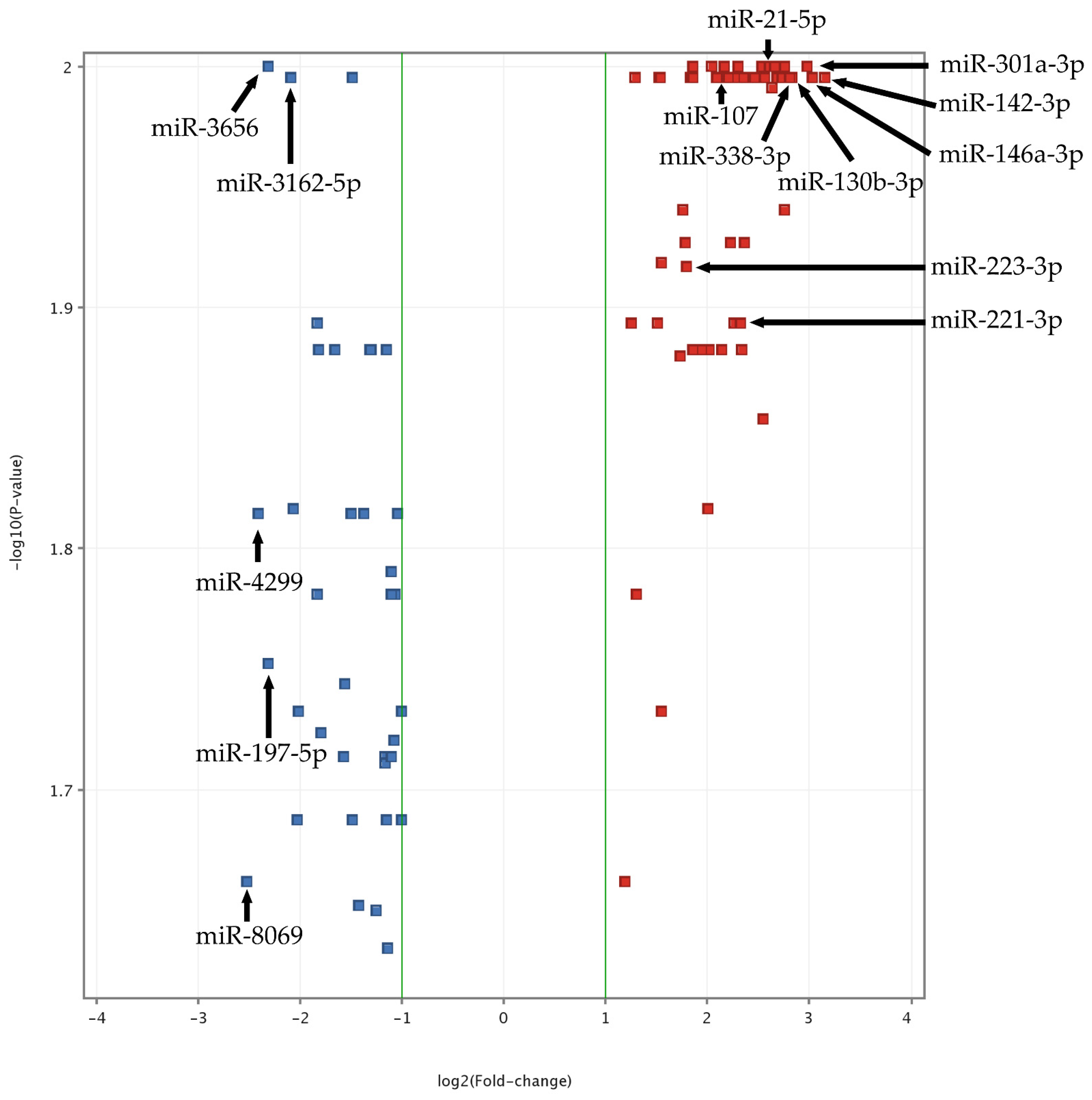


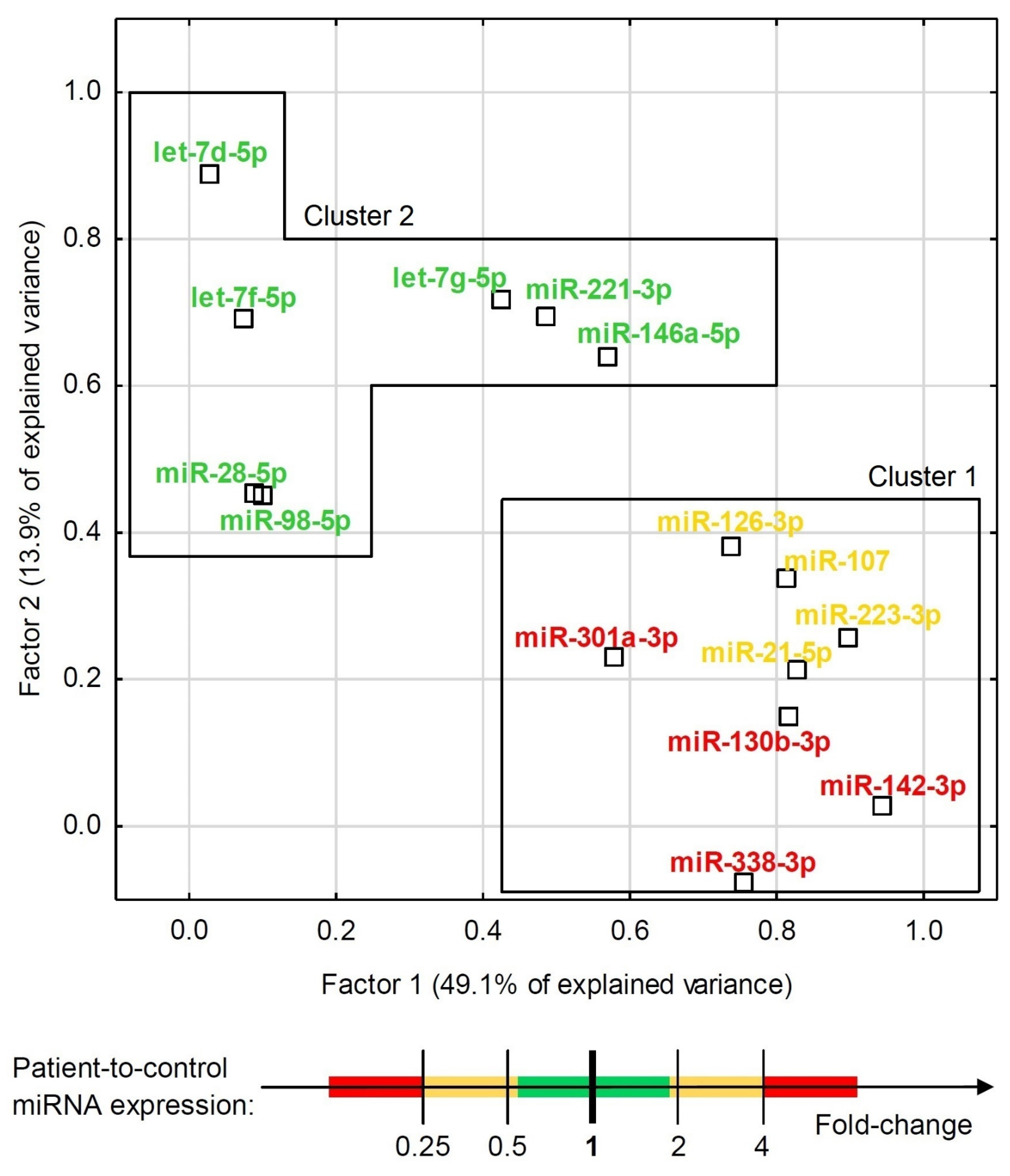

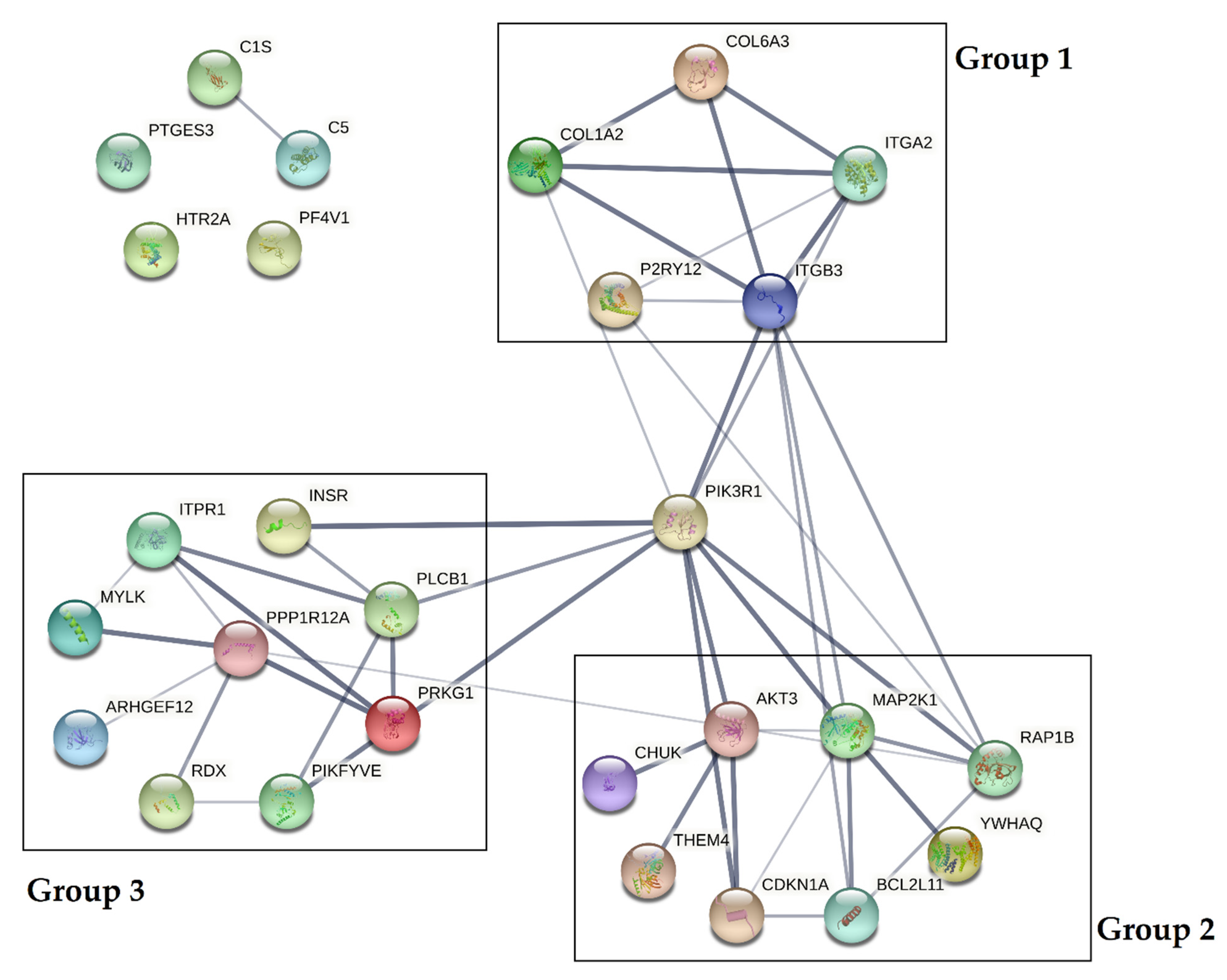
| Clinical Parameter | Study Group | Control Group | References |
|---|---|---|---|
| Age | 54 (46–59.25) | 50 (40.75–57.5) | - |
| Male | 43 | 40 | - |
| Female | 7 | 10 | - |
| Erythrocytes (106/µL) | 4.57 (4.283–4.988) | 4.95 (4.545–5.27) | 4.2–6.1 |
| Leukocytes (103/µL) | 8.6 (7.333–9.648) | 6.165 (4.828–7.935) | 4–11 |
| Blood platelets (103/µL) | 251.5 (201.8–281.8) | 250 (215–299.8) | 150–400 |
| Glucose (mmol/l) | 6 (5.365–6.535) | 5.055 (4.745–5.563) | 4.1–5.1 |
| Creatinine (µmol/l) | 82.5 (74.5–91) | 74.26 (68.51–86.85) | 64–104 |
| GFR (ml/min/1.73m2) | 95.2 (79.13–101) | 86.33 (79.93–99.01) | >60 |
| Cholesterol (mmol/l) | 5.645 (4.475–6.81) | 4.8 (4.075–5.303) | 3–5 |
| HDL (mmol/l) | 1.145 (1–1.333) | 1.350 (1.120–1.810) | >1 |
| LDL (mmol/l) | 3.21 (2.373–4.41) | 2.780 (2.220–3.238) | - |
| Triglycerides (mmol/l) | 1.805 (1.155–2.815) | 1.195 (0.938–1.688) | <1.7 |
| AST (U/I) | 32.00 (25.75–43.65) | 19.55 (16.30–25.85) | 0–50 |
| ALT (U/I) | 29 (21–39) | 20.85 (14.73–33.35) | 0–50 |
| TSH (mIU/l) | 1.8 (1.183–2.75) | 2.055 (1.413–2.863) | 0.27–4.20 |
| BMI | <35 | <35 | <35 |
| Model Characteristics | Model Based on AST | Model Based on miR-142-3p and AST |
|---|---|---|
| Odds ratio (95%CI), p-value | ||
| AST (Box–Cox transformed*100) 1 | 2.08 (1.54–2.79), p < 0.0001 | 2.57 (1.73–3.81), p < 0.0001 |
| miR-142-3p (−ΔCt) | N/A | 1.91 (1.37–2.67), p = 0.0001 |
| Coefficients of determination: | ||
| Cox–Snell R2 | 32.5% | 48.2% |
| Nagelkerke R2 | 43.4% | 64.2% |
| Information criteria: | ||
| AIC | 103.3 | 78.9 |
| BIC | 108.5 | 86.7 |
| Goodness-of-fit: | ||
| Hosmer–Lemeshow χ2(df), p-value | 6.60 (8), p = 0.58 | 14.84 (8), p = 0.062 |
| MicroRNA | mRNA Targets 1 | Kegg Pathway 2 | Associated Genes |
|---|---|---|---|
| miR-223-3p | 46 | PI3K-Akt signaling pathway | AKT3, CREB1 *, CHUK, FGF2 *, ITGA2, PDGFRA * |
| Platelet activation | AKT3, RASGRP1 *, ITGA2, P2RY12 | ||
| miR-142-3p | 41 | Platelet activation | AKT3, RASGRP1 *, ARHGEF12, COL24A1 *, ITGA2, MYLK, PLCB1, PPP1R12A |
| Regulation of actin cytoskeleton | ARHGEF12, FGF2 *, ITGA2, MYLK, PPP1R12A | ||
| Focal adhesion | AKT3, COL24A1 *, ITGA2, MYLK, PPP1R12A | ||
| Vascular smooth muscle contraction | ARHGEF12, MYLK, PLCB1, PPP1R12A | ||
| miR-21-5p | 58 | Platelet activation | AKT3, RAP1B, RASGRP1 *, ARHGEF12, COL3A1, COL24A1 *, ITPR1, ITGA2, ITGB3, PIK3R1, PLCB1, PRKG1 |
| PI3K-Akt signaling pathway | AKT3, PHLPP2 *, COL3A1, COL24A1 *, CHUK, FGF2 *, ITGA2, ITGB3, PIK3R1, PDGFRA *, | ||
| miR-107 | 92 | PI3K-Akt signaling pathway | AKT3, BCL2L11 *, PHLPP2 *, CREB1 *, CHRM2 *, COL3A1, COL6A3, COL24A1 *, FGF2 *, INSR, ITGA2, MAP2K1, PIK3R1, VEGFA * |
| Platelet activation | AKT3, COL3A1, COL24A1 *, ITPR1, ITGA2, MYLK, PIK3R1, PLCB1, PPP1R12A | ||
| Focal adhesion | AKT3, COL3A1, COL6A3, COL24A1 *, ITGA2, MAP2K1, MYLK, PIK3R1, PPP1R12A, VEGFA * | ||
| cAMP signaling pathway | AKT3, ADRB2 *, CREB1*, CHRM2 *, GRIN2A *, MAP2K1, PIK3R1, PPP1R12A | ||
| Regulation of actin cytoskeleton | CHRM2 *, FGF2 *, ITGA2, MAP2K1, MYLK, PIK3R1, PPP1R12A | ||
| Calcium signaling pathway | HTR2A, ADRB2 *, CHRM2 *, GRIN2A *, ITPR1, MYLK, PLCB1 | ||
| Circadian entrainment | CREB1 *, GRIN2A *, ITPR1, PLCB1, PRKG1 | ||
| Phosphatidylinositol signaling system | ITPR1, PIK3R1, PLCB1 | ||
| Chemokine signaling pathway | AKT3, MAP2K1, PIK3R1, PLCB1, PF4V1 | ||
| miR-221-3p | 71 | PI3K-Akt signaling pathway | AKT3, BCL2L11 *, PHLPP2 *, CREB1 *, CHRM2 *, ITGA2, ITGB3, MAP2K1, PIK3R1, PDGFRA *, THEM4, YWHAQ |
| Regulation of actin cytoskeleton | CHRM2 *, ITGA2, ITGB3, MAP2K1, PIKFYVE, PIK3R1, PDGFRA *, RDX | ||
| Platelet activation | AKT3, RAP1B, RASGRP1 *, ITGA2, ITGB3, PIK3R1, PLCB1, PRKG1 | ||
| Focal adhesion | AKT3, RAP1B, ITGA2, ITGB3, MAP2K1, PIK3R1, PDGFRA * | ||
| cAMP signaling pathway | AKT3, RAP1B, CREB1 *, CHRM2 *, MAP2K1, PIK3R1 | ||
| miR-301a-3p | 91 | PI3K-Akt signaling pathway | AKT3, BCL2L11, PHLPP2 *, CREB1 *, CHRM2 *, COL1A2, COL6A3, CHUK, CDKN1A, INSR, MAP2K1, PIK3R1, PDGFRA * |
| Platelet activation | AKT3, ARHGEF12, COL1A2, ITPR1, PIK3R1, PLCB1, PRKG1, PLAU * | ||
| cAMP signaling pathway | AKT3, RAPGEF4 *, ADRB1 *, CREB1 *, CHRM2 *, MAP2K1, PIK3R1 | ||
| Focal adhesion | AKT3, COL1A2, COL6A3, MAP2K1, PIK3R1, PDGFRA * | ||
| Regulation of actin skeleton | ARHGEF12, CHRM2 *, MAP2K1, PIKFYVE, PIK3R1, PDGFRA *, RDX | ||
| Vascular smooth muscle contraction | ARHGEF12, ITPR1, MAP2K1, PLCB1, PRKG1 | ||
| Calcium signaling pathway | ADRB1 *, CHRM2 *, ITPR1, PLCB1, PDGFRA * | ||
| Phosphatidylinositol signaling system | ITPR1, PIKFYVE, PIK3R1, PLCB1 | ||
| Arachidonic acid metabolism | PTGES3 | ||
| miR-130b-3p | 93 | PI3K-Akt signaling pathway | AKT3, BCL2L11, PHLPP2 *, CREB1 *, CHRM2 *, COL1A2, COL6A3, CHUK, CDKN1A, INSR, MAP2K1, PDGFRA * |
| Platelet activation | AKT3, RAP1B, ARHGEF12, COL1A2, ITPR1, MYLK, PLCB1, PRKG1 | ||
| Regulation of actin cytoskeleton | ARHGEF12, CHRM2 *, MAP2K1, MYLK, PIKFYVE, PDGFRA *, RDX | ||
| cAMP signaling pathway | AKT3, RAP1B, RAPGEF4 *, ADRB1 *, CREB1 *, CHRM2 *, MAP2K1 | ||
| Vascular smooth muscle contraction | ARHGEF12M ITPR1, MAP2K1, MYLK, PLCB1, PRKG1 | ||
| Complement and coagulation cascade | F3 *, C1S, C5, PLAU* | ||
| Arachidonic acid metabolism | PTGES3 | ||
| miR-338-3p | 58 | PI3K-Akt signaling pathway | AKT3, PHLPP2 *, CREB1 *, CHRM2 *, COL6A3, FGF2 *, ITGB3, PDGFRA * |
| Regulation of actin cytoskeleton | ARHGEF12, CHRM2 *, FGF2 *, ITGB3, PDGFRA *, RDX | ||
| Platelet activation | AKT3, ARHGEF12, ITPR1, ITGB3, PRKG1 | ||
| Calcium signaling pathway | ADRB2 *, CHRM2 *, GRIN2A *, ITPR1, PDGFRA * | ||
| cAMP signaling pathway | AKT3, ADRB2 *, CREB1 *, CHRM2 *, GRIN2A * | ||
| Focal adhesion | AKT3, COL6A3, ITGB3, PDGFRA * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelenberger, R.; Karbownik, M.S.; Kacprzak, M.; Maciak, K.; Bijak, M.; Zielińska, M.; Czarny, P.; Śliwiński, T.; Saluk-Bijak, J. Screening Analysis of Platelet miRNA Profile Revealed miR-142-3p as a Potential Biomarker in Modeling the Risk of Acute Coronary Syndrome. Cells 2021, 10, 3526. https://doi.org/10.3390/cells10123526
Szelenberger R, Karbownik MS, Kacprzak M, Maciak K, Bijak M, Zielińska M, Czarny P, Śliwiński T, Saluk-Bijak J. Screening Analysis of Platelet miRNA Profile Revealed miR-142-3p as a Potential Biomarker in Modeling the Risk of Acute Coronary Syndrome. Cells. 2021; 10(12):3526. https://doi.org/10.3390/cells10123526
Chicago/Turabian StyleSzelenberger, Rafał, Michał Seweryn Karbownik, Michał Kacprzak, Karina Maciak, Michał Bijak, Marzenna Zielińska, Piotr Czarny, Tomasz Śliwiński, and Joanna Saluk-Bijak. 2021. "Screening Analysis of Platelet miRNA Profile Revealed miR-142-3p as a Potential Biomarker in Modeling the Risk of Acute Coronary Syndrome" Cells 10, no. 12: 3526. https://doi.org/10.3390/cells10123526
APA StyleSzelenberger, R., Karbownik, M. S., Kacprzak, M., Maciak, K., Bijak, M., Zielińska, M., Czarny, P., Śliwiński, T., & Saluk-Bijak, J. (2021). Screening Analysis of Platelet miRNA Profile Revealed miR-142-3p as a Potential Biomarker in Modeling the Risk of Acute Coronary Syndrome. Cells, 10(12), 3526. https://doi.org/10.3390/cells10123526








