Protein Kinase A (PRKA) Activity Is Regulated by the Proteasome at the Onset of Human Sperm Capacitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals and Reagents
2.3. Culture Media
2.4. Semen Collection
2.5. Sperm Selection
2.6. SDS-PAGE and Immunoblotting
2.7. Stripping the PVDF Membranes
2.8. Immunofluorescence Assessment of Spermatozoa
2.9. Statistical Analyses
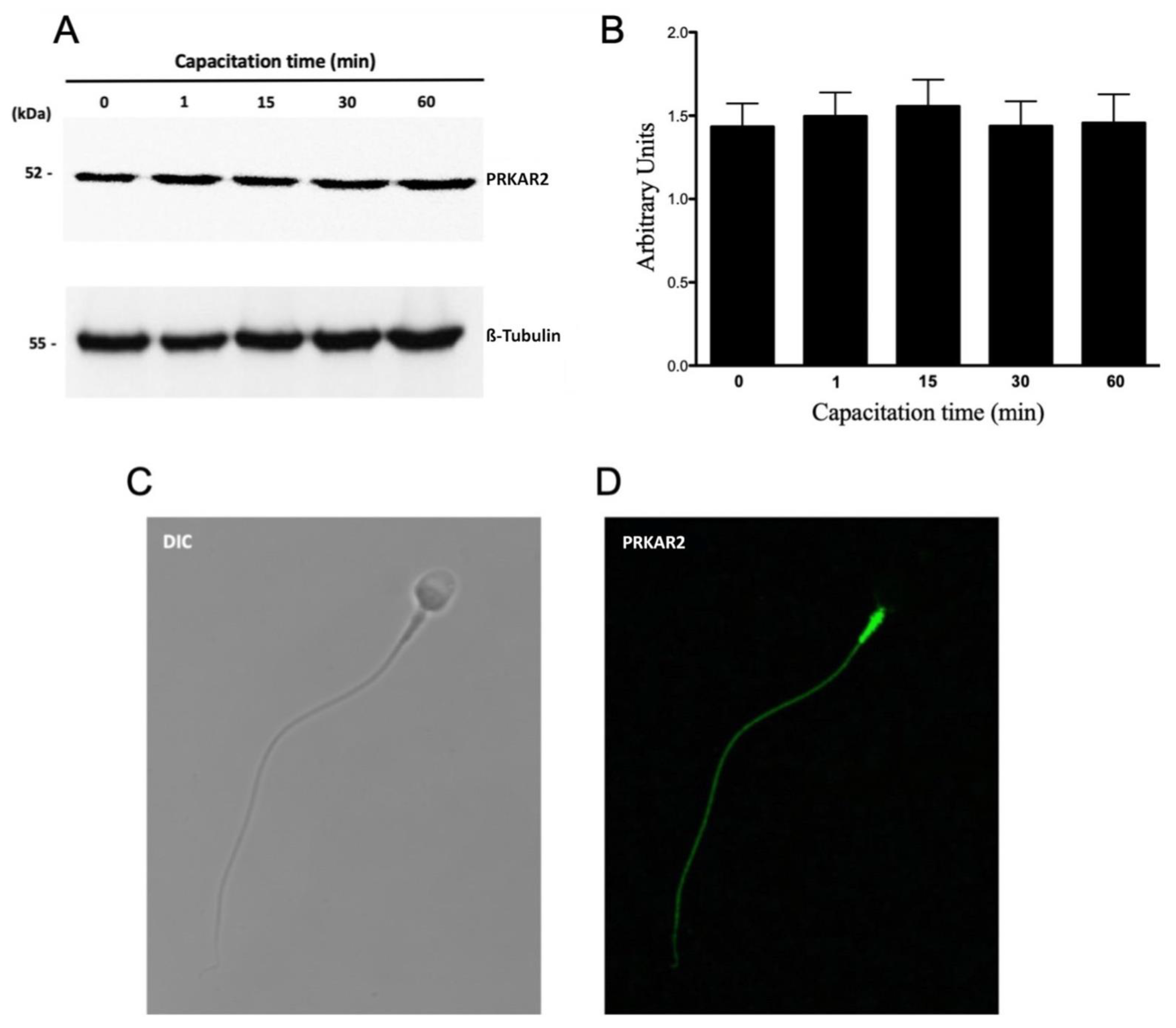
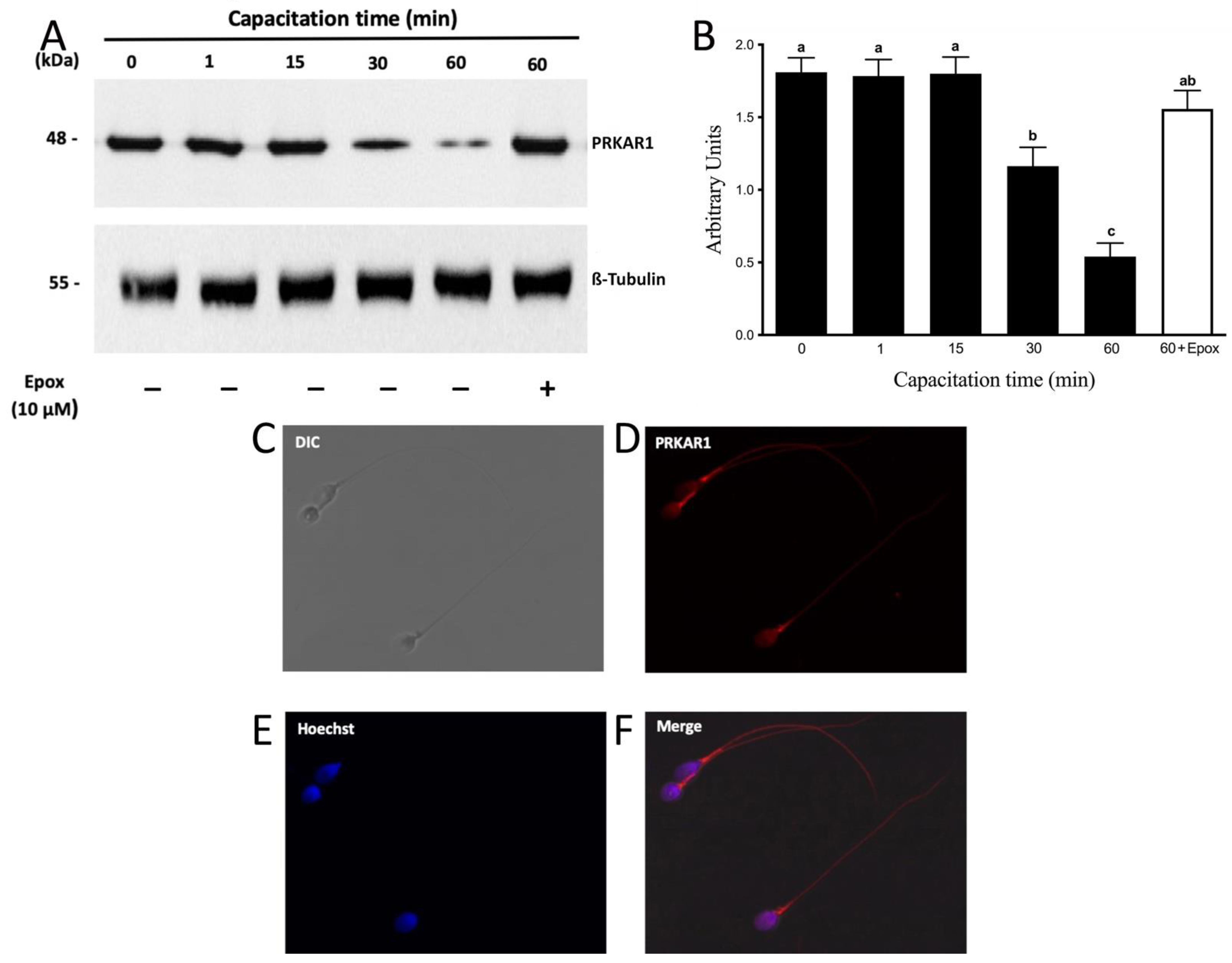
3. Results
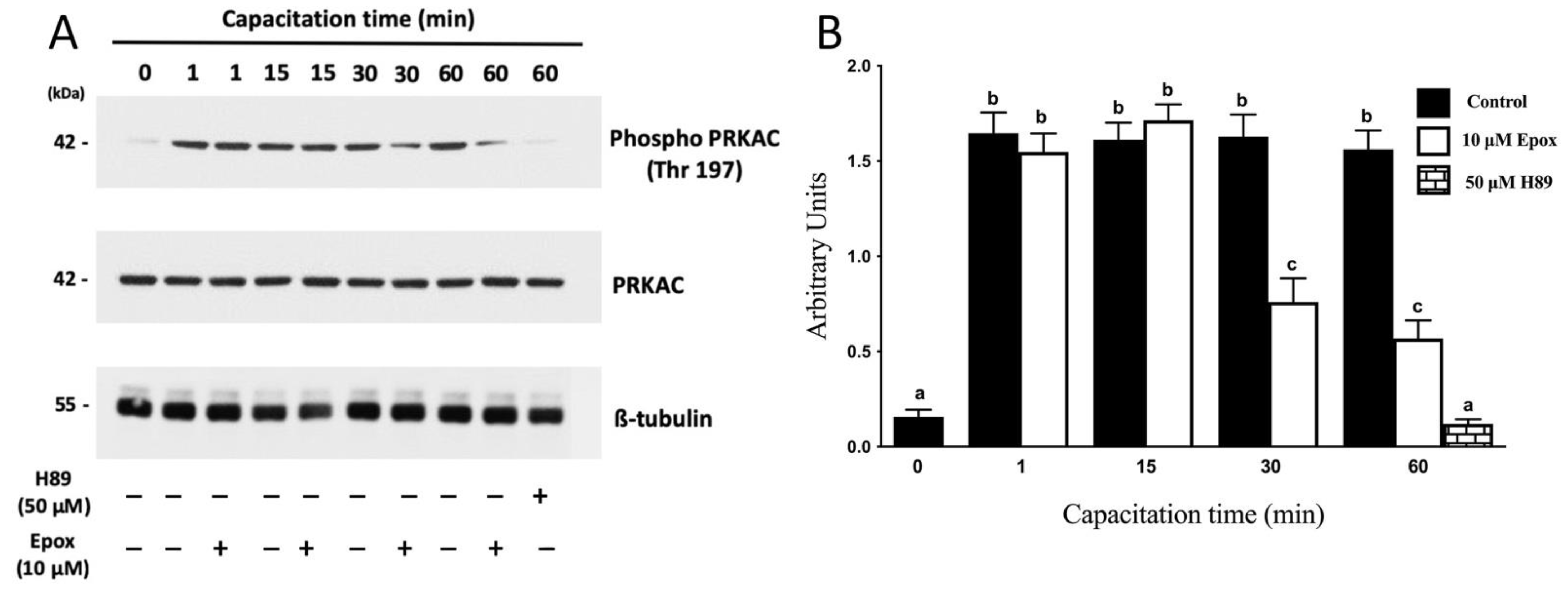
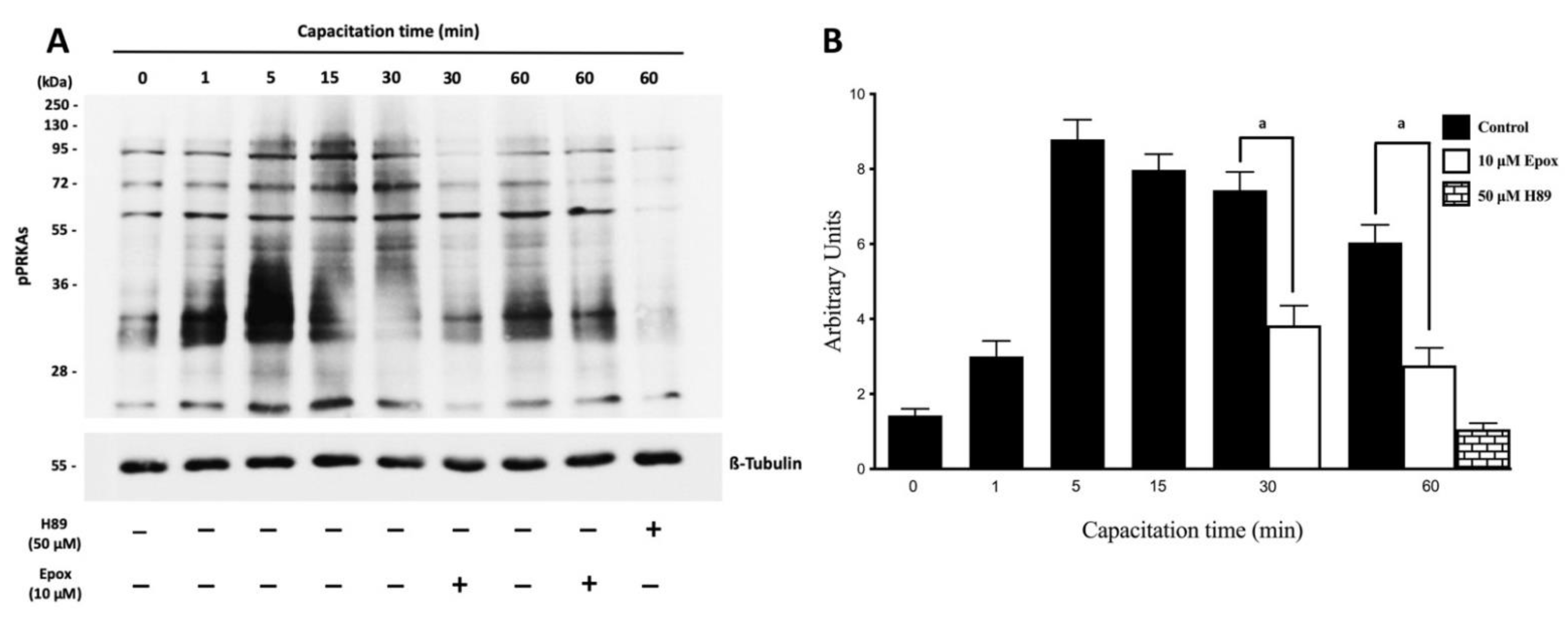
3.1. The Proteasome Is Required for PRKA Signaling during Human Sperm Capacitation
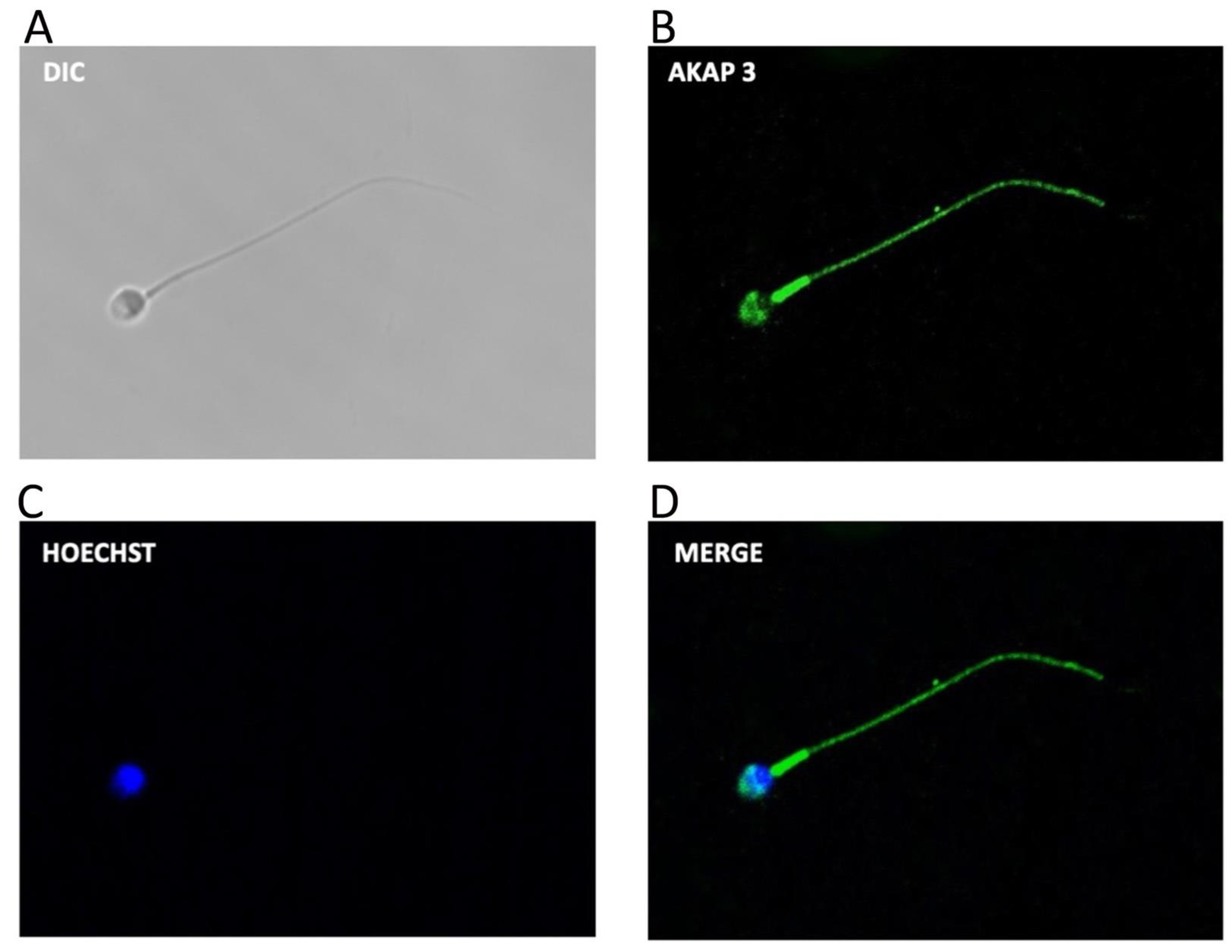
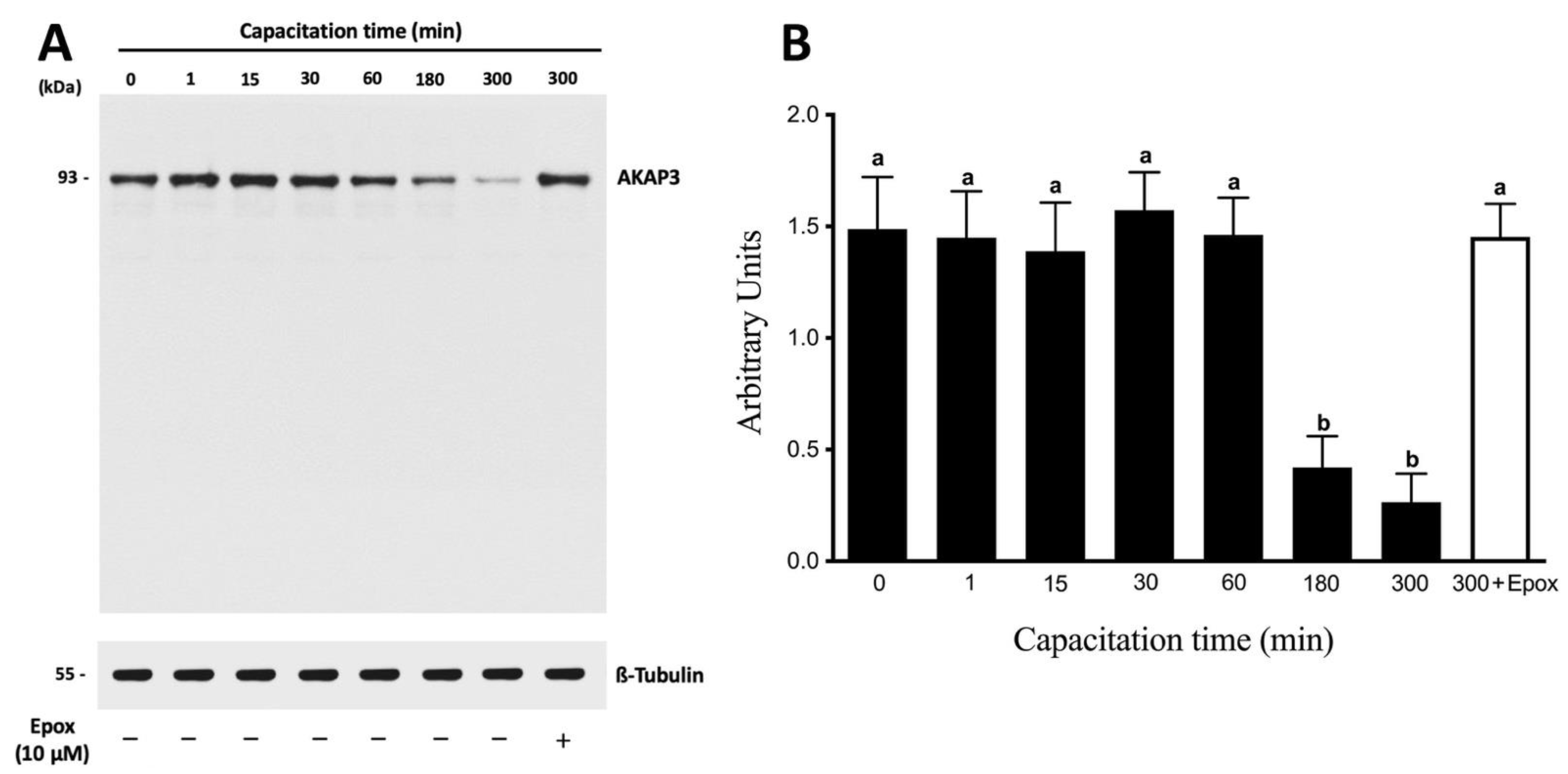
3.2. AKAP3 Is Degraded during Sperm Capacitation
3.3. PRKAR1 Is Degraded during Sperm Capacitation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, A.L.; Ciechanover, A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 73–96. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C. The ubiquitin–proteasome system in kidney physiology and disease. Nat. Rev. Nephrol. 2019, 15, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.H.; Goldstein, G.; Niall, H.D. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry 1975, 14, 2214–2218. [Google Scholar] [CrossRef]
- Bebington, C.; Doherty, F.J.; Fleming, S.D. The possible biological and reproductive functions of ubiquitin. Hum. Reprod. Update 2001, 7, 102–111. [Google Scholar] [CrossRef]
- Ciechanover, a Intracellular protein degradation: From a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 2005, 12, 1178–1190. [CrossRef] [PubMed]
- Heinemeyer, W.; Kleinschmidt, J.A.; Saidowsky, J.; Escher, C.; Wolf, D.H. Proteinase yscE, the yeast proteasome/multicatalytic-multifunctional proteinase: Mutants unravel its function in stress induced proteolysis and uncover its necessity for cell survival. EMBO J. 1991, 10, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Velichutina, I.; Connerly, P.L.; Arendt, C.S.; Li, X.; Hochstrasser, M. Plasticity in eucaryotic 20S proteasome ring assembly revealed by a subunit deletion in yeast. EMBO J. 2004, 23, 500–510. [Google Scholar] [CrossRef]
- Jung, T.; Catalgol, B.; Grune, T. The proteasomal system. Mol. Asp. Med. 2009, 30, 191–296. [Google Scholar] [CrossRef]
- Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 2004, 5, 417–421. [Google Scholar] [CrossRef]
- Jung, T.; Grune, T. Structure of the proteasome. Prog. Mol. Biol. Transl. Sci. 2012, 109, 1–39. [Google Scholar] [CrossRef]
- Bousquet-Dubouch, M.-P.; Fabre, B.; Monsarrat, B.; Burlet-Schiltz, O. Proteomics to study the diversity and dynamics of proteasome complexes: From fundamentals to the clinic. Expert Rev. Proteom. 2011, 8, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Thibaudeau, T.A.; Smith, D.M. A practical review of proteasome pharmacology. Pharmacol. Rev. 2019, 71, 170–197. [Google Scholar] [CrossRef]
- Tipler, C.P.; Hutchon, S.P.; Hendil, K.; Tanaka, K.; Fishel, S.; Mayer, R.J. Purification and characterization of 26S proteasomes from human and mouse spermatozoa. Mol. Hum. Reprod. 1997, 3, 1053–1060. [Google Scholar] [CrossRef][Green Version]
- Wojcik, C.; Benchaib, M.; Lornage, J.; Czyba, J.C.; Guerin, J.F. Proteasomes in human spermatozoa. Int. J. Androl. 2000, 23, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Kong, M.; Pizarro, E.; Pasten, C. Participation of the sperm proteasome in human fertilization. Hum. Reprod. 2003, 18, 1010–1017. [Google Scholar] [CrossRef]
- Zapata-Carmona, H.; Barón, L.; Zuñiga, L.M.; Díaz, E.S.; Kong, M.; Drobnis, E.Z.; Sutovsky, P.; Morales, P. The activation of the chymotrypsin-like activity of the proteasome is regulated by soluble adenyl cyclase/cAMP/protein kinase A pathway and required for human sperm capacitation. Mol. Hum. Reprod. 2019, 25, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Kerns, K.; Morales, P.; Sutovsky, P. Regulation of Sperm Capacitation by the 26S Proteasome: An Emerging New Paradigm in Spermatology. Biol. Reprod. 2016, 94, 117. [Google Scholar] [CrossRef]
- Zigo, M.; Manaskova-Postlerova, P.; Jonakova, V.; Kerns, K.; Sutovsky, P. Compartmentalization of the proteasome-interacting proteins during sperm capacitation. Sci. Rep. 2019, 9, 12583. [Google Scholar] [CrossRef]
- Austin, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Zapata-Carmona, H.; Soriano-Úbeda, C.; París-Oller, E.; Matás, C. Periovulatory oviductal fluid decreases sperm protein kinase A activity, tyrosine phosphorylation, and in vitro fertilization in pig. Andrology 2020, 8, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ Signaling in Mammalian Spermatozoa. Mol. Cell Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.A.; Babcock, D.F.; Wennemuth, G.; Brown, W.; Burton, K.A.; McKnight, G.S. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 13483–13488. [Google Scholar] [CrossRef] [PubMed]
- Shabb, J.B. Physiological substrates of cAMP-dependent protein kinase. Chem. Rev. 2001, 101, 2381–2411. [Google Scholar] [CrossRef]
- Bruce, J.I.E.; Shuttleworth, T.J.; Giovannucci, D.R.; Yule, D.I. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells: A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J. Biol. Chem. 2002, 277, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Moseley, F.L.C.; Jha, K.N.; Björndahl, L.; Brewis, I.A.; Publicover, S.J.; Barratt, C.L.R.; Lefièvre, L. Protein tyrosine phosphorylation, hyperactivation and progesterone-induced acrosome reaction are enhanced in IVF media: An effect that is not associated with an increase in protein kinase A activation. Mol. Hum. Reprod. 2005, 11, 523–529. [Google Scholar] [CrossRef]
- Martínez-León, E.; Osycka-Salut, C.; Signorelli, J.; Pozo, P.; Pérez, B.; Kong, M.; Morales, P.; Pérez-Martínez, S.; Díaz, E.S. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum. Reprod. 2015, 30, 2138–2151. [Google Scholar] [CrossRef]
- Battistone, M.A.; Da Ros, V.G.; Salicioni, A.M.; Navarrete, F.A.; Krapf, D.; Visconti, P.E.; Cuasnicú, P.S. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol. Hum. Reprod. 2013, 19, 570–580. [Google Scholar] [CrossRef]
- Björndahl, L.; Barratt, C.L.R.; Mortimer, D.; Jouannet, P. “How to count sperm properly”: Checklist for acceptability of studies based on human semen analysis. Hum. Reprod. 2016, 31, 227–232. [Google Scholar] [CrossRef]
- WHO World Health Orgnization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO World Health Orgnization: Geneva, Switzerland, 2010; ISBN 9789241547789. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Jin, H.X.; Wu, T.X.; Jiang, Y.J.; Zou, J.W.; Zhuang, S.L.; Mao, X.; Yu, Q. Sen Role of phosphorylated Thr-197 in the catalytic subunit of cAMP-dependent protein kinase. J. Mol. Struct. THEOCHEM 2007, 805, 9–15. [Google Scholar] [CrossRef]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef]
- Moore, M.J.; Kanter, J.R.; Jones, K.C.; Taylor, S.S. Phosphorylation of the catalytic subunit of protein kinase A: Autophosphorylation versus phosphorylation by phosphoinositide-dependent kinase-1. J. Biol. Chem. 2002, 277, 47878–47884. [Google Scholar] [CrossRef]
- Hillman, P.; Ickowicz, D.; Vizel, R.; Breitbart, H. Dissociation between AKAP3 and PKARII Promotes AKAP3 Degradation in Sperm Capacitation. PLoS ONE 2013, 8, e68873. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: Modulation and protein kinase A dependency. Mol. Hum. Reprod. 2004, 10, 355–363. [Google Scholar] [CrossRef]
- Nassar, A.; Mahony, M.; Morshedi, M.; Lin, M.H.; Srisombut, C.; Oehninger, S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil. Steril. 1999, 71, 919–923. [Google Scholar] [CrossRef]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef]
- Neuhaus, E.M.; Mashukova, A.; Barbour, J.; Wolters, D.; Hatt, H. Novel function of β-arrestin2 in the nucleus of mature spermatozoa. J. Cell Sci. 2006, 119, 3047–3056. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Nixon, B.; Baker, M.A.; Aitken, R.J. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. MHR Basic Sci. Reprod. Med. 2008, 14, 235–243. [Google Scholar] [CrossRef]
- Vetter, M.M.; Zenn, H.M.; Méndez, E.; Van Den Boom, H.; Herberg, F.W.; Skålhegg, B.S. The testis-specific Cα2 subunit of PKA is kinetically indistinguishable from the common C1 subunit of PKA. BMC Biochem. 2011, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A.; Carr, D.W.; Meizel, S. Involvement of Protein Kinase A and A Kinase Anchoring Protein in the Progesterone-Initiated Human Sperm Acrosome Reaction. Biol. Reprod. 2000, 820, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol. Reprod. 1999, 60, 1402–1409. [Google Scholar] [CrossRef]
- Kong, M.; Diaz, E.S.; Morales, P. Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol. Reprod. 2009, 80, 1026–1035. [Google Scholar] [CrossRef]
- Hegde, A.N. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Prog. Neurobiol. 2004, 73, 311–357. [Google Scholar] [CrossRef] [PubMed]
- Lignitto, L.; Carlucci, A.; Sepe, M.; Stefan, E.; Cuomo, O.; Nisticò, R.; Scorziello, A.; Savoia, C.; Garbi, C.; Annunziato, L.; et al. Control of PKA stability and signalling by the RING ligase praja2. Nat. Cell Biol. 2011, 13, 412–422. [Google Scholar] [CrossRef]
- Scott, J.D.; DelľAcqua, M.L.; Fraser, I.D.C.; Tavalin, S.J.; Lester, L.B. Coordination of cAMP Signaling Events through PKA Anchoring. Adv. Pharmacol. 1999, 47, 175–207. [Google Scholar] [CrossRef]
- Vizel, R.; Hillman, P.; Ickowicz, D.; Breitbart, H. AKAP3 degradation in sperm capacitation is regulated by its tyrosine phosphorylation. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Barón, L.; Fara, K.; Zapata-Carmona, H.; Zuñiga, L.; Kong, M.; Signorelli, J.; Díaz, E.S.; Morales, P. Participation of protein kinases and phosphatases in the progesterone-induced acrosome reaction and calcium influx in human spermatozoa. Andrology 2016, 4, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Newhall, K.J.; Cummings, D.E.; Nolan, M.A.; McKnight, G.S. Deletion of the RIIβ-subunit of protein kinase A decreases body weight and increases energy expenditure in the obese, leptin-deficient ob/ob mouse. Mol. Endocrinol. 2005, 19, 982–991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vijayaraghavan, S.; Liberty, G.A.; Mohan, J.; Winfrey, V.P.; Olson, G.E.; Carr, D.W. Isolation and molecular characterization of AKAP110, a novel, sperm-specific protein kinase A-anchoring protein. Mol. Endocrinol. 1999, 5, 705–717. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Olson, G.E.; NagDas, S.; Winfrey, V.P.; Carr, D.W. Subcellular localization of the regulatory subunits of cyclic adenosine 3’,5’-monophosphate-dependent protein kinase in bovine spermatozoa. Biol. Reprod. 1997, 57, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Biały, L.P.; Ziemba, H.T.; Marianowski, P.; Fracki, S.; Bury, M.; Wójcik, C. Localization of a proteasomal antigen in human spermatozoa: Immunohistochemical electron microscopic study. Folia Histochem. Cytobiol. 2001, 39, 129–130. [Google Scholar] [PubMed]
- Morales, P.; Pizarro, E.; Kong, M.; Jara, M. Extracellular localization of proteasomes in human sperm. Mol. Reprod. Dev. 2004, 68, 115–124. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapata-Carmona, H.; Barón, L.; Kong, M.; Morales, P. Protein Kinase A (PRKA) Activity Is Regulated by the Proteasome at the Onset of Human Sperm Capacitation. Cells 2021, 10, 3501. https://doi.org/10.3390/cells10123501
Zapata-Carmona H, Barón L, Kong M, Morales P. Protein Kinase A (PRKA) Activity Is Regulated by the Proteasome at the Onset of Human Sperm Capacitation. Cells. 2021; 10(12):3501. https://doi.org/10.3390/cells10123501
Chicago/Turabian StyleZapata-Carmona, Héctor, Lina Barón, Milene Kong, and Patricio Morales. 2021. "Protein Kinase A (PRKA) Activity Is Regulated by the Proteasome at the Onset of Human Sperm Capacitation" Cells 10, no. 12: 3501. https://doi.org/10.3390/cells10123501
APA StyleZapata-Carmona, H., Barón, L., Kong, M., & Morales, P. (2021). Protein Kinase A (PRKA) Activity Is Regulated by the Proteasome at the Onset of Human Sperm Capacitation. Cells, 10(12), 3501. https://doi.org/10.3390/cells10123501







