Transcriptomic Profiles Reveal Downregulation of Low-Density Lipoprotein Particle Receptor Pathway Activity in Patients Surviving Severe COVID-19
Abstract
:1. Introduction
2. Materials and Methods
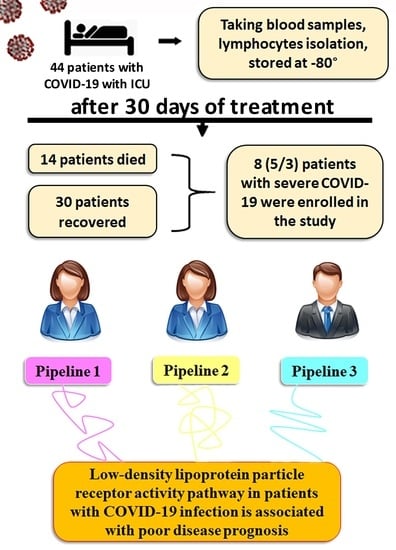
2.1. Patients
2.2. Isolation of Peripheral Blood Mononuclear Cells
2.3. RNA Isolation, Library Preparation, and Sequencing
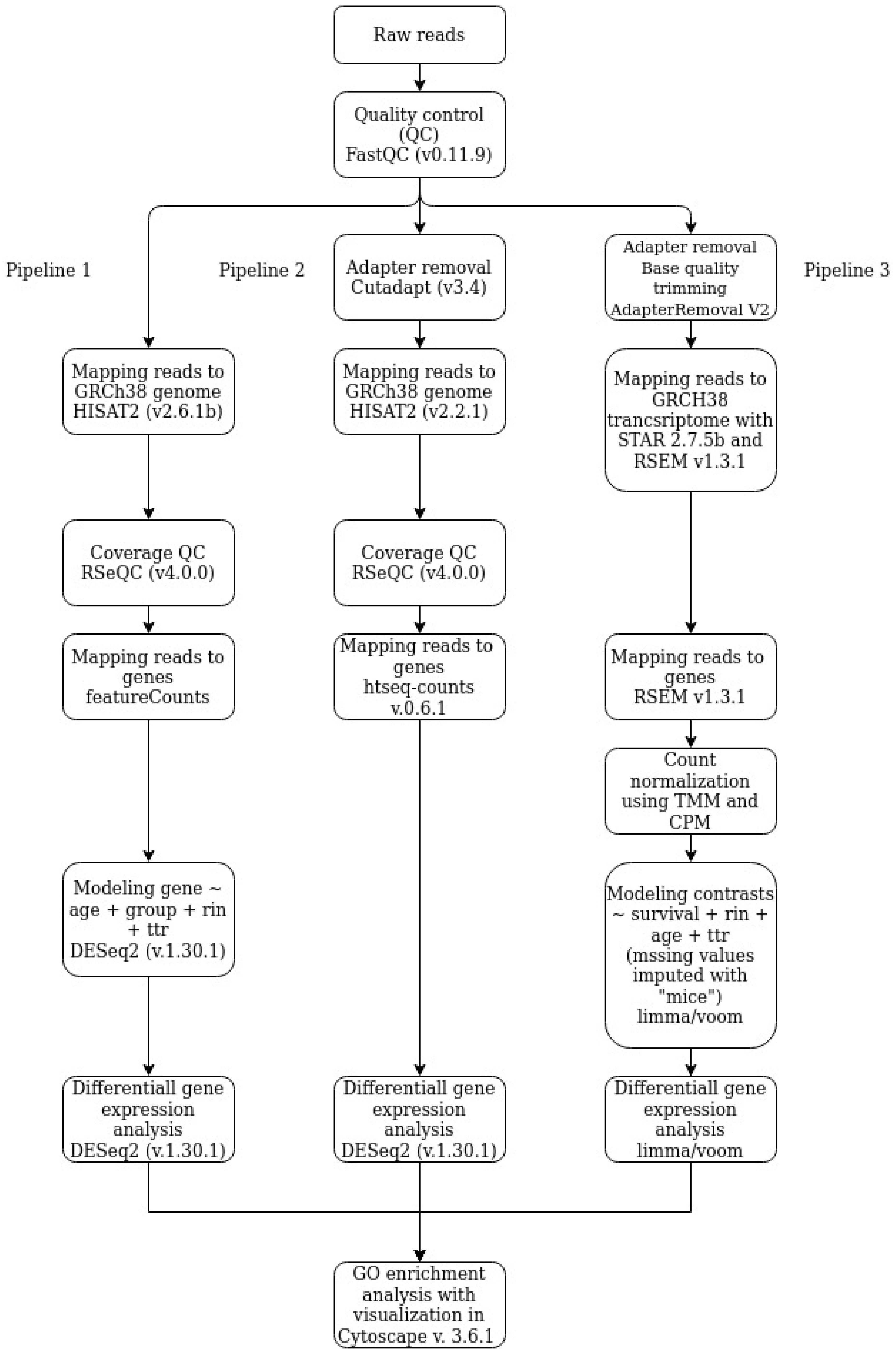
2.4. Data Processing and Analysis
2.4.1. Pipeline 1
2.4.2. Pipeline 2
2.4.3. Pipeline 3
2.4.4. Go Enrichment Analysis
3. Results
3.1. RNA-Seq Experiments
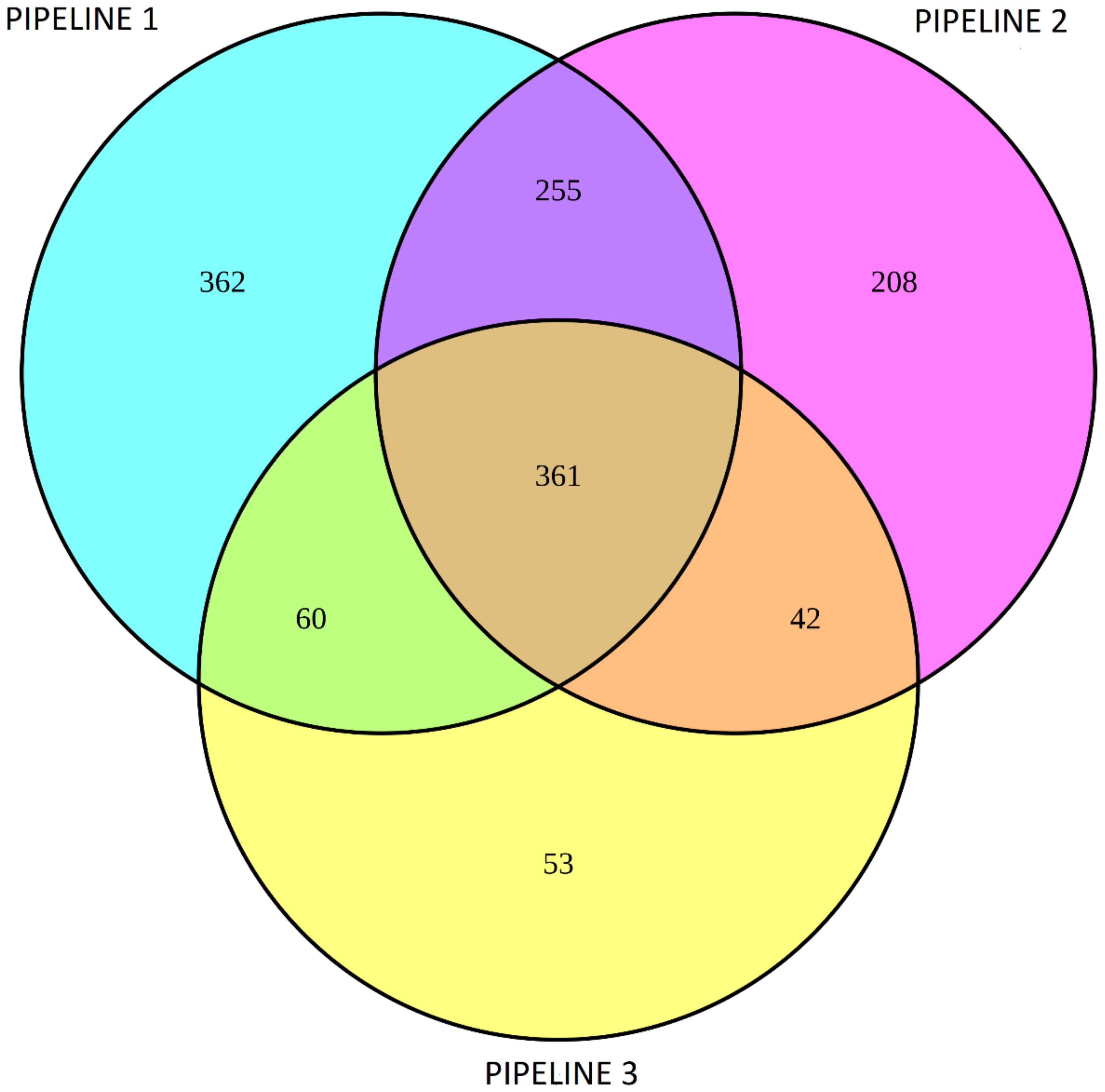
3.2. Differential Gene Expression Analysis Using DESeq2 and Limma/Voom
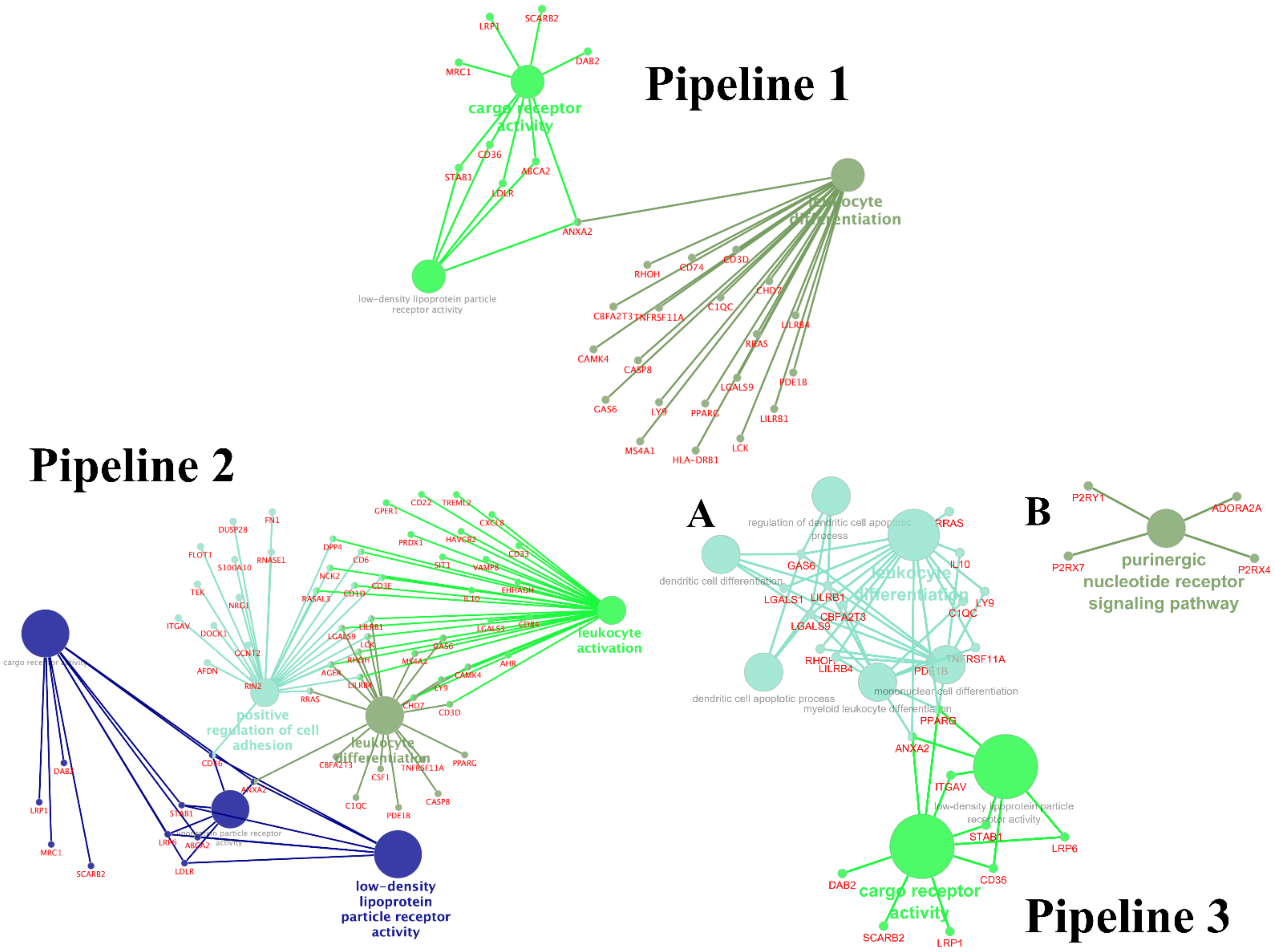
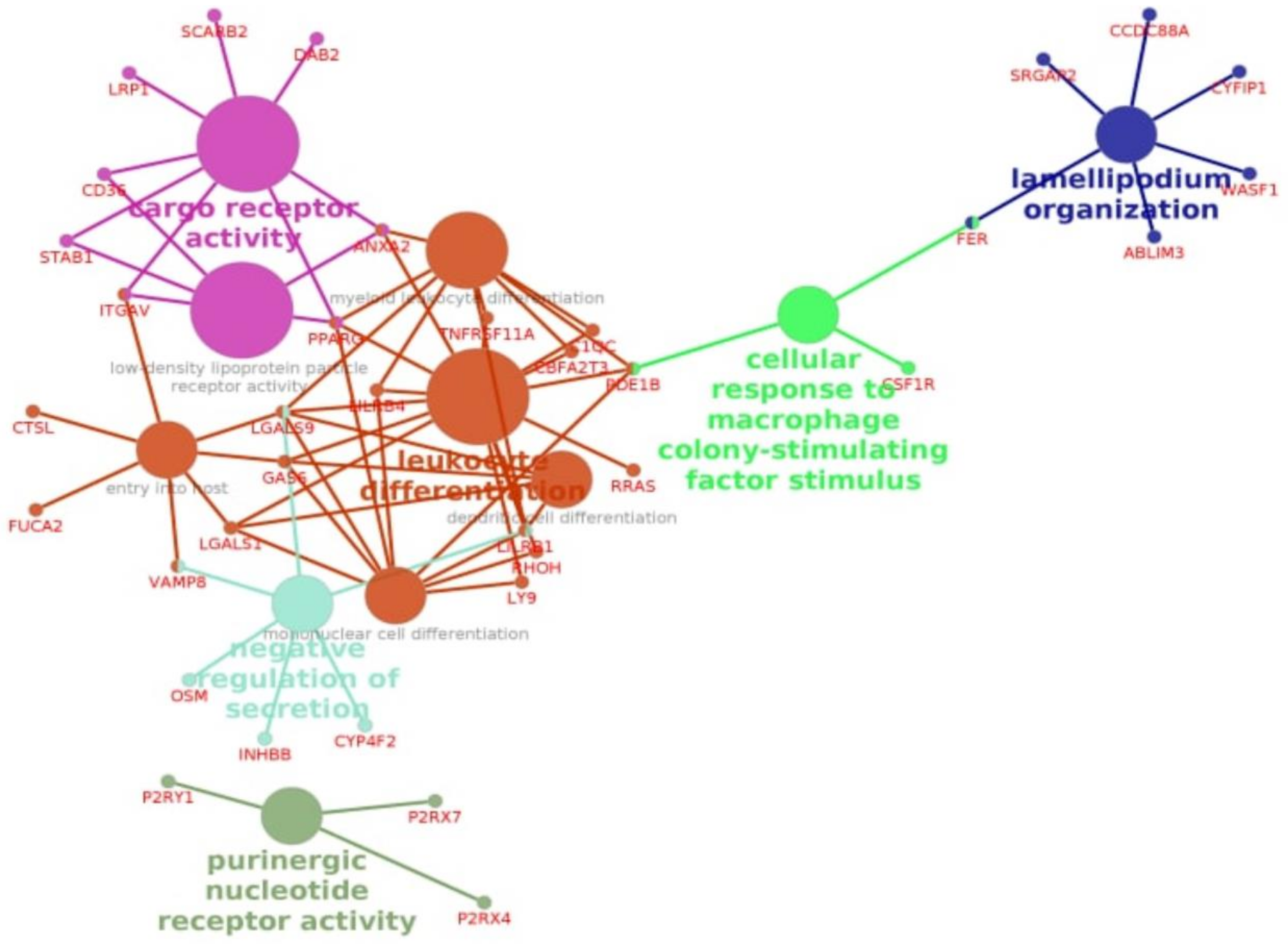
3.3. Enrichment Gene Ontology (GO) Analysis
3.4. Enrichment Gene Ontology (GO) Analysis of Overlapping DEGs between Three Pipelines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berekaa, M.M. Insights into the COVID-19 pandemic: Origin, pathogenesis, diagnosis, and therapeutic interventions. Front. Biosci. 2021, 13, 117–139. [Google Scholar]
- Boban, M. Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments. Int. J. Clin. Pract. 2021, 75, e13868. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripathi, T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Wu, J.; Deng, W.; Li, S.; Yang, X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell. Mol. Life Sci. CMLS 2021, 78, 531–544. [Google Scholar] [CrossRef]
- Amirfakhryan, H.; Safari, F. Outbreak of SARS-CoV2: Pathogenesis of infection and cardiovascular involvement. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2021, 62, 13–23. [Google Scholar] [CrossRef]
- Kumar, P.; Sah, A.K.; Tripathi, G.; Kashyap, A.; Tripathi, A.; Rao, R.; Mishra, P.C.; Mallick, K.; Husain, A.; Kashyap, M.K. Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19. Mol. Cell. Biochem. 2021, 476, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb. Pathog. 2021, 150, 104673. [Google Scholar] [CrossRef]
- Alharthy, A.; Aletreby, W.; Faqihi, F.; Balhamar, A.; Alaklobi, F.; Alanezi, K.; Jaganathan, P.; Tamim, H.; Alqahtani, S.A.; Karakitsos, D.; et al. Clinical Characteristics and Predictors of 28-Day Mortality in 352 Critically Ill Patients with COVID-19: A Retrospective Study. J. Epidemiol. Glob. Health 2021, 11, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.L.; Su, H.C. A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell 2020, 181, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y. Herpes simplex virus encephalitis of childhood: Inborn errors of central nervous system cell-intrinsic immunity. Hum. Genet. 2020, 139, 911–918. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef]
- Li, C.X.; Chen, J.; Lv, S.K.; Li, J.H.; Li, L.L.; Hu, X. Whole-Transcriptome RNA Sequencing Reveals Significant Differentially Expressed mRNAs, miRNAs, and lncRNAs and Related Regulating Biological Pathways in the Peripheral Blood of COVID-19 Patients. Mediat. Inflamm. 2021, 2021, 6635925. [Google Scholar] [CrossRef]
- Gardinassi, L.G.; Souza, C.O.S.; Sales-Campos, H.; Fonseca, S.G. Immune and Metabolic Signatures of COVID-19 Revealed by Transcriptomics Data Reuse. Front. Immunol. 2020, 11, 1636. [Google Scholar] [CrossRef]
- Jang, J.G.; Hur, J.; Hong, K.S.; Lee, W.; Ahn, J.H. Prognostic Accuracy of the SIRS, qSOFA, and NEWS for Early Detection of Clinical Deterioration in SARS-CoV-2 Infected Patients. J. Korean Med. Sci. 2020, 35, e234. [Google Scholar] [CrossRef]
- Zaidi, H.; Bader-El-Den, M.; McNicholas, J. Using the National Early Warning Score (NEWS/NEWS 2) in different Intensive Care Units (ICUs) to predict the discharge location of patients. BMC Public Health 2019, 19, 1231. [Google Scholar] [CrossRef] [Green Version]
- Kanof, M.E.; Smith, P.D.; Zola, H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr. Protoc. Immunol. 2001, 7, 7.1. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 8 January 2019).
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Lindgreen, S.; Orlando, L. AdapterRemoval v2: Rapid adapter trimming, identification, and read merging. BMC Res. Notes 2016, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 67. [Google Scholar] [CrossRef] [Green Version]
- The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [CrossRef] [Green Version]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Zeng, W.; Su, J.; Wan, H.; Yu, X.; Cao, X.; Tan, W.; Wang, H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Karathanasis, S.K.; Yang, Z.H.; Freeman, L.; Kotani, K.; Remaley, A.T. COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 9843–9853. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Ye, G.; Cao, X.; Xu, X.; Tan, W.; Zhang, Y. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metab. Clin. Exp. 2020, 107, 154243. [Google Scholar] [CrossRef]
- Hu, X.; Chen, D.; Wu, L.; He, G.; Ye, W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 510, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Žarković, N.; Orehovec, B.; Milković, L.; Baršić, B.; Tatzber, F.; Wonisch, W.; Tarle, M.; Kmet, M.; Mataić, A.; Jakovčević, A.; et al. Preliminary Findings on the Association of the Lipid Peroxidation Product 4-Hydroxynonenal with the Lethal Outcome of Aggressive COVID-19. Antioxidants 2021, 10, 1341. [Google Scholar] [CrossRef]
- Orlowski, S.; Mourad, J.J.; Gallo, A.; Bruckert, E. Coronaviruses, cholesterol and statins: Involvement and application for Covid-19. Biochimie 2021, 189, 51–64. [Google Scholar] [CrossRef]
- Sanders, D.W.; Jumper, C.C.; Ackerman, P.J.; Bracha, D.; Donlic, A.; Kim, H.; Kenney, D.; Castello-Serrano, I.; Suzuki, S.; Tamura, T.; et al. SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation. eLife 2021, 10. [Google Scholar] [CrossRef]
- Petersen, J.; Drake, M.J.; Bruce, E.A.; Riblett, A.M.; Didigu, C.A.; Wilen, C.B.; Malani, N.; Male, F.; Lee, F.H.; Bushman, F.D.; et al. The major cellular sterol regulatory pathway is required for Andes virus infection. PLoS Pathog. 2014, 10, e1003911. [Google Scholar] [CrossRef]
- Charlton, F.W.; Hover, S.; Fuller, J.; Hewson, R.; Fontana, J.; Barr, J.N.; Mankouri, J. Cellular cholesterol abundance regulates potassium accumulation within endosomes and is an important determinant in bunyavirus entry. J. Biol. Chem. 2019, 294, 7335–7347. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Simoneau, C.R.; Kulsuptrakul, J.; Bouhaddou, M.; Travisano, K.A.; Hayashi, J.M.; Carlson-Stevermer, J.; Zengel, J.R.; Richards, C.M.; Fozouni, P.; et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell 2021, 184, 106–119.e114. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ahn, J.H.; Park, H.H.; Kim, H.N.; Kim, H.; Yoo, Y.; Shin, H.; Hong, K.S.; Jang, J.G.; Park, C.G.; et al. COVID-19-activated SREBP2 disturbs cholesterol biosynthesis and leads to cytokine storm. Signal Transduct. Target. Ther. 2020, 5, 186. [Google Scholar] [CrossRef]
- Lim, H.I.; Hajjar, K.A. Annexin A2 in Fibrinolysis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2021, 22, 6836. [Google Scholar] [CrossRef]
- Fairoozy, R.H.; Cooper, J.; White, J.; Giambartolomei, C.; Folkersen, L.; Wannamethee, S.G.; Jefferis, B.J.; Whincup, P.; Ben-Shlomo, Y.; Kumari, M.; et al. Identifying low density lipoprotein cholesterol associated variants in the Annexin A2 (ANXA2) gene. Atherosclerosis 2017, 261, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, K.; Saavedra, Y.G.; Canuel, M.; Routhier, S.; Desjardins, R.; Hamelin, J.; Mayne, J.; Lazure, C.; Seidah, N.G.; Day, R. Annexin A2 reduces PCSK9 protein levels via a translational mechanism and interacts with the M1 and M2 domains of PCSK9. J. Biol. Chem. 2014, 289, 17732–17746. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.R.; Skeate, J.G.; Kast, W.M. Annexin A2 in Virus Infection. Front. Microbiol. 2018, 9, 2954. [Google Scholar] [CrossRef] [Green Version]
- Erol, A. Role of oxidized LDL-induced “trained macrophages” in the pathogenesis of COVID-19 and benefits of pioglitazone: A hypothesis. Diabetes Metab. Syndr. 2020, 14, 713–714. [Google Scholar] [CrossRef]
- Kzhyshkowska, J. Multifunctional receptor stabilin-1 in homeostasis and disease. Sci. World J. 2010, 10, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Krusell, L. Cross-talk between endocytic clearance and secretion in macrophages. Immunobiology 2009, 214, 576–593. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.; Hajjar, D.P.; Silverstein, R.L. CD36: A class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Investig. 2001, 108, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Neculai, D.; Kain, K.C. CD36 and malaria: Friends or foes? A decade of data provides some answers. Trends Parasitol. 2014, 30, 436–444. [Google Scholar] [CrossRef]
- Erdman, L.K.; Cosio, G.; Helmers, A.J.; Gowda, D.C.; Grinstein, S.; Kain, K.C. CD36 and TLR interactions in inflammation and phagocytosis: Implications for malaria. J. Immunol. 2009, 183, 6452–6459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Means, T.K.; Mylonakis, E.; Tampakakis, E.; Colvin, R.A.; Seung, E.; Puckett, L.; Tai, M.F.; Stewart, C.R.; Pukkila-Worley, R.; Hickman, S.E.; et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J. Exp. Med. 2009, 206, 637–653. [Google Scholar] [CrossRef]
- Park, L.; Zhou, J.; Zhou, P.; Pistick, R.; El Jamal, S.; Younkin, L.; Pierce, J.; Arreguin, A.; Anrather, J.; Younkin, S.G.; et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc. Natl. Acad. Sci. USA 2013, 110, 3089–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatz, J.F.; Luiken, J.J. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie 2017, 136, 21–26. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, P.; Zhao, L.; Chen, Y.; Zhang, X.; Zeng, S.; Wei, L.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; et al. CD36 plays a negative role in the regulation of lipophagy in hepatocytes through an AMPK-dependent pathway. J. Lipid Res. 2019, 60, 844–855. [Google Scholar] [CrossRef] [Green Version]
- Chevrier, S.; Zurbuchen, Y.; Cervia, C.; Adamo, S.; Raeber, M.E.; de Souza, N.; Sivapatham, S.; Jacobs, A.; Bachli, E.; Rudiger, A.; et al. A distinct innate immune signature marks progression from mild to severe COVID-19. Cell Rep. Med. 2021, 2, 100166. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Khanna, P. Covid and cholesterol (C&C): Something to worry about or much ado about nothing? Trends Anaesth. Crit. Care 2021, 36, 39–40. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Huckriede, J.; Anderberg, S.B.; Morales, A.; de Vries, F.; Hultstrom, M.; Bergqvist, A.; Ortiz-Perez, J.T.; Sels, J.W.; Wichapong, K.; Lipcsey, M.; et al. Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients. Sci. Rep. 2021, 11, 15701. [Google Scholar] [CrossRef]
- Patel, D.; Jana, A.; Roy, A.; Pahan, K. Cinnamon and its Metabolite Protect the Nigrostriatum in a Mouse Model of Parkinson’s Disease Via Astrocytic GDNF. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2019, 14, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Kook, M.C.; Lee, J.H.; Shin, J.Y.; Park, J.; Bae, Y.K.; Choi, I.J.; Ryu, K.W.; Kim, Y.W. Peroxisome proliferator-activated receptor gamma upregulates galectin-9 and predicts prognosis in intestinal-type gastric cancer. Int. J. Cancer 2015, 136, 810–820. [Google Scholar] [CrossRef]
- Meoni, G.; Ghini, V.; Maggi, L.; Vignoli, A.; Mazzoni, A.; Salvati, L.; Capone, M.; Vanni, A.; Tenori, L.; Fontanari, P.; et al. Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 2021, 17, e1009243. [Google Scholar] [CrossRef] [PubMed]
| ID Patients | Discharged Alive or Died | Age | Gender | T °C | Oxygen Saturation | Respiration Rate | CT-SKAN * | Therapy | Day of Death |
|---|---|---|---|---|---|---|---|---|---|
| 38 34939 | Dead | 66 | Male | 37.1 | 65 | 26 | 4 | Tocilizumab 400 mg CVVH 1470 min | 28 |
| 98 36367 | Dead | 72 | Male | 37.5 | 88 | 22 | 2 | Ruxolitinib 10 mg pd | 19 |
| 101 37339 | Dead | 63 | Male | 36.9 | 80 | 25 | 4 | Tocilizumab 400 mg CVVH 1520 min | 30 |
| 114 37483 | Alive | 79 | Male | 36.7 | 92 | 22 | 3 | Baricitinib 4 mg pd | - |
| 51 35875 | Alive | 72 | Male | 38.0 | 92 | 22 | 4 | Tocilizumab 400 mg CVVH 1440 min | - |
| 96 36891 | Alive | 59 | Male | 37.6 | 86 | 27 | 4 | Tocilizumab 400 mg | - |
| 99 36444 | Alive | 63 | Male | 36.6 | 88 | 21 | 4 | Tocilizumab 400 mg CVVH min 1490 min | - |
| 126 37998 | Alive | 74 | Male | 36.8 | 89 | 22 | 4 | Baricitinib 4 mg pd | - |
| Gene | Pipeline 1 | Pipeline 2 | Pipeline 3 | |||
|---|---|---|---|---|---|---|
| log2FC | padj | log2FC | padj | log2FC | padj | |
| STAB1 | −1.67 | 5.75 × 10−9 | −1.69 | 4.02 × 10−9 | −1.83 | 5.15 × 10−6 |
| PPARG | −1.68 | 0.001 | −1.74 | 0.0045 | −1.86 | 0.0007 |
| CD36 | −1.22 | 3.92 × 10−5 | −1.25 | 5.47 × 10−5 | −1.38 | 9.89 × 10−5 |
| ITGAV | −0.93 | 0.0059 | −1.17 | 0.0008 | −1.15 | 0.0004 |
| ANXA2 | −1.15 | 4.83 × 10−5 | −1.23 | 0.0004 | −1.23 | 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlasov, I.; Panteleeva, A.; Usenko, T.; Nikolaev, M.; Izumchenko, A.; Gavrilova, E.; Shlyk, I.; Miroshnikova, V.; Shadrina, M.; Polushin, Y.; et al. Transcriptomic Profiles Reveal Downregulation of Low-Density Lipoprotein Particle Receptor Pathway Activity in Patients Surviving Severe COVID-19. Cells 2021, 10, 3495. https://doi.org/10.3390/cells10123495
Vlasov I, Panteleeva A, Usenko T, Nikolaev M, Izumchenko A, Gavrilova E, Shlyk I, Miroshnikova V, Shadrina M, Polushin Y, et al. Transcriptomic Profiles Reveal Downregulation of Low-Density Lipoprotein Particle Receptor Pathway Activity in Patients Surviving Severe COVID-19. Cells. 2021; 10(12):3495. https://doi.org/10.3390/cells10123495
Chicago/Turabian StyleVlasov, Ivan, Alexandra Panteleeva, Tatiana Usenko, Mikhael Nikolaev, Artem Izumchenko, Elena Gavrilova, Irina Shlyk, Valentina Miroshnikova, Maria Shadrina, Yurii Polushin, and et al. 2021. "Transcriptomic Profiles Reveal Downregulation of Low-Density Lipoprotein Particle Receptor Pathway Activity in Patients Surviving Severe COVID-19" Cells 10, no. 12: 3495. https://doi.org/10.3390/cells10123495
APA StyleVlasov, I., Panteleeva, A., Usenko, T., Nikolaev, M., Izumchenko, A., Gavrilova, E., Shlyk, I., Miroshnikova, V., Shadrina, M., Polushin, Y., Pchelina, S., & Slonimsky, P. (2021). Transcriptomic Profiles Reveal Downregulation of Low-Density Lipoprotein Particle Receptor Pathway Activity in Patients Surviving Severe COVID-19. Cells, 10(12), 3495. https://doi.org/10.3390/cells10123495