Neuromolecular Underpinnings of Negative Cognitive Bias in Depression
Abstract
1. Introduction
2. Pessimistic Judgment Bias
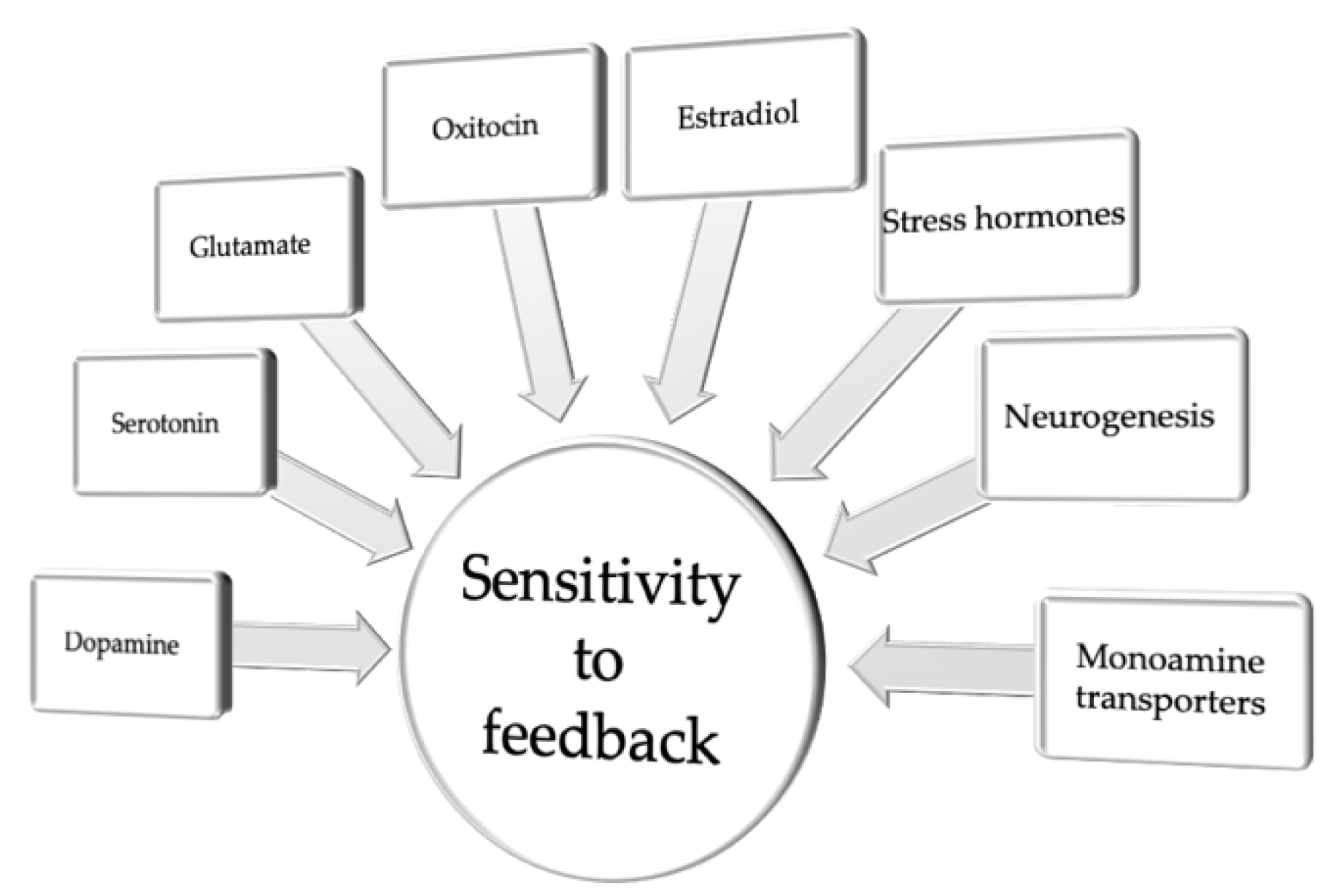
3. Biased Sensitivity to Feedback
4. Implications for Treatment
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 180–181. [Google Scholar] [CrossRef]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Pike, C.T.; Kessler, R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 2015, 76, 155–162. [Google Scholar] [CrossRef]
- DSM-5. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Chamberlain, S.R.; Sahakian, B.J. Cognition in mania and depression: Psychological models and clinical implications. Curr. Psychiatry Rep. 2004, 6, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Brittlebank, A.D.; Scott, J.; Williams, J.M.; Ferrier, I.N. Autobiographical memory in depression: State or trait marker? Br. J. Psychiatry 1993, 162, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, D.R.; Post, R.M. Impaired recognition of affect in facial expression in depressed patients. Biol. Psychiatry 1992, 31, 947–953. [Google Scholar] [CrossRef]
- Murphy, F.C.; Sahakian, B.J.; Rubinsztein, J.S.; Michael, A.; Rogers, R.D.; Robbins, T.W.; Paykel, E.S. Emotional bias and inhibitory control processes in mania and depression. Psychol. Med. 1999, 29, 1307–1321. [Google Scholar] [CrossRef]
- Douglas, K.M.; Porter, R.J. Recognition of disgusted facial expressions in severe depression. Br. J. Psychiatry 2010, 197, 156–157. [Google Scholar] [CrossRef]
- Maniglio, R.; Gusciglio, F.; Lofrese, V.; Belvederi Murri, M.; Tamburello, A.; Innamorati, M. Biased processing of neutral facial expressions is associated with depressive symptoms and suicide ideation in individuals at risk for major depression due to affective temperaments. Compr. Psychiatry 2014, 55, 518–525. [Google Scholar] [CrossRef]
- Beats, B.C.; Sahakian, B.J.; Levy, R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol. Med. 1996, 26, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Sahakian, B.J.; Herrod, J.J.; Robbins, T.W.; Paykel, E.S. Abnormal response to negative feedback in unipolar depression: Evidence for a diagnosis specific impairment. J. Neurol Neurosurg. Psychiatry 1997, 63, 74–82. [Google Scholar] [CrossRef]
- Clark, L.; Chamberlain, S.R.; Sahakian, B.J. Neurocognitive mechanisms in depression: Implications for treatment. Annu. Rev. Neurosci. 2009, 32, 57–74. [Google Scholar] [CrossRef]
- Noonan, M.P.; Kolling, N.; Walton, M.E.; Rushworth, M.F. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur. J. Neurosci. 2012, 35, 997–1010. [Google Scholar] [CrossRef]
- Wheeler, E.Z.; Fellows, L.K. The human ventromedial frontal lobe is critical for learning from negative feedback. Brain 2008, 131, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.; Dayan, P.; Schultz, J.; Deichmann, R.; Friston, K.; Dolan, R.J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 2004, 304, 452–454. [Google Scholar] [CrossRef]
- Palminteri, S.; Justo, D.; Jauffret, C.; Pavlicek, B.; Dauta, A.; Delmaire, C.; Czernecki, V.; Karachi, C.; Capelle, L.; Durr, A.; et al. Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron 2012, 76, 998–1009. [Google Scholar] [CrossRef]
- Tricomi, E.; Balleine, B.W.; O’Doherty, J.P. A specific role for posterior dorsolateral striatum in human habit learning. Eur. J. Neurosci. 2009, 29, 2225–2232. [Google Scholar] [CrossRef]
- Carver, C.S.; Scheier, M.F. Dispositional optimism. Trends Cogn. Sci. 2014, 18, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Scheier, M.F.; Carver, C.S. Dispositional optimism and physical health: A long look back, a quick look forward. Am. Psychol. 2018, 73, 1082. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F.; Seligman, M.E. Learned helplessness: Theory and evidence. J. Exp. Psychol. Gen. 1976, 105, 3–46. [Google Scholar] [CrossRef]
- Abramson, L.Y.; Seligman, M.E.; Teasdale, J.D. Learned helplessness in humans: Critique and reformulation. J. Abnorm. Psychol. 1978, 87, 49–74. [Google Scholar] [CrossRef]
- Beck, A.T. Depression: Clinical, Experimental, and Theoretical Aspects; Harper and Row: New York, NY, USA, 1967. [Google Scholar]
- Beck, A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 2008, 165, 969–977. [Google Scholar] [CrossRef]
- Alloy, L.B.; Ahrens, A.H. Depression and pessimism for the future: Biased use of statistically relevant information in predictions for self versus others. J. Pers. Soc. Psychol. 1987, 52, 366–378. [Google Scholar] [CrossRef]
- Korn, C.W.; Sharot, T.; Walter, H.; Heekeren, H.R.; Dolan, R.J. Depression is related to an absence of optimistically biased belief updating about future life events. Psychol. Med. 2014, 44, 579–592. [Google Scholar] [CrossRef]
- Zenger, M.; Glaesmer, H.; Höckel, M.; Hinz, A. Pessimism Predicts Anxiety, Depression and Quality of Life in Female Cancer Patients. Jpn. J. Clin. Oncol. 2010, 41, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Papciak, J.; Popik, P.; Fuchs, E.; Rygula, R. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav. Brain Res. 2013, 256, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Rygula, R.; Papciak, J.; Popik, P. Trait pessimism predicts vulnerability to stress-induced anhedonia in rats. Neuropsychopharmacology 2013, 38, 2188–2196. [Google Scholar] [CrossRef]
- Pessiglione, M.; Seymour, B.; Flandin, G.; Dolan, R.J.; Frith, C.D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 2006, 442, 1042–1045. [Google Scholar] [CrossRef]
- Schultz, W.; Tremblay, L.; Hollerman, J.R. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology 1998, 37, 421–429. [Google Scholar] [CrossRef]
- Sharot, T.; Guitart-Masip, M.; Korn, C.W.; Chowdhury, R.; Dolan, R.J. How dopamine enhances an optimism bias in humans. Curr. Biol. 2012, 22, 1477–1481. [Google Scholar] [CrossRef]
- Vellani, V.; de Vries, L.P.; Gaule, A.; Sharot, T. A selective effect of dopamine on information-seeking. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Enkel, T.; Gholizadeh, D.; von Bohlen Und Halbach, O.; Sanchis-Segura, C.; Hurlemann, R.; Spanagel, R.; Gass, P.; Vollmayr, B. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 2010, 35, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.J.; Paul, E.S.; Mendl, M. Animal behaviour: Cognitive bias and affective state. Nature 2004, 427, 312. [Google Scholar] [CrossRef]
- Rygula, R.; Papciak, J.; Popik, P. The effects of acute pharmacological stimulation of the 5-HT, NA and DA systems on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2014, 24, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.A.; Houghton, C.J.; Robinson, E.S.J. Behavioural and computational methods reveal differential effects for how delayed and rapid onset antidepressants effect decision making in rats. Eur. Neuropsychopharmacol. 2017, 27, 1268–1280. [Google Scholar] [CrossRef]
- Rygula, R.; Szczech, E.; Papciak, J.; Nikiforuk, A.; Popik, P. The effects of cocaine and mazindol on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. Behav. Brain Res. 2014, 270, 206–212. [Google Scholar] [CrossRef]
- Golebiowska, J.; Rygula, R. Effects of acute dopaminergic and serotonergic manipulations in the ACI paradigm depend on the basal valence of cognitive judgement bias in rats. Behav. Brain Res. 2017, 327, 133–143. [Google Scholar] [CrossRef]
- Sharp, T.; Foster, G.A. In vivo measurement using microdialysis of the release and metabolism of 5-hydroxytryptamine in raphe neurones grafted to the rat hippocampus. J. Neurochem. 1989, 53, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, J.S.; Aghajanian, G.K. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1987, 1, 3–9. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Zhang, W.; Carter, P.A.; Shaw, J.; Chernet, E.; Phebus, L.; Wong, D.T.; Perry, K.W. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology 2002, 160, 353–361. [Google Scholar] [CrossRef]
- Koch, S.; Perry, K.W.; Nelson, D.L.; Conway, R.G.; Threlkeld, P.G.; Bymaster, F.P. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: An in vivo microdialysis and receptor binding study. Neuropsychopharmacology 2002, 27, 949–959. [Google Scholar] [CrossRef]
- Invernizzi, R.; Belli, S.; Samanin, R. Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex. Brain Res 1992, 584, 322–324. [Google Scholar] [CrossRef]
- Pozzi, L.; Invernizzi, R.; Garavaglia, C.; Samanin, R. Fluoxetine increases extracellular dopamine in the prefrontal cortex by a mechanism not dependent on serotonin: A comparison with citalopram. J. Neurochem. 1999, 73, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.H.; Munafo, M.R.; Robinson, E.S. Investigating the psychopharmacology of cognitive affective bias in rats using an affective tone discrimination task. Psychopharmacology 2013, 226, 601–613. [Google Scholar] [CrossRef]
- Doyle, R.E.; Hinch, G.N.; Fisher, A.D.; Boissy, A.; Henshall, J.M.; Lee, C. Administration of serotonin inhibitor p-Chlorophenylalanine induces pessimistic-like judgement bias in sheep. Psychoneuroendocrinology 2011, 36, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Stracke, J.; Otten, W.; Tuchscherer, A.; Puppe, B.; Düpjan, S. Serotonin depletion induces pessimistic-like behavior in a cognitive bias paradigm in pigs. Physiol. Behav. 2017, 174, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Curzytek, K.; Kubera, M.; Trojan, E.; Wojcik, K.; Basta-Kaim, A.; Detka, J.; Maes, M.; Rygula, R. The effects of pessimism on cell-mediated immunity in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 295–303. [Google Scholar] [CrossRef]
- Drozd, R.; Cieslak, P.E.; Rychlik, M.; Rodriguez Parkitna, J.; Rygula, R. Cognitive Judgment Bias Interacts with Risk Based Decision Making and Sensitivity to Dopaminergic Challenge in Male Rats. Front. Behav. Neurosci. 2016, 10, 163. [Google Scholar] [CrossRef]
- Drozd, R.; Rojek-Sito, K.; Rygula, R. The trait ‘pessimism’ does not interact with cognitive flexibility but makes rats more vulnerable to stress-induced motivational deficits: Results from the attentional set-shifting task. Behav. Brain Res. 2017, 335, 199–207. [Google Scholar] [CrossRef]
- Rafa, D.; Kregiel, J.; Popik, P.; Rygula, R. Effects of optimism on gambling in the rat slot machine task. Behav. Brain Res. 2016, 300, 97–105. [Google Scholar] [CrossRef]
- Rygula, R.; Golebiowska, J.; Kregiel, J.; Kubik, J.; Popik, P. Effects of optimism on motivation in rats. Front. Behav. Neurosci. 2015, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Espigares, F.; Abad-Tortosa, D.; Varela, S.A.M.; Ferreira, M.G.; Oliveira, R.F. Short telomeres drive pessimistic judgement bias in zebrafish. Biol. Lett. 2021, 17, 20200745. [Google Scholar] [CrossRef]
- Roy, B.; Diez-Roux, A.V.; Seeman, T.; Ranjit, N.; Shea, S.; Cushman, M. Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA). Psychosom. Med. 2010, 72, 134–140. [Google Scholar] [CrossRef]
- Beevers, C.G.; Wells, T.T.; Ellis, A.J.; McGeary, J.E. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J. Abnorm Psychol. 2009, 118, 670–681. [Google Scholar] [CrossRef]
- Fox, E.; Ridgewell, A.; Ashwin, C. Looking on the bright side: Biased attention and the human serotonin transporter gene. Proc. Biol. Sci. 2009, 276, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Kloke, V.; Schreiber, R.S.; Bodden, C.; Möllers, J.; Ruhmann, H.; Kaiser, S.; Lesch, K.-P.; Sachser, N.; Lewejohann, L. Hope for the best or prepare for the worst? Towards a spatial cognitive bias test for mice. PLoS ONE 2014, 9, e105431. [Google Scholar] [CrossRef][Green Version]
- Krakenberg, V.; von Kortzfleisch, V.T.; Kaiser, S.; Sachser, N.; Richter, S.H. Differential Effects of Serotonin Transporter Genotype on Anxiety-Like Behavior and Cognitive Judgment Bias in Mice. Front. Behav. Neurosci. 2019, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Boddington, R.; Gómez Dunlop, C.A.; Garnham, L.C.; Ryding, S.; Abbey-Lee, R.N.; Kreshchenko, A.; Løvlie, H. The relationship between monoaminergic gene expression, learning, and optimism in red junglefowl chicks. Anim. Cogn. 2020, 23, 901–911. [Google Scholar] [CrossRef]
- Spasojević, J.; Alloy, L.B. Rumination as a common mechanism relating depressive risk factors to depression. Emotion 2001, 1, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.C.; Michael, A.; Robbins, T.W.; Sahakian, B.J. Neuropsychological impairment in patients with major depressive disorder: The effects of feedback on task performance. Psychol. Med. 2003, 33, 455–467. [Google Scholar] [CrossRef]
- Taylor Tavares, J.V.; Clark, L.; Furey, M.L.; Williams, G.B.; Sahakian, B.J.; Drevets, W.C. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 2008, 42, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Rygula, R.; Noworyta-Sokolowska, K.; Drozd, R.; Kozub, A. Using rodents to model abnormal sensitivity to feedback in depression. Neurosci. Biobehav. Rev. 2018, 95, 336–346. [Google Scholar] [CrossRef]
- Henriques, J.B.; Glowacki, J.M.; Davidson, R.J. Reward fails to alter response bias in depression. J. Abnorm Psychol. 1994, 103, 460–466. [Google Scholar] [CrossRef]
- McFarland, B.R.; Klein, D.N. Emotional reactivity in depression: Diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety 2009, 26, 117–122. [Google Scholar] [CrossRef]
- Robinson, O.J.; Cools, R.; Carlisi, C.O.; Sahakian, B.J.; Drevets, W.C. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am. J. Psychiatry 2012, 169, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Joormann, J. Cognition and depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef] [PubMed]
- Roiser, J.P.; Sahakian, B.J. Hot and cold cognition in depression. CNS Spectr. 2013, 18, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.D.; Kumar, P.; Ebmeier, K.P. Blunted response to feedback information in depressive illness. Brain 2007, 130, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Goetz, E.; Ostacher, M.; Iosifescu, D.V.; Perlis, R.H. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol. Psychiatry 2008, 64, 162–168. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Iosifescu, D.; Hallett, L.A.; Ratner, K.G.; Fava, M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J. Psychiatr. Res. 2008, 43, 76–87. [Google Scholar] [CrossRef]
- Frank, M.J.; Seeberger, L.C.; O’Reilly, R.C. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science 2004, 306, 1940–1943. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J. Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J. Cogn. Neurosci. 2005, 17, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Eisenegger, C.; Naef, M.; Linssen, A.; Clark, L.; Gandamaneni, P.K.; Müller, U.; Robbins, T.W. Role of dopamine D2 receptors in human reinforcement learning. Neuropsychopharmacology 2014, 39, 2366–2375. [Google Scholar] [CrossRef]
- Frank, M.J.; Hutchison, K. Genetic contributions to avoidance-based decisions: Striatal D2 receptor polymorphisms. Neuroscience 2009, 164, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.M.; Frank, M.J.; Larcher, K.; Fellows, L.K.; Clark, C.A.; Leyton, M.; Dagher, A. Striatal D1 and D2 signaling differentially predict learning from positive and negative outcomes. Neuroimage 2015, 109, 95–101. [Google Scholar] [CrossRef]
- Hikida, T.; Kimura, K.; Wada, N.; Funabiki, K.; Nakanishi, S. Distinct Roles of Synaptic Transmission in Direct and Indirect Striatal Pathways to Reward and Aversive Behavior. Neuron 2010, 66, 896–907. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Tye, L.D.; Kreitzer, A.C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat. Neurosci. 2012, 15, 816–818. [Google Scholar] [CrossRef]
- Alsiö, J.; Phillips, B.U.; Sala-Bayo, J.; Nilsson, S.R.O.; Calafat-Pla, T.C.; Rizwand, A.; Plumbridge, J.M.; López-Cruz, L.; Dalley, J.W.; Cardinal, R.N.; et al. Dopamine D2-like receptor stimulation blocks negative feedback in visual and spatial reversal learning in the rat: Behavioural and computational evidence. Psychopharmacology 2019, 236, 2307–2323. [Google Scholar] [CrossRef]
- Verharen, J.P.H.; Adan, R.A.H.; Vanderschuren, L. Differential contributions of striatal dopamine D1 and D2 receptors to component processes of value-based decision making. Neuropsychopharmacology 2019, 44, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J.; O’Reilly, R.C. A mechanistic account of striatal dopamine function in human cognition: Psychopharmacological studies with cabergoline and haloperidol. Behav. Neurosci. 2006, 120, 497–517. [Google Scholar] [CrossRef]
- Lim, T.V.; Cardinal, R.N.; Bullmore, E.T.; Robbins, T.W.; Ersche, K.D. Impaired Learning From Negative Feedback in Stimulant Use Disorder: Dopaminergic Modulation. Int. J. Neuropsychopharmacol. 2021, 24, 867–878. [Google Scholar] [CrossRef]
- McCabe, C.; Huber, A.; Harmer, C.J.; Cowen, P.J. The D2 antagonist sulpiride modulates the neural processing of both rewarding and aversive stimuli in healthy volunteers. Psychopharmacology 2011, 217, 271–278. [Google Scholar] [CrossRef]
- Jocham, G.; Klein, T.A.; Ullsperger, M. Differential modulation of reinforcement learning by D2 dopamine and NMDA glutamate receptor antagonism. J. Neurosci. 2014, 34, 13151–13162. [Google Scholar] [CrossRef]
- Cools, R.; Frank, M.J.; Gibbs, S.E.; Miyakawa, A.; Jagust, W.; D’Esposito, M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J. Neurosci. 2009, 29, 1538–1543. [Google Scholar] [CrossRef]
- Frank, M.J.; Moustafa, A.A.; Haughey, H.M.; Curran, T.; Hutchison, K.E. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc. Natl. Acad. Sci. USA 2007, 104, 16311–16316. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.A.; Neumann, J.; Reuter, M.; Hennig, J.; von Cramon, D.Y.; Ullsperger, M. Genetically determined differences in learning from errors. Science 2007, 318, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Jocham, G.; Klein, T.A.; Neumann, J.; von Cramon, D.Y.; Reuter, M.; Ullsperger, M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J. Neurosci. 2009, 29, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, S.R.; Muller, U.; Blackwell, A.D.; Clark, L.; Robbins, T.W.; Sahakian, B.J. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 2006, 311, 861–863. [Google Scholar] [CrossRef]
- Skandali, N.; Rowe, J.B.; Voon, V.; Deakin, J.B.; Cardinal, R.N.; Cormack, F.; Passamonti, L.; Bevan-Jones, W.R.; Regenthal, R.; Chamberlain, S.R.; et al. Dissociable effects of acute SSRI (escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology 2018, 43, 2645–2651. [Google Scholar] [CrossRef]
- Evers, E.A.; Cools, R.; Clark, L.; van der Veen, F.M.; Jolles, J.; Sahakian, B.J.; Robbins, T.W. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology 2005, 30, 1138–1147. [Google Scholar] [CrossRef]
- Cools, R.; Robinson, O.J.; Sahakian, B. Acute tryptophan depletion in healthy volunteers enhances punishment prediction but does not affect reward prediction. Neuropsychopharmacology 2008, 33, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Den Ouden, H.E.; Daw, N.D.; Fernandez, G.; Elshout, J.A.; Rijpkema, M.; Hoogman, M.; Franke, B.; Cools, R. Dissociable effects of dopamine and serotonin on reversal learning. Neuron 2013, 80, 1090–1100. [Google Scholar] [CrossRef]
- Willeit, M.; Praschak-Rieder, N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: A review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. Neuroimage 2010, 53, 878–892. [Google Scholar] [CrossRef]
- Bari, A.; Theobald, D.E.; Caprioli, D.; Mar, A.C.; Aidoo-Micah, A.; Dalley, J.W.; Robbins, T.W. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 2010, 35, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, C.; Sigrist, H.; Spinelli, S.; Lesch, K.P.; Sautter, E.; Seifritz, E.; Pryce, C.R. Establishing a probabilistic reversal learning test in mice: Evidence for the processes mediating reward-stay and punishment-shift behaviour and for their modulation by serotonin. Neuropharmacology 2012, 63, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Rygula, R.; Clarke, H.F.; Cardinal, R.N.; Cockcroft, G.J.; Xia, J.; Dalley, J.W.; Robbins, T.W.; Roberts, A.C. Role of Central Serotonin in Anticipation of Rewarding and Punishing Outcomes: Effects of Selective Amygdala or Orbitofrontal 5-HT Depletion. Cereb. Cortex. 2015, 25, 3064–3076. [Google Scholar] [CrossRef]
- Phillips, B.U.; Dewan, S.; Nilsson, S.R.O.; Robbins, T.W.; Heath, C.J.; Saksida, L.M.; Bussey, T.J.; Alsio, J. Selective effects of 5-HT2C receptor modulation on performance of a novel valence-probe visual discrimination task and probabilistic reversal learning in mice. Psychopharmacology 2018, 235, 2101–2111. [Google Scholar] [CrossRef]
- Cools, R.; Roberts, A.C.; Robbins, T.W. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 2008, 12, 31–40. [Google Scholar] [CrossRef]
- Robinson, O.J.; Cools, R.; Sahakian, B.J. Tryptophan depletion disinhibits punishment but not reward prediction: Implications for resilience. Psychopharmacology 2012, 219, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Bollen, E.; Rygula, R. Ketamine decreases sensitivity of male rats to misleading negative feedback in a probabilistic reversal-learning task. Psychopharmacology 2017, 234, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Seib, D.R.; Espinueva, D.F.; Floresco, S.B.; Snyder, J.S. A role for neurogenesis in probabilistic reward learning. Behav. Neurosci. 2020, 134, 283–295. [Google Scholar] [CrossRef]
- Veselic, S.; Jocham, G.; Gausterer, C.; Wagner, B.; Ernhoefer-Reßler, M.; Lanzenberger, R.; Eisenegger, C.; Lamm, C.; Losecaat Vermeer, A. A causal role of estradiol in human reinforcement learning. Horm. Behav. 2021, 134, 105022. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.Z.; Young, J.W.; He, Y.V.; Cope, Z.A.; Shilling, P.D.; Feifel, D. Oxytocin improves probabilistic reversal learning but not effortful motivation in Brown Norway rats. Neuropharmacology 2019, 150, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Bryce, C.A.; Floresco, S.B. Central CRF and acute stress differentially modulate probabilistic reversal learning in male and female rats. Behav. Brain Res. 2021, 397, 112929. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, A.; Srivastava, P.; Sharif, A.; Stech, K.; Floeder, J.; Yohn, S.E.; Samuels, B.A. Chronic corticosterone administration induces negative valence and impairs positive valence behaviors in mice. Transl. Psychiatry 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Goodwin, G.M.; Cowen, P.J. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br. J. Psychiatry 2009, 195, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, B.R. Cognitive neuropsychological theory: Reconciliation of psychological and biological approaches for depression. Pharm. Ther. 2019, 197, 38–51. [Google Scholar] [CrossRef]
- Beck, A.T. Cognitive therapy. A 30-year retrospective. Am. Psychol. 1991, 46, 368–375. [Google Scholar] [CrossRef]
- Serra, M.; Salgado-Pineda, P.; Delaveau, P.; Fakra, E.; Gasto, C.; Blin, O. Effects of antidepressant drugs on emotion. Clin. Neuropharmacol. 2006, 29, 170–185. [Google Scholar] [CrossRef]
- Godlewska, B.R.; Harmer, C.J. Cognitive neuropsychological theory of antidepressant action: A modern-day approach to depression and its treatment. Psychopharmacology 2021, 238, 1265–1278. [Google Scholar] [CrossRef]
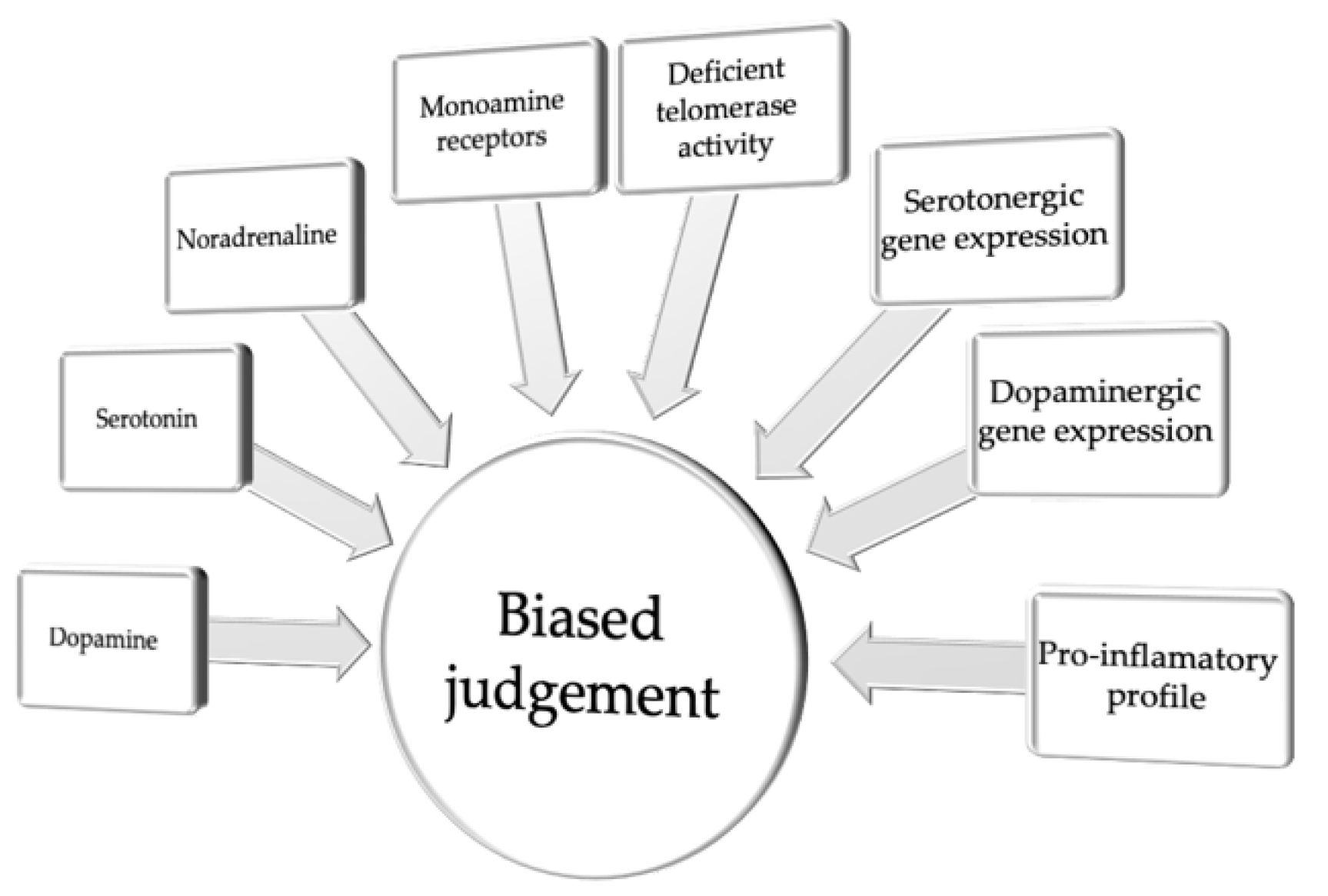
| Pessimistic Judgment Bias | |||||
|---|---|---|---|---|---|
| Target System | Drug Used | Study Subject | Test | Behavioral Outcome | Reference |
| DA | L-DOPA | Human | BUT | Impaired ability to update belief in response to undesirable information about the future, higher optimism | [31] |
| DA | L-DOPA | Human | SMT | Shift bias toward the information about gains | [32] |
| DA | d-amph | Rat | ACI | Optimism | [35] |
| DA | L-DOPA | Rat | ACI | Pessimistic shift in animals classified as optimistic | [38] |
| DA | Halo | Rat | ACI | Optimists became more pessimistic, while pessimists became more optimistic | [38] |
| 5-HT | Escit | Rat | ACI | Pessimistic shift in animals classified as optimistic | [38] |
| 5-HT | Cit | Human | BUT | No effect | [31] |
| 5-HT | Cit | Rat | ACI | Negative interpretation of ambiguous cues (a low dose) or optimistic judgment bias (a high dose) | [35] |
| 5-HT | Flx | Rat | ATDT | Pro-optimistic effects of chronic treatment | [45] |
| 5-HT | Flx | Rat | ACI | Pro-optimistic effects of chronic treatment | [36] |
| 5-HT | pCPA | Sheep | SDT | Pessimistic judgment bias | [46] |
| 5-HT | pCPA | Pig | ACI | Pessimistic judgment bias | [47] |
| NA | Desi | Rat | ACI | Pessimistic judgment bias | [35] |
| NA/DA | Mazin | Rat | ACI | Pessimistic judgment bias | [37] |
| NA | Rbx | Rat | ACI | Decrease in the positive processing | [45] |
| NA | Rbx, Cort | Rat | ACI | Pessimistic judgment bias | [33] |
| Sensitivity to feedback | |||||
| Target System | Drug Used | Study Subject | Test | Behavioral Outcome | Reference |
| DA | L-DOPA | Human | PLT | Higher sensitivity to positive than negative outcomes in PD patients on medication | [72] |
| DA | L-DOPA, Halo | Human | G/NG | Subjects treated with L-DOPA have a greater propensity to choose the most rewarding action relative to subjects treated with haloperidol | [29] |
| DA | Sulp | Human | RLT | Impairment in reward choice performance | [74] |
| DA | APTD | Human | PST | Improved learning from negative outcomes | [76] |
| DA | Quin | Rat | SPRL | Impaired learning from negative feedback | [79] |
| DA | Raclo, Quin | Rat | PRL | Negative feedback learning depends on D2R signaling, whereas learning from positive feedback depends on D1R signaling | [80] |
| DA | Halo | Human | PST | Increased DA release during positive feedback enhanced Go learning for good choices | [81] |
| DA | Ami, Prami | Human | RL | Impaired learning from negative feedback | [82] |
| 5-HT | Cit | Human | PRL | Low dose increased tendency to switch the response following negative feedback | [89] |
| 5-HT | Escit | Human | PRL | Impaired learning with uncertain reinforcement and enhanced responsivity to negative feedback | [90] |
| 5-HT | ATD | Human | PRL | Increased punishment prediction | [92] |
| 5-HT | SB 242084 | Mice | PRL | Reduced sensitivity to positive feedback | [98] |
| 5-HT | WAY 163909 | Mice | PRL | Increased sensitivity to positive feedback and decreased sensitivity to negative feedback | [98] |
| glu | Ket | Rat | PRL | Diminished the sensitivity of rats to negative feedback | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noworyta, K.; Cieslik, A.; Rygula, R. Neuromolecular Underpinnings of Negative Cognitive Bias in Depression. Cells 2021, 10, 3157. https://doi.org/10.3390/cells10113157
Noworyta K, Cieslik A, Rygula R. Neuromolecular Underpinnings of Negative Cognitive Bias in Depression. Cells. 2021; 10(11):3157. https://doi.org/10.3390/cells10113157
Chicago/Turabian StyleNoworyta, Karolina, Agata Cieslik, and Rafal Rygula. 2021. "Neuromolecular Underpinnings of Negative Cognitive Bias in Depression" Cells 10, no. 11: 3157. https://doi.org/10.3390/cells10113157
APA StyleNoworyta, K., Cieslik, A., & Rygula, R. (2021). Neuromolecular Underpinnings of Negative Cognitive Bias in Depression. Cells, 10(11), 3157. https://doi.org/10.3390/cells10113157






