Inhibition of Human Osteoclast Differentiation by Kynurenine through the Aryl-Hydrocarbon Receptor Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Cell Isolation
2.3. Osteoclast Differentiation
2.4. TRAP Stain and F-Actin Ring
2.5. MTT (3-(4,5-Dimethylthiazol-2-yl)) and LDH (Lactate Dehydrogenase) Assay
2.6. RNA Extraction and Real-Time Quantitative (RT-q PCR)
2.7. Immunoblotting (IB)
2.8. Immunofluorescence
2.9. Transfection
2.10. Statistical Analysis
3. Results
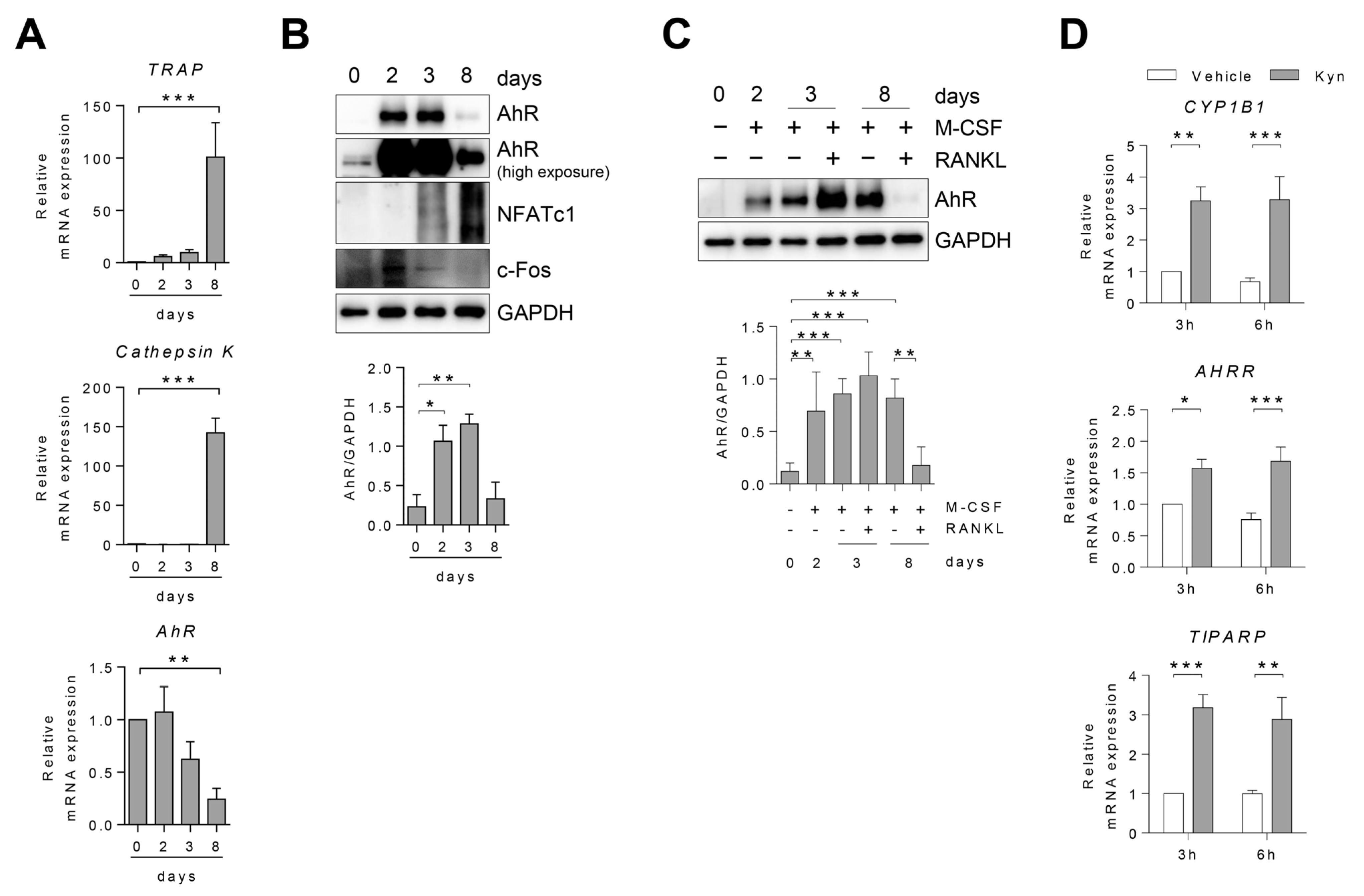
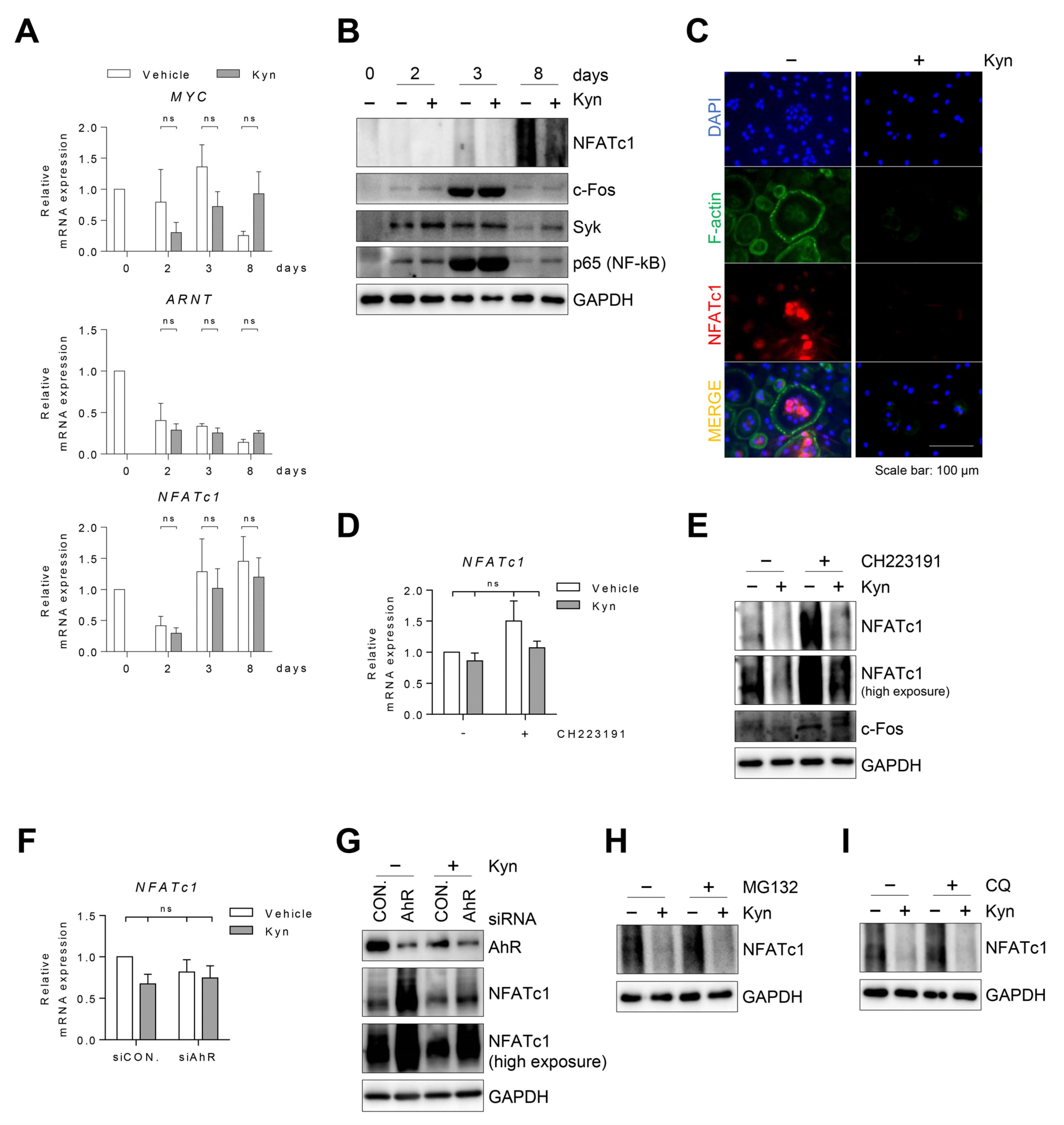
3.1. AhR Expression Changes in Human Osteoclast during Differentiation
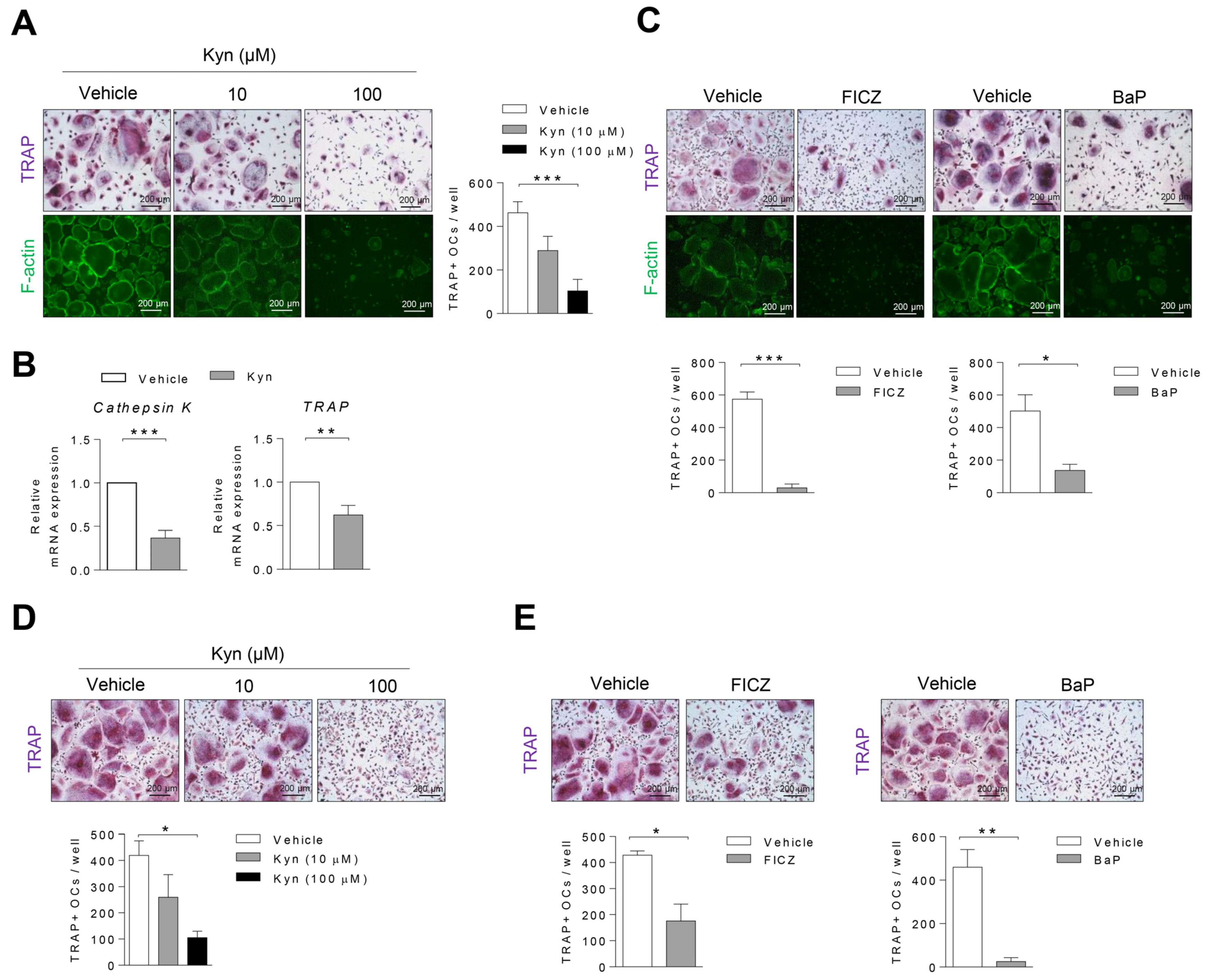
3.2. AhR Agonists Inhibit Osteoclast Differentiation in Human PBMC and RA SFMC CD14+ Cells
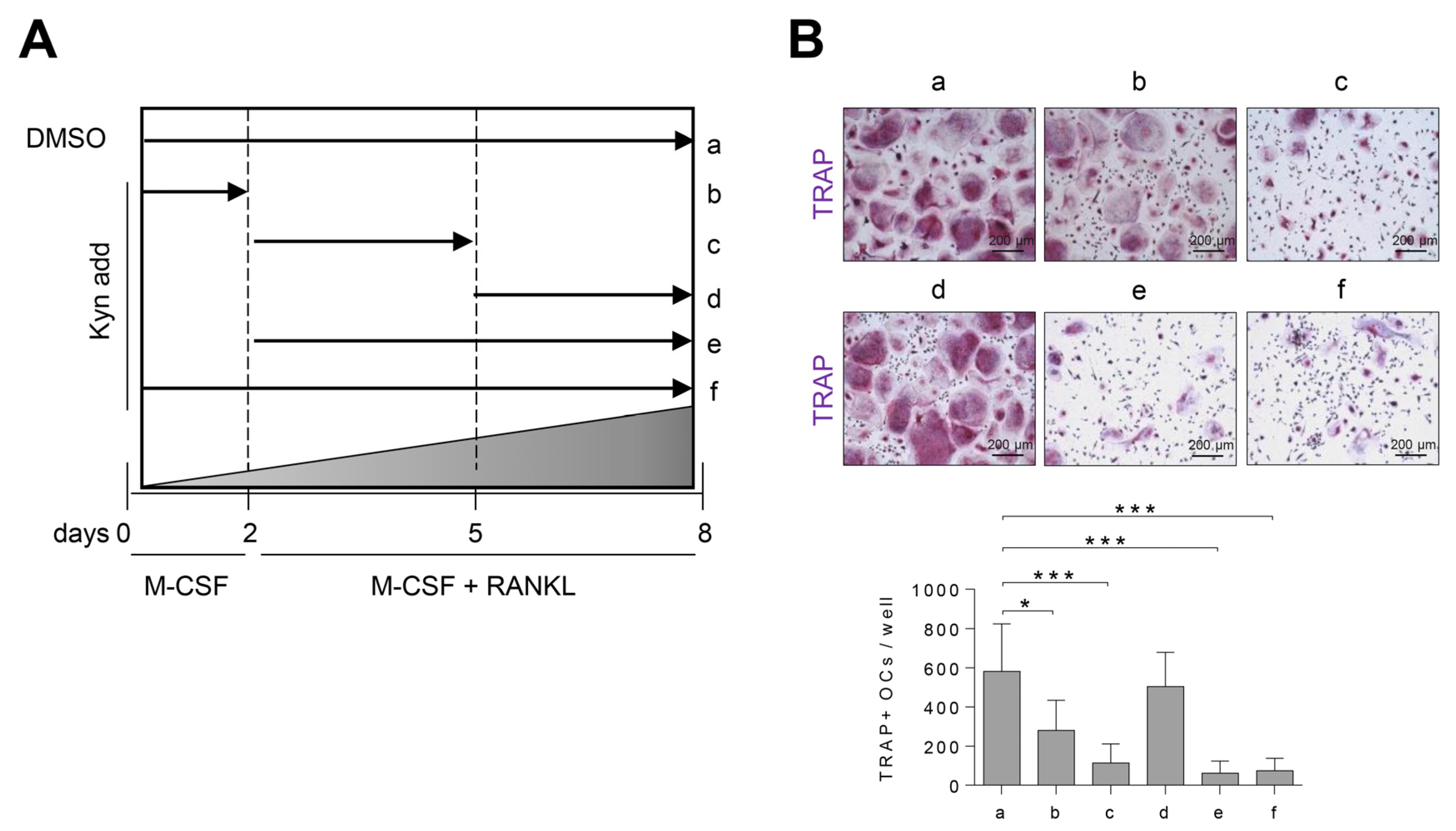
3.3. Treatment of Kyn in the Early Stage of Osteoclastogenesis Effectively Suppresses Osteoclast Formation
3.4. Blockade of AhR Signaling Reverses Kyn-Induced Inhibition of Osteoclastogenesis
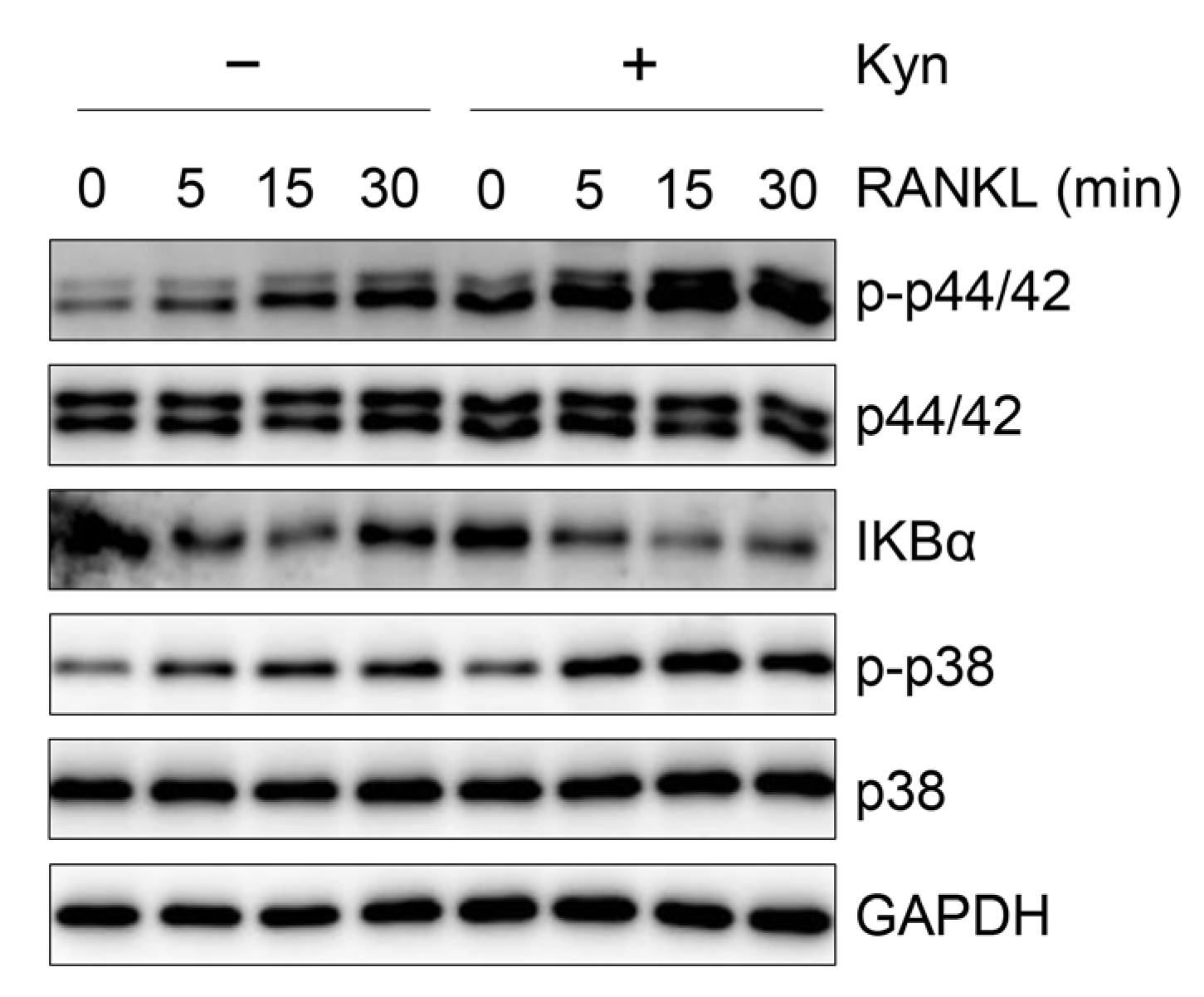
3.5. Kyn Does Not Regulate RANKL-Induced Signaling in OCPs
3.6. Kyn Downregulates NFATc1 Protein in Human Osteoclasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Gambari, L.; Grassi, F.; Roseti, L.; Grigolo, B.; Desando, G. Learning from Monocyte-Macrophage Fusion and Multinucleation: Potential Therapeutic Targets for Osteoporosis and Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 6001. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Tanaka, Y. RANKL as a therapeutic target of rheumatoid arthritis. J. Bone Miner. Metab. 2021, 39, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Vazquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, S. Smoking as a Preventable Risk Factor for Rheumatoid Arthritis: Rationale for Smoking Cessation Treatment in Patients with Rheumatoid Arthritis. J. Rheum. Dis. 2019, 26, 12. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Sun, L.; Cao, J.; Yuen, T.; Lu, P.; Bab, I.; Leu, N.A.; Srinivasan, S.; Wagage, S.; Hunter, C.A.; et al. Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proc. Nat. Acad. Sci. USA 2013, 110, 11115–11120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donate, P.B.; Alves de Lima, K.; Peres, R.S.; Almeida, F.; Fukada, S.Y.; Silva, T.A.; Nascimento, D.C.; Cecilio, N.T.; Talbot, J.; Oliveira, R.D.; et al. Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Peres, R.S.; Pinto, L.G.; Oliveira, R.D.R.; Lima, K.A.; Donate, P.B.; Silva, J.R.; Ryffel, B.; Cunha, T.M.; Alves-Filho, J.C.; et al. Smoking-Induced aggravation of experimental arthritis is dependent of aryl hydrocarbon receptor activation in Th17 cells. Arthritis Res. Ther. 2018, 20, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Jiang, L.; Wan, B.; Zhang, W.; Yao, L.; Che, T.; Gan, C.; Su, N.; He, J.; Huang, J.; et al. The role of aryl hydrocarbon receptor in bone remodeling. Prog. Biophys. Mol. Biol. 2018, 134, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Madhavaram, S.; Ji, J.D. The Role of Aryl-Hydrocarbon Receptor (AhR) in Osteoclast Differentiation and Function. Cells 2020, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J. Mol. Med. 2005, 83, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, J.H.; Jung, J.H.; Kim, T.H.; Ji, J.D. TREM-1, a negative regulator of human osteoclastogenesis. Immuno. Lett. 2016, 171, 50–59. [Google Scholar] [CrossRef]
- Park, J.; Park, H.; Lee, Y.L.; Weon, S.; Kim, Y.G.; Yang, J.H.; Nam, B.; Jo, S.; Kim, T.H. Blocking TNFalpha attenuates progressive cartilage matrix degradation in inflammatory arthritis. Exp. Ther. Med. 2021, 22, 808. [Google Scholar] [CrossRef]
- Jo, S.; Nam, B.; Lee, Y.L.; Park, H.; Weon, S.; Choi, S.-H.; Park, Y.-S.; Kim, T.-H. The TNF-NF-κB-DKK1 Axis Promoted Bone Formation in the Enthesis of Ankylosing Spondylitis. J. Rheum. Dis. 2021, 28, 216–224. [Google Scholar] [CrossRef]
- Jo, S.; Wang, S.E.; Lee, Y.L.; Kang, S.; Lee, B.; Han, J.; Sung, I.H.; Park, Y.S.; Bae, S.C.; Kim, T.H. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. 2018, 20, 115. [Google Scholar] [CrossRef]
- Kim, S.H.; Henry, E.C.; Kim, D.K.; Kim, Y.H.; Shin, K.J.; Han, M.S.; Lee, T.G.; Kang, J.K.; Gasiewicz, T.A.; Ryu, S.H.; et al. Novel compound 2-Methyl-2H-Pyrazole-3-Carboxylic acid (2-Methyl-4-o-Tolylazo-Phenyl)-Amide (CH-223191) prevents 2,3,7,8-TCDD-Induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol. Pharmacol. 2006, 69, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Battaglino, R.; Kim, D.; Fu, J.; Vaage, B.; Fu, X.Y.; Stashenko, P. c-Myc is required for osteoclast differentiation. J. Bone Miner. Res. 2002, 17, 763–773. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Murray, T.J.; Mitchell, G.C.; Hesterman, E.V.; Karchner, S.I.; Merson, R.R.; Hahn, M.E.; Sherr, D.H. The aryl hydrocarbon receptor constitutively represses c-Myc transcription in human mammary tumor cells. Oncogene 2005, 24, 7869–7881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.C.; Shyu, J.F.; Lim, P.S.; Fang, T.C.; Lu, C.L.; Zheng, C.M.; Hou, Y.C.; Wu, C.C.; Lin, Y.F.; Lu, K.C. Concentration and Duration of Indoxyl Sulfate Exposure Affects Osteoclastogenesis by Regulating NFATc1 via Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 3486. [Google Scholar] [CrossRef]
- Jia, Y.; Tao, Y.; Lv, C.; Xia, Y.; Wei, Z.; Dai, Y. Tetrandrine enhances the ubiquitination and degradation of Syk through an AhR-c-Src-C-Cbl pathway and consequently inhibits osteoclastogenesis and bone destruction in arthritis. Cell. Death Dis. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Acosta, O.; Vega, L.; Estrada-Muniz, E.; Rodriguez, M.S.; Gonzalez, F.J.; Elizondo, G. Activation of aryl hydrocarbon receptor regulates the LPS/IFNgamma-Induced inflammatory response by inducing ubiquitin-Proteosomal and lysosomal degradation of RelA/p65. Biochem. Pharmacol. 2018, 155, 141–149. [Google Scholar] [CrossRef]
- Yu, T.Y.; Pang, W.J.; Yang, G.S. Aryl hydrocarbon receptors in osteoclast lineage cells are a negative regulator of bone mass. PLoS ONE 2015, 10, e0117112. [Google Scholar] [CrossRef]
- Izawa, T.; Arakaki, R.; Mori, H.; Tsunematsu, T.; Kudo, Y.; Tanaka, E.; Ishimaru, N. The Nuclear Receptor AhR Controls Bone Homeostasis by Regulating Osteoclast Differentiation via the RANK/c-Fos Signaling Axis. J. Immunol. 2016, 197, 4639–4650. [Google Scholar] [CrossRef]
- Fu, J.; Nogueira, S.V.; Drongelen, V.V.; Coit, P.; Ling, S.; Rosloniec, E.F.; Sawalha, A.H.; Holoshitz, J. Shared epitope-Aryl hydrocarbon receptor crosstalk underlies the mechanism of gene-Environment interaction in autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2018, 115, 4755–4760. [Google Scholar] [CrossRef] [Green Version]
- Eisa, N.H.; Reddy, S.V.; Elmansi, A.M.; Kondrikova, G.; Kondrikov, D.; Shi, X.M.; Novince, C.M.; Hamrick, M.W.; McGee-Lawrence, M.E.; Isales, C.M.; et al. Kynurenine Promotes RANKL-Induced Osteoclastogenesis In Vitro by Activating the Aryl Hydrocarbon Receptor Pathway. Int. J. Mol. Sci. 2020, 21, 7931. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Zhao, B.; Triantafyllopoulou, A.; Park-Min, K.H.; Ivashkiv, L.B. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-Mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheumatol. 2010, 62, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Colonna, M.; Turnbull, I.; Klesney-Tait, J. The enigmatic function of TREM-2 in osteoclastogenesis. Adv. Exp. Med. Biol. 2007, 602, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Dai, Y. Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 2020, 126, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, J.; Lu, C.; Xie, D.; Liu, J.; Zhong, C.; Wu, X.; Dai, R.; Zhang, H.; Guan, D.; et al. HIF1alpha inhibition facilitates Leflunomide-AHR-CRP signaling to attenuate bone erosion in CRP-Aberrant rheumatoid arthritis. Nat. Commun. 2019, 10, 4579. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Okamoto, H.; Iwamoto, T.; Toyama, Y.; Tomatsu, T.; Yamanaka, H.; Momohara, S. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology 2008, 47, 1317–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| AhR | ACATCACCTACGCCAGTCG | TCTATGCCGCTTGGAAGGAT |
| Cathepsin K | CTCTTCCATTTCTTCCACGAT | ACACCAACTCCCTTCCAAAG |
| TRAP | TGGCTTTGCCTATGTGGA | CCTGGTCTTAAAGAGGGACTT |
| NFATc1 | GCATCACAGGGA AGACCGTGTC | GAAGTTCAATGTCGGAGTTTCTGAG |
| CYP1B1 | AGTTCTTGAGGCACTGCGAA | GTGATAGTGGCCGGTACGTT |
| AHRR | ACCGCGGATGCAAAAGTAAAAG | CTCCTTCCTGCTGAGTAATTG |
| TIPARP | CACCCTCTAGCAATGTCAACTC | AGACTCGGGATACTCTCTCC |
| C-Myc | CAGCGAGGATATCTGGAAGA | CTTCTCTGAGACGAGCTT |
| ARNT1 | ACTACCCGCTCAGGCTTTTC | ATGGAGTCTGAAAGCTGCCC |
| GAPDH | CAAGATCATCAGCAATGCC | CTGTGGTCATGAGTCCTTCC |
| Antigen | Manufacturer | Catalog Number |
|---|---|---|
| AhR | Cell Signaling, Danvers, MA, USA | 83200 |
| NFATc1 | BD biosciences, San Jose, CA, USA | 556602 |
| c-Fos | Abcam, Cambridge, MA, USA | ab190289 |
| p-p44/42 | Cell Signaling, Danvers, MA, USA | 9101 |
| p44/42 | Cell Signaling, Danvers, MA, USA | 9102 |
| IKBα | Cell Signaling, Danvers, MA, USA | 9242 |
| p-p38 | Cell Signaling, Danvers, MA, USA | 9215 |
| p38 | Santa Cruz, Dallas, CA, USA | 535 |
| Syk | Cell Signaling, Danvers, MA, USA | 2712 |
| p65 | Santa Cruz, Dallas, CA, USA | 372 |
| GAPDH | Cell Signaling, Danvers, MA, USA | 2118 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Oh, Y.; Jo, S.; Ji, J.-D.; Kim, T.-H. Inhibition of Human Osteoclast Differentiation by Kynurenine through the Aryl-Hydrocarbon Receptor Pathway. Cells 2021, 10, 3498. https://doi.org/10.3390/cells10123498
Kim S-Y, Oh Y, Jo S, Ji J-D, Kim T-H. Inhibition of Human Osteoclast Differentiation by Kynurenine through the Aryl-Hydrocarbon Receptor Pathway. Cells. 2021; 10(12):3498. https://doi.org/10.3390/cells10123498
Chicago/Turabian StyleKim, So-Yeon, Younseo Oh, Sungsin Jo, Jong-Dae Ji, and Tae-Hwan Kim. 2021. "Inhibition of Human Osteoclast Differentiation by Kynurenine through the Aryl-Hydrocarbon Receptor Pathway" Cells 10, no. 12: 3498. https://doi.org/10.3390/cells10123498
APA StyleKim, S.-Y., Oh, Y., Jo, S., Ji, J.-D., & Kim, T.-H. (2021). Inhibition of Human Osteoclast Differentiation by Kynurenine through the Aryl-Hydrocarbon Receptor Pathway. Cells, 10(12), 3498. https://doi.org/10.3390/cells10123498






