Abstract
Depression is one of the most common mental disorders in the general population, and multiple mechanisms are involved in the etiology of this disease, including myelination. According to the Developmental Origins of Health and Disease (DOHaD) hypothesis, maternal diet affects the lifetime of the individual during adulthood and may contribute to the development of neuropsychiatric disorders. Additionally, the intensive processes of myelination contribute to the development of the central nervous system in the perinatal period, while any alterations during this crucial process providing the physiological functioning of neurons may lead to neuropsychiatric disorders in the next generation. The present review summarizes the current knowledge on the role of the myelin-related changes in depression, as well as the crosstalk among maternal malnutrition, myelination, and depression in preclinical and clinical settings.
1. Introduction
Depression is one of the most common mental disorders in the general population and affects all aspects of human life; it is characterized by sadness, guilt, loneliness, low self-worth, disturbed sleep or appetite, and loss of pleasure or concentration. Multiple potential mechanisms are involved in the etiology of depression, indicating the large heterogeneity of this disease [1]. It is now known that the etiology of this mental disorder is much more complex and related to several agents (i.e., reduced brain monoamine levels, stress, infections, genes, inflammation, etc.) [1,2], as well as disturbance within myelination during the prenatal period.
Maternal nutrition, stress, physical activity, substance use disorder, and food preferences seem to be crucial during the fetal period. The Developmental Origins of Health and Disease (DOHaD) hypothesis posits that the in utero and early postnatal experience of the maternal diet affects the lifetime of the individual during adulthood and may contribute to the development of neuropsychiatric disorders [3]. In the perinatal period, the intensive processes of myelination contribute to the development of the central nervous system (CNS) [4], while any alterations during this crucial process providing the physiological functioning of neurons may lead to neurological disorders, including depression [5].
Over the past decade, several studies have suggested that diet could play an important role in the treatment and prevention of depression, as well as the potential effect of maternal nutritional imbalance during gestation on the risk of depressive-like behavior in offspring [5,6,7,8,9,10,11]. The neonatal nervous tissue is extremely sensitive to alterations in local homeostasis, which may be interrupted by several agents, including the maternal diet during gestation and lactation. In the perinatal period, due to intensive processes of neurogenesis and gliogenesis, a huge number of neural progenitors arise, which contribute to the development of the CNS. The newly born neuroblasts give rise to motoneurons, sensory neurons, which are specialized neurons responsible for behavioral and cognitive functions, or interneurons responsible for the interactions between those cells [12,13]. Any alterations during the crucial processing and transmitting of signals within the nervous system providing the physiological functioning of neurons may lead to neurological disorders, including depression [5].
The present review summarizes the current knowledge on the role of the myelin-related changes in depression, as well as the crosslink among maternal malnutrition, myelination, and depression, and it discusses new directions in studies of depression based on a more comprehensive understanding of molecular determinants related to the myelination that participates in this brain disorder.
2. Myelin—Structure and Function
Myelin is a lipid-rich membrane structure produced by myelinating glial cells, the oligodendrocytes in the CNS, and Schwann cells in the peripheral nervous system. The myelin sheets are composed of (i) tightly packed spiraling layers of the membrane of mature oligodendrocytes that lack a cytoplasm, called compact myelin, and (ii) cytoplasmic noncompact regions connecting the oligodendrocyte cell body to the axonal side of the wraps and the nodes of Ranvier [14]. Unlike other biological membranes, compact myelin is made largely of lipids (70–80%) and is enriched in several proteins (20–30%) [15]. Myelin is a compact multilamellar and highly organized structure [14], which insulates nerve cell axons and facilitates signal conduction along with bigger distances as an evolutionary adaptation of the increasing body size of animals. The discontinuous structure of the myelin sheath results in saltatory conduction, whereby the action potential “jumps” from one node of Ranvier over a long-myelinated stretch of the axon. The nodes of Ranvier, as nonmyelinated axonal tracts, provide the regeneration of the neuronal action potential via voltage-sensitive sodium (Nav) channels required for the genesis of the action potential and subsequent repolarization using paranodal fast potassium (K+) channels [16]. Then, this electrical signal provokes the release of neurotransmitters [17] from both excitatory [18] and inhibitory [19] neurons, which bind to receptors on the adjacent postsynaptic cell in synapses. In addition to increasing the velocity of the action potential, myelin provides several critical functions in the nervous system, including mechanistic protection of the axon isolating the electrical signal, axonal growth, metabolism, integrity, and survival [20,21], regulation of neurotransmission [22], neuronal circuits [23], and synaptic plasticity [24].
3. Oligodendrocyte Differentiation, Maturation, and Myelination
Oligodendrocytes derive from highly migratory and proliferative neonatal progenitors, which appear in the developing nervous system starting from approximate midgestation. During development, oligodendrocytes undergo a multistep process of maturation to obtain the capacity for myelination. The majority of neonatal oligodendrocyte progenitors differentiate into mature oligodendrocytes, but a small number of neonatal progenitors remain and transform into adult oligodendrocyte progenitors [25,26,27,28].
The maturation of oligodendrocytes is characterized by several overlapping markers, whose expression is specific to the maturation steps [29] (Figure 1). The capability for myelination is related to the activation of several genes coding for specific protein and lipid components. Most oligodendrocytes develop during embryogenesis and early postnatal life from restricted periventricular germinal regions [30], and glial fibrillary acidic protein (GFAP)-positive astrocytes (type B cells) also generate a small number of nonmyelinating oligodendrocyte precursor cells and mature myelinating oligodendrocytes [31]. Oligodendroglia-biased neural stem cells present the A2B5 marker on the surface [32], while oligodendroglial progenitors are characterized by the presence of transcription factors, i.e., the homeodomain protein NK2 homeobox 2 (Nkx2.2) [33], oligodendrocyte transcription factor 1 and 2 (Olig1/Olig2) [34], SOX10, and myelin regulatory factor (Myrf) [35]. Oligodendrocyte progenitor cells also express platelet-derived growth factor receptor α (PDGFRα) [27] and the transmembrane chondroitin sulfate proteoglycans represented by neuron/glial antigen 2 (NG2) [36], which are involved in cell migration and the reaction in pathological states. Immature oligodendrocyte progenitor cells NG2-positive (NG2+) with branched cell processes may remain in their undifferentiated state with a high proliferative potential in the CNS [37]. Differentiation of oligodendrocyte progenitor cells, which are the ultimate precursors of myelinating cells, is characterized by the sulfatide O4 and O1 markers (pre-oligodendrocytes) [38]. After this stage, the differentiated oligodendrocytes form premyelinating and myelinated oligodendrocytes. Maturing cells are recognized by their two most characteristic markers: the intracellular presence of 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase), contributing to myelin synthesis and maintenance, and galactosylceramidase (GalC), providing the hydrolysis of certain galactolipids, which are integrative myelin molecules, while the presence of O4 in these cells vanishes [39]. Mature oligodendrocytes and the formed myelin sheaths may be recognized by the following markers: myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG) [40] (Figure 1). Myelin sheaths are very variable in patterning and coverage due to the fact that oligodendrocytes may form different numbers of myelin sheaths characterized by different lengths and thicknesses [14].
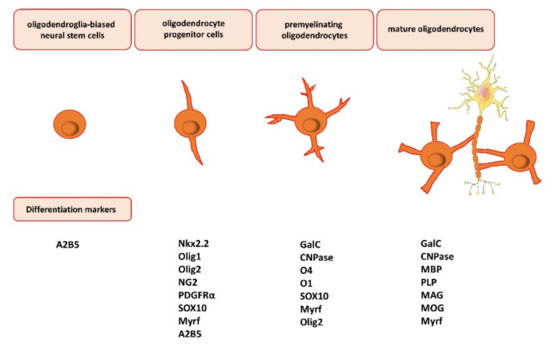
Figure 1.
Oligodendrocyte differentiation, maturation, and myelination. The maturation of oligodendrocytes is characterized by several overlapping markers, whose expression is specific to the maturation steps. Oligodendroglia-biased neural stem cells present the A2B5 marker on the surface. Oligodendrocyte progenitor cells express NK2 homeobox 2 (Nkx2.2), oligodendrocyte transcription factor 1 and 2 (Olig1/Olig2) (nuclear or cytosolic), chondroitin sulfate proteoglycans, represented by neuron/glial antigen 2 (NG2), platelet-derived growth factor receptor α (PDGFRα), transcription factor SOX10, and myelin regulatory factor (Myrf). Next, premyelinating oligodendrocytes express the intracellular presence of 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase) and galactosylceramidase (GalC). Lastly, mature oligodendrocytes may be recognized by the following markers: myelin basic protein (MBP), proteolipid protein (PLP), myelin-associated glycoprotein (MAG), and myelin oligodendrocyte glycoprotein (MOG).
In the CNS, myelination is accomplished by oligodendrocytes that are subjected to several processes including flattening, wrapping around the axons, and widening and elongating to form a myelin internode [41]. Polymerization of actin-related protein 2/3 complex (Arp2/3) is required for the first step of myelination [42], while actin depolymerization factor (ADF)/cofilin-1 is needed for the wrapping of the myelin [43]. The latter process is also endorsed by the CNPase, which prevents myelin compaction for extension of the myelin sheath [44]. Myelin thickness is regulated by the growth factors brain-derived neurotrophic factor (BDNF) and basic fibroblast growth factor (FGF2). FGF2, via the activation of the extracellular signal-related kinase (ERK) 1/2, targets the transcription factor Myrf, in addition to activating the mechanistic target of rapamycin complex 1 (mTORC1) in the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mTOR pathway [45]. The variability of myelin is also dependent on other glial cells, i.e., astrocytes, microglia, and cell types of the vasculature, by providing materials for myelin building and promoting the differentiation of oligodendrocytes [46].
In humans, myelination begins early in the third trimester, but the peak of myelination occurs primarily during the first 2 years after birth. It is mostly complete at 5 years of age but continues until early adulthood in some regions such as the frontal cortex [47]. Myelination follows a fixed spatiotemporal pattern; firstly, myelination appears in the brain stem, followed by the cerebellum and, finally, the cerebral cortex.
A large reservoir of oligodendrocyte progenitor cells persists in the adult brain after the myelination. Oligodendrocyte precursors express a wide array of neurotransmitter and neuroactive ligand receptors. Oligodendrocyte progenitor cells modulate interactions with neurons providing a prominent role in the neuronal activity in the brain [48]. First of all, these cells contribute to physical contact with neurons; secondly, oligodendrocyte progenitor cells are directly connected with glutamatergic and γ-aminobutyric acid (GABA)-ergic neurons that regulate its proliferation. NG2+ cells increase the frequency and amplitude of spontaneous glutamatergic inputs during the first 3 postnatal weeks [49], while transmission between GABAergic interneurons and NG2+ cells diminished during development [50]. Moreover, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-dependent signaling in oligodendrocyte lineage cells promotes oligodendrocyte development and myelination during postnatal development [48]. The expression of AMPA receptors in the oligodendrocyte progenitor cells helps to rapidly respond to synaptic input through membrane depolarization and local calcium influx [51]. An increase in the calcium permeability of AMPA receptors via modification of the amino acid composition of the GluA2 subunit enhanced the proliferation of the oligodendrocyte progenitor cells and reduced the number of mature oligodendrocytes [52], while loss of AMPA receptor signaling did not alter the proliferation of the oligodendrocyte progenitor cells but decreased the survival of mature oligodendrocytes [53]. In addition to neurotransmitter receptors to amino acids (glutamate and GABA), oligodendrocyte progenitor cells express receptors for other neuromodulators (ATP, acetylcholine, histamine, norepinephrine, serotonin, dopamine) and neuropeptides (substance P, angiotensin II, bradykinin). Activation of these receptors induces intracellular calcium signaling, suggesting that oligodendroglia can sense and respond to numerous neuronal-activity-dependent signals and that the microenvironment may regulate their cellular behavior [48]. Taken together, myelin and oligodendrocyte lineage cells through different effects on cellular processes may function as a central player to link previously proposed theories of depression and may play a significant role in the pathogenesis of this disease.
4. Myelin-Related Changes in Depression
4.1. Preclinical Studies
At the preclinical level, impaired myelination is linked to a “depressive-like” phenotype. In fact, in a model induced by chronic unpredictable stress (CUS), a lower volume of white matter [54] and the medial prefrontal cortex [55], along with reduced length and volume of the myelinated fibers and myelin sheath thickness [54], was observed, while a reduced number of oligodendrocytes was seen in the prelimbic cortex [56] in depressed rats. It seems that stress induces myelin structure alterations, which in turn may trigger depression, rather than the depression itself provoking myelin dysfunction. At the same time, the protein expression of CNPase and MBP and the MBP intensity were reduced in the medial prefrontal cortex [55], whereas reduced protein levels of MBP and Olig2 were observed in the hippocampus [57] in these rats (Table 1). Interestingly, a running exercise reversed the depression-like phenotype in stressed animals and had a positive effect on the differentiation of oligodendrocytes, myelinated fibers, and myelin-forming ability in these brain regions [54,55,57].

Table 1.
Myelin-related changes in depression: animal studies.
A similar myelin-related structural effect of chronic stress was seen in the medial prefrontal cortex of mice [60] and rat pups after maternal separation [65]. In fact, hypomyelination and a reduction in the number of oligodendroglial lineage cells and mature oligodendrocytes were presented in this structure, which impaired cognitive functions in mice subjected to chronic stress [60] and in rat pups separated from their dam 3 h daily during the first three postnatal weeks [65]. The lack of maternal care during early life alters myelination in the medial prefrontal cortex immediately after stress exposition and even in adult rats [65]. It should be highlighted that maternal separation reduces histone deacetylases 1/2 (HDAC1/2), and this reduction promotes the Wnt signaling pathway, which in turn negatively regulates the development of oligodendrocytes and impairs the oligodendrocyte precursor cell proliferation and oligodendrocyte maturation [65]. These data highlight the different neurobiological effects of chronic stress exposure on myelin disruption, which may contribute to behavioral changes. Additionally, 4-week chronic variable stress evoked downregulation of genes related to oligodendrocytes and myelin in the medial prefrontal cortex and nucleus accumbens of mice, which was observed even after 1 week of stress with weaker behavioral effects [62]. However, upregulation of myelin-related transcripts was observed in the corpus callosum of mice following 4 weeks of stress [62]. This finding conflicts with data in mice, in which 3 weeks of stress evoked narrowing of the nodes and paranodes of Ranvier, redistribution of contactin-associated protein (Caspr) and voltage-dependent potassium channel (Kv1.1) in the corpus callosum, and decreased activity in white matter, suggesting the association of morphological changes in oligodendrocytes with an inhibition of axonal activity [61] (Table 1). Additionally, using an oligodendrocyte primary culture subjected to chronic stress, it was shown that altered organization of the nodes of Ranvier was related to inhibition of the transcription of metabotropic glutamate receptors [61].
In a very recent study by Kurokawa and coworkers (2020), major myelin proteins and mature oligodendrocytes were decreased in the hippocampus of stress-maladaptive but not stress-adaptive mice following chronic exposure to restraint stress [70]. On the other hand, stress related to maternal separation 3 h daily during the first three postnatal weeks did not change myelination in the corpus callosum, striatum, or hippocampus at postnatal days (PNDs) 21 and 60 [65], whereas myelination was not altered following 8-week social isolation in the corpus callosum and nucleus accumbens of isolated mice [63]. Prolonged social isolation in mice induced behavioral, transcriptional, and ultrastructural changes in oligodendrocytes in the prefrontal cortex [63,64], in addition to provoking changes within nuclear chromatin condensation as an additional marker related to the maturation of oligodendrocytes, which in turn contributed to impaired adult myelination of the prefrontal cortex [63]. The latter changes were normalized after reintroduction into a social environment only when isolation was in juvenile and adult mice [63]. Contrastingly, mice isolated immediately after weaning evoked myelin alterations in the prefrontal cortex and changes in the prefrontal cortex-dependent cognitive behavior that did not recover upon reintroduction to a regular environment, suggesting that the disturbed myelination during this critical period has functional consequences in adulthood [64] (Table 1).
Reductions in myelin thickness, length of internodes, and myelinated segments in the medial prefrontal cortex were specific to mice that displayed social avoidance behavior (“susceptible” mice) during chronic social defeat stress, while decreased MBP labeling was observed in mice both susceptible and resilient to stress in the nucleus accumbens [69]. Additionally, in the medial prefrontal cortex of susceptible mice, reduced numbers of mature oligodendrocytes were correlated with a decrease in repressive histone methylation marks associated with differentiation (histone 3 lysine 9 trimethylation; H3K9me3) of oligodendrocytes [69]. Another study also confirmed the structural changes in the prefrontal cortex following chronic social defeat stress; the authors highlighted that this kind of stress provokes downregulation of myelin-related genes, and they provided evidence for the molecular basis of altered myelination in this structure [68]. These data parallel observations in the hippocampus and prefrontal cortex in stress-induced (social defeat stress) and genetic (learned helplessness) [67] animal models of depression, where reduced levels of oligodendrocyte precursor cells were observed. It should be noted that social defeat stress reduced NG2 glial secretion of FGF2 in stress-susceptible mice, and the latter change by itself is sufficient to induce depression-like behavior, which was presented using the transgenic line of mice with NG2 ablation, comparable to stress-induced behavior [67]. These data highlight the different myelin-related effects of chronic stress exposure, which could lead to a depression-like phenotype. Moreover, clemastine, an antimuscarinic compound, administered orally for 2 weeks in adult mice following social isolation showed antidepressant-like effects via enhancement of oligodendrocyte progenitor differentiation and epigenetic changes (higher levels of H3K9me3) in the oligodendrocytes of the prefrontal cortex [72]. These data not only link the oligodendrocyte dysfunction with depressive-like behavior but also strongly support the engagement of myelin-related changes in depression and the efficacy of a promyelinating drug in this disorder in preclinical studies.
According to these observations, in which the myelination is damped during depression (above), antidepressant drugs should reverse and/or normalize this alteration. In fact, it was shown that fluoxetine, a selective serotonin reuptake inhibitor, administered chronically reversed the suppressive effect of chronic unpredictable mild stress (CUMS) on myelin-related gene expression in the amygdala [58,59] and cingulate cortex [59]. In contrast, neither chronic defeat stress nor chronic fluoxetine treatment affected the level of newly generated cells differentiated into oligodendrocyte precursor cells (NG2+) in the medial prefrontal cortex of rats [66]. Moreover, fluoxetine administered to rhesus monkeys did not change the packing of oligodendrocytes and size of oligodendrocyte cell bodies; however, this drug was administered to naïve animals not introduced into the depression model [73]. Furthermore, imipramine reversed the impaired myelination (reduced levels of myelin-related proteins, number of nodes of Ranvier, and numbers of mature and immature oligodendrocytes) induced by removal of the olfactory bulbs in mice and improved depression-like behavior [71] (Table 1). These data seem to support the participation of the disturbance of myelin function and myelination in the pathophysiology of depression. Because there have been no studies with other antidepressant drugs, further research is urgently needed to validate the exact relationship between antidepressant effects and myelination.
4.2. Human Studies
Myelin-related changes in depression were also documented in human studies. In fact, in patients with major depressive disorder (MDD), myelin levels were reduced in the whole brain and nucleus accumbens, whereas, in the lateral prefrontal cortex, myelin levels were decreased in depressed patients with a greater number of depressive episodes [74]. Depression was related to lower white-matter microstructural integrity and gray-matter loss in several brain areas [75,76,77,78]. Additionally, a reduction in the integrity of the macromolecular protein was seen in patients with late-life MDD in white-matter tracts and subcortical nuclei [79], as well as in multiple left-hemisphere frontostriatal and limbic regions, the thalamus, the corpus callosum, and occipital white matter [61,80] compared with controls. Similarly, patients with treatment-resistant depression had a reduced integrity of myelin in task-positive network regions (bilateral precentral gyrus and left middle occipital lobe) and in default-mode network regions (left precuneus and left temporal lobe) [81]. On the other hand, antidepressant treatment evoked greater functional connectivity in the frontal precentral gyrus compared to never depressed old adults, but there was no difference between groups concerning white-matter hyperintensity burden points [82]. A meta-analysis of studies examining white-matter hyperintensities in mood disorder patients showed that depressed patients (i.e., unipolar and bipolar) with a history of suicide had higher deep-white matter hyperintensities and periventricular hyperintensities compared to patients without attempted suicide [83].
Postmortem studies on the brains of depressed patients confirmed the lower intensity of myelin staining in the dorsolateral prefrontal cortex regions in MDD individuals [84] and unipolar and bipolar affective disorders [85]. The reduction in the number of glial cells was significant in subgroups of subjects with MDD or bipolar disorder with a clear family history of depression in the subgenual part of the prefrontal cortex [86]. Additionally, MDD subjects had a greater mean myelin cross-sectional area and myelin thickness per axon in the corpus callosum genu [87], whereas a reduced level of mean myelin cross-sectional area was seen in the splenium of the corpus callosum [88]. A morphometric study also showed a reduction in the numerical density of oligodendroglial cells in the dorsolateral prefrontal cortex [73,89,90,91], frontopolar cortices [92], CA1 pyramidal layer of the hippocampus [93], and amygdala [94] of individuals with MDD, as well as in the prefrontal cortex [89,90,91], caudate nucleus [95], and CA1 pyramidal layer and left alveus of the hippocampus [93] of brains from subjects with bipolar disorder (Table 2). These changes provide evidence for the lowered density of oligodendroglial cells in depression that may contribute to the atrophy of neurons and play a key role in the pathophysiology of this disorder.

Table 2.
Myelin-related changes in depression: human postmortem studies.
The molecular data from human depressed brains show that reduced levels of oligodendroglial cells correlate with downregulation of myelin-related genes and proteins related to transcription factors [67,97,99], oligodendrocyte function [97], myelin synthesis [59,97], and structural components of myelin sheaths [59,73,91,96,97,98,101] (Table 2). In contrast, increased expression of CNPase, OLIG1, and MOG mRNA was observed in the ventral prefrontal cortex of MDD subjects [73]. The authors suggested that these increased levels of gene expression probably compensated for the lower PLP1 level, which is responsible for about 50% of myelin in the CNS [102]. Interestingly, sex-dependent differences and brain-region-specific gene expression for oligodendrocytes were observed in the postmortem depressed brain [100]. Thus, the levels of these genes were either increased in the dorsolateral prefrontal cortex and subgenual anterior cingulate cortex or decreased in the amygdala of men with MDD, whereas, in women with MDD, these effects were opposite (reduced expression in the dorsolateral prefrontal cortex and subgenual anterior cingulate cortex but increased expression in the amygdala) [100]. These data show the opposite molecular signatures of MDD in men and women, which is consistent with the sex-specific changes in incidence, symptomatology, and neuroimaging in MDD patients [103].
Compared to the preclinical study in which genetic ablation of oligodendrocyte precursor cells provoked the depressive-like behavior in mice [67], human studies seem to be less convincing. Thus, studies in humans are correlative but did not demonstrate directly that myelin alterations are causally implicated in the pathogenesis of depression. However, these findings suggest that depressive disorders may be associated with abnormalities in the structural oligodendroglia, while altered gene expression represents a potential molecular mechanism for the degeneration of axons and the dysfunctional maturation of oligodendrocytes in depression.
5. Crosstalk among Maternal Malnutrition, Myelination, and Depression
5.1. Preclinical Studies
From the preclinical point of view, a modified maternal diet during pregnancy and lactation seems to play a critical role in producing a depression-like phenotype in offspring [6,7,8,9,10]. Since most of the brain development occurs prenatally and during lactation, while oligodendrocytes are extremely sensitive to the alteration in local homeostasis, and since its survival and/or maturation may be limited and arrested as a result of various kinds of pathological signals including environmental effects, the modified diet in mothers may be a crucial factor for programming severe changes in offspring. However, little is known about the effect of diet during gestation and lactation on the myelination in offspring brain.
A maternal high-fat diet 5 weeks before mating and during pregnancy and lactation reduced the level of oligodendrocyte precursors in the lateral cortex of offspring at PND 7 [104]. At the same time, a modified maternal diet evoked decreases in myelination in the medial cortex of male but not female offspring at PND 21 [104]. The latter changes were associated with disruption of iron regulation and inflammatory cytokine homeostasis in the offspring. Interestingly, these structural changes correlated with altered learning and memory behavior [104]. The latter changes were associated with changes in the iron-regulatory proteins (hepcidin, ferroportin, and l-ferritin) and disruption of iron regulation in pups born to high-fat diet-fed dams, which is a crucial component in the progression of myelination in developing neurons. Next, maternal-obesity-induced hepcidin dysregulation was associated with neuroinflammation and oxidative stress in pups at the critical period from birth through 21 days, when active brain growth, extension of neuronal processes, migrating oligodendrocytes, and myelination were the most extensive. Furthermore, an iron-deficient diet during gestation and lactation in female rats evoked a decrease in the diameter of myelinated axons and peak amplitudes of compound action potentials specific to these axons in the corpus callosum of offspring at PND 40, whereas a reduction in the dendritic length of pyramidal neurons in the hippocampus and a decrease in branching complexity in the cortex were observed at PND 21 [105]. Parallel to the structural myelin-related changes, these rats presented deficits in recognition memory at PND 40 [105]. Another study showed that these memory deficits were attenuated by prenatal choline supplementation and were related to the restoration of reduced hippocampal Mbp mRNA levels [106]. These data parallel observations in mouse offspring, which presented loss of social memory and sensorimotor gating deficits at the behavioral level, related to exposure to a high-fat diet in mothers for 4 weeks before mating, during gestation, and until weaning [107]. Reductions in the number and area of myelin cytosolic channels in the rostrum of the corpus callosum of adolescent male mouse offspring were found, whereas, in the hippocampus, a key projection region of the corpus callosum, the myelination-associated transcripts and myelin-promoting growth factors were decreased [107]. The above sex-specific transcriptomic changes are in line with the evidence suggesting that maternal-diet-induced epigenetic regulation in the brain is often sex-specific and depends on estrogen receptor expression, neuroinflammatory signals, neonatal hormone exposure, and cellular differences in genetic sex [108]. Additionally, reduced numbers of mature lysosomes and increased synaptic contacts were observed in the corpus callosum of offspring following a maternal high-fat diet without effects on the processes involved in the density, distribution, or maturation of oligodendrocytes, which suggests that maternal-diet-induced changes within the microglia may be involved in the modified myelination [107]. On the other hand, alcohol exposure during gestation and lactation in dams induced an alteration of myelin damage biomarkers in the prefrontal cortex, and this effect was mitigated by high-fat diet feeding during childhood and adolescence in female offspring, suggesting that the fatty diet may supply extra lipids that could overcome the deleterious effects of alcohol on myelination [109]. On the other hand, a diet enriched in n-3 polyunsaturated fatty acids (ꞷ-3 acids) in mice 2 months before conception and continued throughout lactation dose-dependently increased the expression levels of MBP in pup mice at PND 21 and 42 [110] and decreased apoptosis and hypomyelination in the lipopolysaccharide-induced white-matter injury [111], suggesting that maternal ꞷ-3 acids promote early brain development. Higher levels of docosahexaenoic acid (DHA) in the maternal diet increased the myelin content, altered the lipid composition of rat pup myelin, and increased latencies of the auditory startle response in rat pups [112]. Cotreatment with essential fatty acids and zinc during pregnancy led to greater MBP levels in the brainstem of piglets compared to the control or each supplement alone [113]. Similarly, lactoferrin, a component of maternal milk, supplemented in maternal food during lactation displayed neuroprotective effects on reduced lipopolysaccharide-induced ventriculomegaly, brain tissue loss, and microstructural modifications, including myelination deficit in mice pups [114].
In the latest study by Trujillo-Villarreal and coworkers (2021), it was shown that a cafeteria diet in mothers for 9 weeks (pre-pregnancy, pregnancy, and lactation) might prime depression-like behavior in the offspring, observed as a deficient motivation for natural rewards [115]. Structural studies presented a reduction in the local volume of the hippocampus, nucleus accumbens, and thalamus in offspring [115]. At the same time, a reduction in synaptic terminals, cell number, and myelin staining was seen in the hippocampus, which was accompanied by dysregulation in the hippocampal glutamatergic system [115]. These data seem to support the participation of maternal malnutrition in the myelin-related changes and depression-like phenotype in offspring.
Interestingly, chronic nutritional stress using a low-calorie protein diet in mothers during pregnancy and lactation produced long-term changes in network parameters in offspring brain [116] and in the optic nerve [117]; however, this altered diet did not change the long integrative myelinated tracks in mice pups, but it reduced the frequency of short tracks in the central brain regions [116]. The rats fed an iodine-deficient diet from 3 months before pregnancy to the end of lactation altered the hippocampal myelin at PND 14 and 21, whereas hypothyroxinemia reduced the expression of Olig2 and myelin-related proteins in these rats, suggesting that impairment of hippocampal myelinated growth may cause the neurological deficits and alterations of brain function in offspring [118].
In summary, since most brain development occurs prenatally and during lactation, maternal nutrition has been identified as a key factor for brain growth and maturation in offspring. According to preclinical research, a modified maternal diet provokes different myelin-related changes in rodents including gene and/or protein expression related to myelin, maturation of oligodendrocytes, and myelin structure changes, as a cellular mechanism that may contribute to behavioral alterations in offspring. Moreover, further research is needed to elucidate the molecular mechanisms related to myelin responsible for the development of depressive-like behavior in offspring exposed to a modified maternal diet, as well as search for contributory factors in the development of mental brain disorders.
5.2. Human Studies
In humans, there are several lines of evidence that maternal malnutrition is implicated in myelin-related alterations. A case study on a 9-month-old girl with vitamin B12 deficiency, whose mother was a strict vegetarian with a low socioeconomic status, showed psychomotor regression, hypotonia, and lethargy with cerebral atrophy and delayed myelination [119]. A similar study on a 5-month-old Italian male infant hospitalized because of poor weight gain, feeding difficulties, severe pallor, muscle hypotonia, and somnolence led to a diagnosis of having vitamin B12 and iron deficiency due to nutritional inadequacy (mother was a vegan treated with a multivitamin oral preparation during the second and third trimesters). This maternal malnutrition provoked mild dilatation of the lateral ventricles with diffuse delayed myelination in the brain [120], suggesting that altered myelin formation and integrity are related to dysregulated incorporation of fatty acids, as well as an accumulation of lactate or neurotoxic cytokines. These data only link maternal diet with myelination; however, further research is urgently needed to validate the clinical relationship between maternal diet and risk of depression in children.
The behavioral changes observed in early childhood may be associated with morphological, molecular, and functional alterations in the brain after a modified maternal diet. Importantly, several mechanisms including epigenetic (i.e., posttranslational modifications of histone proteins, noncoding RNAs, and DNA methylation), inflammatory, and hormonal may contribute to individual differences in predisposition to depression; however, further investigations are needed. In this regard, the maternal diet during gestation and lactation can lead to long-lasting alterations in the epigenome, which may induce long-term neurobiological modifications affecting synaptic function and structural plasticity.
6. Conclusions
This review can raise awareness regarding the higher risk of developing problems during delivery of children (including the transmission of disorders), thus allowing for a better understanding of the mechanisms of such transmission and ultimately preventing it. In fact, the diet is one of the main sources of developmental brain damage with possible long-life neurodevelopmental disabilities; thus, understanding the effects of maternal diet on the developing brain as well as on depression is an important public health concern worldwide. Poor antenatal and postnatal nutrition further prevents infants from attaining their full developmental potential and developing affective cognition and increases their susceptibility to psychiatric disorders. As presented in this review, preclinical and clinical studies show that depression is associated with myelin-related changes. Additionally, maternal malnutrition during pregnancy and lactation is associated with specific alterations in the myelination processes in offspring, which seem to contribute to neurobehavioral changes in adolescence and adulthood, including a depression-like phenotype. However, more studies are required to determine the exact outcome of this problem to break new ground in our understanding of the impact of a maternal high-fat diet during gestation and lactation on myelination in brain offspring and to provide novel mechanistic strategies underlying myelin modulation in the pathogenesis of depression.
Funding
This research was funded by the National Science Centre, Kraków, Poland, grant number UMO-2020/39/D/NZ5/02581.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the writing of the manuscript.
References
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef] [PubMed]
- Boda, E. Myelin and oligodendrocyte lineage cell dysfunctions: New players in the etiology and treatment of depression and stress-related disorders. Eur. J. Neurosci. 2019, 53, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef]
- Cortés-Albornoz, M.C.; García-Guáqueta, D.P.; Velez-van-Meerbeke, A.; Talero-Gutiérrez, C. Maternal nutrition and neurodevelopment: A scoping review. Nutrients 2021, 13, 3550. [Google Scholar] [CrossRef]
- Gawlinska, K.; Gawlinski, D.; Przegalinski, E.; Filip, M. Maternal high-fat diet during pregnancy and lactation provokes depressive-like behavior and influences the irisin/brain-derived neurotrophic factor axis and inflammatory factors in male and female offspring in rats. J. Physiol. Pharmacol. 2019, 70, 407–417. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Korostyński, M.; Borczyk, M.; Frankowska, M.; Piechota, M.; Filip, M.; Przegaliński, E. Maternal dietary patterns are associated with susceptibility to a depressive-like phenotype in rat offspring. Dev. Cogn. Neurosci. 2020, 47, 100879. [Google Scholar] [CrossRef]
- Gawliński, D.; Gawlińska, K.; Smaga, I. Maternal high-fat diet modulates Cnr1 gene expression in male rat offspring. Nutrients 2021, 13, 2885. [Google Scholar] [CrossRef]
- Bayandor, P.; Farajdokht, F.; Mohaddes, G.; Diba, R.; Hosseindoost, M.; Mehri, K.; Zavvari Oskuye, Z.; Babri, S. The effect of troxerutin on anxiety- and depressive-like behaviours in the offspring of high-fat diet fed dams. Arch. Physiol. Biochem. 2019, 125, 156–162. [Google Scholar] [CrossRef]
- Giriko, C.; Andreoli, C.A.; Mennitti, L.V.; Hosoume, L.F.; Souto Tdos, S.; Silva, A.V.; Mendes-da-Silva, C. Delayed physical and neurobehavioral development and increased aggressive and depression-like behaviors in the rat offspring of dams fed a high-fat diet. Int. J. Dev. Neurosci. 2013, 31, 731–739. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef]
- Budday, S.; Steinmann, P.; Kuhl, E. Physical biology of human brain development. Front. Cell Neurosci. 2015, 9, 257. [Google Scholar] [CrossRef]
- Jiang, X.; Nardelli, J. Cellular and molecular introduction to brain development. Neurobiol. Dis. 2016, 92, 3–17. [Google Scholar] [CrossRef]
- Bakhti, M.; Aggarwal, S.; Simons, M. Myelin architecture: Zippering membranes tightly together. Cell Mol. Life Sci. 2014, 71, 1265–1277. [Google Scholar] [CrossRef]
- Laule, C.; Vavasour, I.M.; Kolind, S.H.; Li, D.K.; Traboulsee, T.L.; Moore, G.R.; MacKay, A.L. Magnetic resonance imaging of myelin. Neurotherapeutics 2007, 4, 460–484. [Google Scholar] [CrossRef]
- Snaidero, N.; Simons, M. Myelination at a glance. J. Cell Sci. 2014, 127, 2999–3004. [Google Scholar] [CrossRef]
- Maheras, K.J.; Peppi, M.; Ghoddoussi, F.; Galloway, M.P.; Perrine, S.A.; Gow, A. Absence of claudin 11 in CNS myelin perturbs behavior and neurotransmitter levels in mice. Sci. Rep. 2018, 8, 3798. [Google Scholar] [CrossRef]
- Tomassy, G.S.; Berger, D.R.; Chen, H.H.; Kasthuri, N.; Hayworth, K.J.; Vercelli, A.; Seung, H.S.; Lichtman, J.W.; Arlotta, P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 2014, 344, 319–324. [Google Scholar] [CrossRef]
- Stedehouder, J.; Couey, J.J.; Brizee, D.; Hosseini, B.; Slotman, J.A.; Dirven, C.M.F.; Shpak, G.; Houtsmuller, A.B.; Kushner, S.A. Fast-spiking parvalbumin interneurons are frequently myelinated in the cerebral cortex of mice and humans. Cereb. Cortex 2017, 27, 5001–5013. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Brady, S.T.; Witt, A.S.; Kirkpatrick, L.L.; de Waegh, S.M.; Readhead, C.; Tu, P.H.; Lee, V.M. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J. Neurosci. 1999, 19, 7278–7288. [Google Scholar] [CrossRef]
- Jang, M.; Gould, E.; Xu, J.; Kim, E.J.; Kim, J.H. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. Elife 2019, 8, e42156. [Google Scholar] [CrossRef]
- Monje, M. Myelin plasticity and nervous system function. Annu. Rev. Neurosci. 2018, 41, 61–76. [Google Scholar] [CrossRef]
- Zemmar, A.; Chen, C.C.; Weinmann, O.; Kast, B.; Vajda, F.; Bozeman, J.; Isaad, N.; Zuo, Y.; Schwab, M.E. Oligodendrocyte- and neuron-specific Nogo-A restrict dendritic branching and spine density in the adult mouse motor cortex. Cereb. Cortex 2018, 28, 2109–2117. [Google Scholar] [CrossRef]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1392–1401. [Google Scholar] [CrossRef]
- Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science 2010, 330, 779–782. [Google Scholar] [CrossRef]
- Janowska, J.; Sypecka, J. Therapeutic strategies for leukodystrophic disorders resulting from perinatal asphyxia: Focus on myelinating oligodendrocytes. Mol. Neurobiol. 2018, 55, 4388–4402. [Google Scholar] [CrossRef]
- Warf, B.C.; Fok-Seang, J.; Miller, R.H. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J. Neurosci. 1991, 11, 2477–2488. [Google Scholar] [CrossRef]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, C.; Yaqubi, M.; Bilodeau, P.A.; Cui, Q.L.; Pernin, F.; Larochelle, C.; Ghadiri, M.; Xu, Y.K.T.; Kennedy, T.E.; Hall, J.; et al. Distinct function-related molecular profile of adult human A2B5-positive pre-oligodendrocytes versus mature oligodendrocytes. J. Neuropathol. Exp. Neurol. 2019, 78, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cai, J.; Wu, Y.; Wu, R.; Lee, J.; Fu, H.; Rao, M.; Sussel, L.; Rubenstein, J.; Qiu, M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 2001, 128, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Tatsumi, K.; Okuda, H.; Shiosaka, S.; Wanaka, A. Olig2-expressing progenitor cells preferentially differentiate into oligodendrocytes in cuprizone-induced demyelinated lesions. Neurochem. Int. 2009, 54, 192–198. [Google Scholar] [CrossRef]
- Hornig, J.; Fröb, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef]
- Hermann, A.; Brandt, M.D.; Loewenbrück, K.F.; Storch, A. “Silenced” polydendrocytes: A new cell type within the oligodendrocyte progenitor cell population? Cell Tissue Res. 2010, 340, 45–50. [Google Scholar] [CrossRef]
- Sommer, I.; Schachner, M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: An immunocytological study in the central nervous system. Dev. Biol. 1981, 83, 311–327. [Google Scholar] [CrossRef]
- Brunner, C.; Lassmann, H.; Waehneldt, T.V.; Matthieu, J.M.; Linington, C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2’,3’-cyclic nucleotide 3’-phosphodiesterase in the CNS of adult rats. J. Neurochem. 1989, 52, 296–304. [Google Scholar] [CrossRef]
- Inouye, H.; Kirschner, D.A. Evolution of myelin ultrastructure and the major structural myelin proteins. Brain Res. 2016, 1641, 43–63. [Google Scholar] [CrossRef]
- Snaidero, M.W.; Czopka, T.; Hekking, L.H.; Mathisen, C.; Verkleij, D.; Goebbels, S.; Edgar, J.; Merkler, D.; Lyons, D.A.; Nave, K.A.; et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 2014, 156, 277–290. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Fu, M.M.; Sloan, S.A.; Ibrahim, A.; Olson, A.; Zaremba, A.; Dugas, J.C.; Wienbar, S.; Caprariello, A.V.; Kantor, C.; et al. CNS myelin wrapping is driven by actin disassembly. Dev. Cell 2015, 34, 152–167. [Google Scholar] [CrossRef]
- Nawaz, S.; Sánchez, P.; Schmitt, S.; Snaidero, N.; Mitkovski, M.; Velte, C.; Brückner, B.R.; Alexopoulos, I.; Czopka, T.; Jung, S.Y.; et al. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev. Cell 2015, 34, 139–151. [Google Scholar] [CrossRef]
- Snaidero, N.; Velte, C.; Myllykoski, M.; Raasakka, A.; Ignatev, A.; Werner, H.B.; Erwig, M.S.; Möbius, W.; Kursula, P.; Nave, K.A.; et al. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017, 18, 314–323. [Google Scholar] [CrossRef]
- Furusho, M.; Ishii, A.; Bansal, R. Signaling by FGF receptor 2, not FGF receptor 1, regulates myelin thickness through activation of ERK1/2-MAPK, which promotes mTORC1 activity in an Akt-independent manner. J. Neurosci. 2017, 37, 2931–2946. [Google Scholar] [CrossRef]
- Hughes, A.N. Glial cells promote myelin formation and elimination. Front. Cell Dev. Biol. 2021, 9, 661486. [Google Scholar] [CrossRef]
- Vancamp, P.; Demeneix, B.A.; Remaud, S. Monocarboxylate transporter 8 deficiency: Delayed or permanent hypomyelination? Front. Endocrinol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Thornton, M.A.; Hughes, E.G. Neuron-oligodendroglia interactions: Activity-dependent regulation of cellular signaling. Neurosci. Lett. 2020, 727, 134916. [Google Scholar] [CrossRef]
- Mangin, J.M.; Kunze, A.; Chittajallu, R.; Gallo, V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J. Neurosci. 2008, 28, 7610–7623. [Google Scholar] [CrossRef]
- Balia, M.; Vélez-Fort, M.; Passlick, S.; Schäfer, C.; Audinat, E.; Steinhäuser, C.; Seifert, G.; Angulo, M.C. Postnatal down-regulation of the GABAA receptor γ2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex 2015, 25, 1114–1123. [Google Scholar] [CrossRef]
- Maldonado, P.P.; Angulo, M.C. Multiple modes of communication between neurons and oligodendrocyte precursor cells. Neuroscientist 2015, 21, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Kula, B.; Nagy, B.; Barzan, R.; Gall, A.; Ehrlich, I.; Kukley, M. In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 2018, 25, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Kougioumtzidou, E.; Shimizu, T.; Hamilton, N.B.; Tohyama, K.; Sprengel, R.; Monyer, H.; Attwell, D.; Richardson, W.D. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. Elife 2017, 6, e28080. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, F.; Luo, Y.; Chen, L.; Chao, F.; Tan, C.; Gao, Y.; Huang, C.; Zhang, L.; Liang, X.; et al. Exercise protects myelinated fibers of white matter in a rat model of depression. J. Comp. Neurol. 2018, 526, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, Q.; Wang, J.; Jiang, L.; Hu, M.; Jiang, Y.; Tang, J.; Liang, X.; Qi, Y.; Dou, X.; et al. Running exercise protects oligodendrocytes in the medial prefrontal cortex in chronic unpredictable stress rat model. Transl. Psychiatry 2019, 9, 322. [Google Scholar] [CrossRef]
- Banasr, M.; Valentine, G.W.; Li, X.Y.; Gourley, S.L.; Taylor, J.R.; Duman, R.S. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol. Psychiatry 2007, 62, 496–504. [Google Scholar] [CrossRef]
- Tang, J.; Liang, X.; Zhang, Y.; Chen, L.; Wang, F.; Tan, C.; Luo, Y.; Xiao, Q.; Chao, F.; Zhang, L.; et al. The effects of running exercise on oligodendrocytes in the hippocampus of rats with depression induced by chronic unpredictable stress. Brain Res. Bull. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Surget, A.; Wang, Y.; Leman, S.; Ibarguen-Vargas, Y.; Edgar, N.; Griebel, G.; Belzung, C.; Sibille, E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology 2009, 34, 1363–1380. [Google Scholar] [CrossRef]
- Sibille, E.; Wang, Y.; Joeyen-Waldorf, J.; Gaiteri, C.; Surget, A.; Oh, S.; Belzung, C.; Tseng, G.C.; Lewis, D.A. A molecular signature of depression in the amygdala. Am. J. Psychiatry 2009, 166, 1011–1024. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Luo, F.; Li, B. Chronic stress regulates NG2⁺ cell maturation and myelination in the prefrontal cortex through induction of death receptor 6. Exp. Neurol. 2016, 277, 202–214. [Google Scholar] [CrossRef]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 2016, 6, 23084. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; Hodes, G.E.; Russo, S.J.; Casaccia, P. Widespread transcriptional alternations in oligodendrocytes in the adult mouse brain following chronic stress. Dev. Neurobiol. 2018, 78, 152–162. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Z.; Tang, H.; Jiao, H.; Sun, X.; Cui, Q.; Luo, F.; Pan, H.; Ma, C.; Li, B. Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of Wnt signaling. Cereb. Cortex 2017, 27, 2871–2884. [Google Scholar] [CrossRef]
- Czéh, B.; Müller-Keuker, J.I.; Rygula, R.; Abumaria, N.; Hiemke, C.; Domenici, E.; Fuchs, E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: Hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology 2007, 32, 1490–1503. [Google Scholar] [CrossRef]
- Birey, F.; Kloc, M.; Chavali, M.; Hussein, I.; Wilson, M.; Christoffel, D.J.; Chen, T.; Frohman, M.A.; Robinson, J.K.; Russo, S.J.; et al. Genetic and stress-induced loss of NG2 glia triggers emergence of depressive-like behaviors through reduced secretion of FGF2. Neuron 2015, 88, 941–956. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Weigel, T.K.; Elkahloun, A.G.; Herkenham, M. Chronic social defeat reduces myelination in the mouse medial prefrontal cortex. Sci. Rep. 2017, 7, 46548. [Google Scholar] [CrossRef]
- Bonnefil, V.; Dietz, K.; Amatruda, M.; Wentling, M.; Aubry, A.V.; Dupree, J.L.; Temple, G.; Park, H.J.; Burghardt, N.S.; Casaccia, P.; et al. Region-specific myelin differences define behavioral consequences of chronic social defeat stress in mice. Elife 2019, 8, e40855. [Google Scholar] [CrossRef]
- Kurokawa, K.; Tsuji, M.; Takahashi, K.; Miyagawa, K.; Mochida-Saito, A.; Takeda, H. Leukemia inhibitory factor participates in the formation of stress adaptation via hippocampal myelination in mice. Neuroscience 2020, 446, 1–13. [Google Scholar] [CrossRef]
- Takahashi, K.; Kurokawa, K.; Hong, L.; Miyagawa, K.; Mochida-Saito, A.; Takeda, H.; Tsuji, M. Disturbance of prefrontal cortical myelination in olfactory bulbectomized mice is associated with depressive-like behavior. Neurochem. Int. 2021, 148, 105112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dupree, J.L.; Gacias, M.; Frawley, R.; Sikder, T.; Naik, P.; Casaccia, P. Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 2016, 36, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Mahajan, G.; Maciag, D.; Sathyanesan, M.; Iyo, A.H.; Moulana, M.; Kyle, P.B.; Woolverton, W.L.; Miguel-Hidalgo, J.J.; Stockmeier, C.A.; et al. Oligodendrocyte morphometry and expression of myelin-related mRNA in ventral prefrontal white matter in major depressive disorder. J. Psychiatr. Res. 2015, 65, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sacchet, M.D.; Gotlib, I.H. Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Sci. Rep. 2017, 7, 2200. [Google Scholar] [CrossRef]
- Smagula, S.F.; Aizenstein, H.J. Brain structural connectivity in late-life major depressive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 271–277. [Google Scholar] [CrossRef]
- Yamada, S.; Takahashi, S.; Ukai, S.; Tsuji, T.; Iwatani, J.; Tsuda, K.; Kita, A.; Sakamoto, Y.; Yamamoto, M.; Terada, M.; et al. Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: A tract-specific analysis study. J. Affect. Disord. 2015, 174, 542–548. [Google Scholar] [CrossRef]
- Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Yamamoto, A.; Kiuchi, K.; Kosaka, J.; Nagatsuka, K.; Iida, H.; Kudo, T. Microstructural differences in the corpus callosum in patients with bipolar disorder and major depressive disorder. J. Clin. Psychiatry 2017, 78, 99–104. [Google Scholar] [CrossRef]
- Zeng, L.L.; Liu, L.; Liu, Y.; Shen, H.; Li, Y.; Hu, D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS ONE 2012, 7, e44248. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Gupta, R.C.; Albert Thomas, M.; Alger, J.; Wyckoff, N.; Hwang, S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004, 130, 131–140. [Google Scholar] [CrossRef]
- Gunning-Dixon, F.M.; Hoptman, M.J.; Lim, K.O.; Murphy, C.F.; Klimstra, S.; Latoussakis, V.; Majcher-Tascio, M.; Hrabe, J.; Ardekani, B.A.; Alexopoulos, G.S. Macromolecular white matter abnormalities in geriatric depression: A magnetization transfer imaging study. Am. J. Geriatr. Psychiatry 2008, 16, 255–262. [Google Scholar] [CrossRef]
- Jia, Z.; Peng, W.; Chen, Z.; Sun, H.; Zhang, H.; Kuang, W.; Huang, X.; Lui, S.; Gong, Q. Magnetization transfer imaging of treatment-resistant depression. Radiology 2017, 284, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, C.; Tudorascu, D.L.; Butters, M.A.; Tamburo, E.; Patel, M.; Price, J.; Karp, J.F.; Reynolds, C.F., III; Aizenstein, H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013, 214, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, M.C.; Seixas, C.; Quarantini, L.C.; Miranda-Scippa, A.; Pompili, M.; Steffens, D.C.; Wenzel, A.; Lacerda, A.L.; de Oliveira, I.R. White matter hyperintensities and their association with suicidality in major affective disorders: A meta-analysis of magnetic resonance imaging studies. CNS Spectr. 2010, 15, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lake, E.M.R.; Steffler, E.A.; Rowley, C.D.; Sehmbi, M.; Minuzzi, L.; Frey, B.N.; Bock, N.A. Altered intracortical myelin staining in the dorsolateral prefrontal cortex in severe mental illness. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 369–376. [Google Scholar] [CrossRef]
- Regenold, W.T.; Phatak, P.; Marano, C.M.; Gearhart, L.; Viens, C.H.; Hisley, K.C. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007, 151, 179–188. [Google Scholar] [CrossRef]
- Ongür, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295. [Google Scholar] [CrossRef]
- Williams, M.R.; Sharma, P.; Fung, K.L.; Pearce, R.K.; Hirsch, S.R.; Maier, M. Axonal myelin increase in the callosal genu in depression but not schizophrenia. Psychol Med. 2015, 45, 2145–2155. [Google Scholar] [CrossRef]
- Williams, M.R.; Sharma, P.; Macdonald, C.; Pearce, R.K.B.; Hirsch, S.R.; Maier, M. Axonal myelin decrease in the splenium in major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 387–395. [Google Scholar] [CrossRef]
- Uranova, N.A.; Vostrikov, V.M.; Orlovskaya, D.D.; Rachmanova, V.I. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004, 67, 269–275. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Uranova, N.A.; Orlovskaya, D.D. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr. Res. 2007, 94, 273–280. [Google Scholar] [CrossRef]
- Kim, S.; Webster, M.J. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol. Psychiatry 2010, 15, 326–336. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nihonmatsu-Kikuchi, N.; Yu, X.; Ishimoto, K.; Hisanaga, S.I.; Tatebayashi, Y. A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol. Psychiatry 2011, 16, 1155–1158. [Google Scholar] [CrossRef]
- Gos, T.; Schroeter, M.L.; Lessel, W.; Bernstein, H.G.; Dobrowolny, H.; Schiltz, K.; Bogerts, B.; Steiner, J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: A postmortem study. J. Psychiatr. Res. 2013, 47, 1694–1699. [Google Scholar] [CrossRef]
- Hamidi, M.; Drevets, W.C.; Price, J.L. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol. Psychiatry 2004, 55, 563–569. [Google Scholar] [CrossRef]
- Vostrikov, V.M.; Uranova, N.A. Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophr. Res. 2020, 215, 211–216. [Google Scholar] [CrossRef]
- Honer, W.G.; Falkai, P.; Chen, C.; Arango, V.; Mann, J.J.; Dwork, A.J. Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience 1999, 91, 1247–1255. [Google Scholar] [CrossRef]
- Aston, C.; Jiang, L.; Sokolov, B.P. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol. Psychiatry 2005, 10, 309–322. [Google Scholar] [CrossRef]
- Novak, G.; Tallerico, T. Nogo A, B and C expression in schizophrenia, depression and bipolar frontal cortex, and correlation of Nogo expression with CAA/TATC polymorphism in 3’-UTR. Brain Res. 2006, 1120, 161–171. [Google Scholar] [CrossRef]
- Klempan, T.A.; Ernst, C.; Deleva, V.; Labonte, B.; Turecki, G. Characterization of QKI gene expression, genetics, and epigenetics in suicide victims with major depressive disorder. Biol. Psychiatry 2009, 66, 824–831. [Google Scholar] [CrossRef]
- Seney, M.L.; Huo, Z.; Cahill, K.; French, L.; Puralewski, R.; Zhang, J.; Logan, R.W.; Tseng, G.; Lewis, D.A.; Sibille, E. Opposite molecular signatures of depression in men and women. Biol. Psychiatry 2018, 84, 18–27. [Google Scholar] [CrossRef]
- Tanti, A.; Kim, J.J.; Wakid, M.; Davoli, M.A.; Turecki, G.; Mechawar, N. Child abuse associates with an imbalance of oligodendrocyte-lineage cells in ventromedial prefrontal white matter. Mol. Psychiatry 2018, 23, 2018–2028. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Sikes-Keilp, C.; Rubinow, D.R. In search of sex-related mediators of affective illness. Biol. Sex Differ. 2021, 12, 55. [Google Scholar] [CrossRef]
- Graf, A.E.; Lallier, S.W.; Waidyaratne, G.; Thompson, M.D.; Tipple, T.E.; Hester, M.E.; Trask, A.J.; Rogers, L.K. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain Behav. Immun. 2016, 58, 369–378. [Google Scholar] [CrossRef]
- Greminger, A.R.; Lee, D.L.; Shrager, P.; Mayer-Pröschel, M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J. Nutr. 2014, 144, 1058–1066. [Google Scholar] [CrossRef]
- Kennedy, B.C.; Dimova, J.G.; Siddappa, A.J.; Tran, P.V.; Gewirtz, J.C.; Georgieff, M.K. Prenatal choline supplementation ameliorates the long-term neurobehavioral effects of fetal-neonatal iron deficiency in rats. J. Nutr. 2014, 144, 1858–1865. [Google Scholar] [CrossRef]
- Bordeleau, M.; Fernández de Cossío, L.; Lacabanne, C.; Savage, J.C.; Vernoux, N.; Chakravarty, M.; Tremblay, M. Maternal high-fat diet modifies myelin organization, microglial interactions, and results in social memory and sensorimotor gating deficits in adolescent mouse offspring. Brain Behav. Immun. Health 2021, 15, 100281. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Duart-Castells, L.; Cantacorps, L.; López-Arnau, R.; Montagud-Romero, S.; Puster, B.; Mera, P.; Serra, D.; Camarasa, J.; Pubill, D.; Valverde, O.; et al. Effects of high-fat diet and maternal binge-like alcohol consumption and their influence on cocaine response in female mice offspring. Int. J. Neuropsychopharmacol. 2021, 24, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Fan, C.; Liu, X.; Xu, F.; Qi, K. Brain histological changes in young mice submitted to diets with different ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy and lactation. Clin. Nutr. 2011, 30, 659–667. [Google Scholar] [CrossRef]
- Tuzun, F.; Kumral, A.; Dilek, M.; Ozbal, S.; Ergur, B.; Yesilirmak, D.C.; Duman, N.; Yilmaz, O.; Ozkan, H. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J. Matern. Fetal Neonatal Med. 2012, 25, 849–854. [Google Scholar] [CrossRef]
- Haubner, L.; Sullivan, J.; Ashmeade, T.; Saste, M.; Wiener, D.; Carver, J. The effects of maternal dietary docosahexaenoic acid intake on rat pup myelin and the auditory startle response. Dev. Neurosci. 2007, 29, 460–467. [Google Scholar] [CrossRef]
- Vallet, J.L.; Rempel, L.A.; Miles, J.R.; Webel, S.K. Effect of essential fatty acid and zinc supplementation during pregnancy on birth intervals, neonatal piglet brain myelination, stillbirth, and preweaning mortality. J. Anim. Sci. 2014, 92, 2422–2432. [Google Scholar] [CrossRef]
- Ginet, V.; van de Looij, Y.; Petrenko, V.; Toulotte, A.; Kiss, J.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation reduces lipopolysaccharide-induced brain injury. Biofactors 2016, 42, 323–336. [Google Scholar] [CrossRef]
- Trujillo-Villarreal, L.A.; Romero-Díaz, V.J.; Marino-Martínez, I.A.; Fuentes-Mera, L.; Ponce-Camacho, M.A.; Devenyi, G.A.; Mallar Chakravarty, M.; Camacho-Morales, A.; Garza-Villarreal, E.E. Maternal cafeteria diet exposure primes depression-like behavior in the offspring evoking lower brain volume related to changes in synaptic terminals and gliosis. Transl. Psychiatry 2021, 11, 53. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Gleiser, P.M.; Bernal, V.; Hallgrímsson, B.; Gonzalez, P.N. Brain structural networks in mouse exposed to chronic maternal undernutrition. Neuroscience 2018, 380, 14–26. [Google Scholar] [CrossRef]
- Almeida, M.F.; Silveira, A.C.; Guedes, R.C.; Hokoç, J.N.; Martinez, A.M. Quantitative ultrastructural evidence of myelin malformation in optic nerves of rats submitted to a multideficient diet. Nutr. Neurosci. 2005, 8, 91–99. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Y.; Dong, J.; Min, H.; Song, B.; Shan, Z.; Teng, W.; Xi, Q.; Chen, J. Hypothyroxinemia induced by maternal mild iodine deficiency impairs hippocampal myelinated growth in lactational rats. Environ. Toxicol 2015, 30, 1264–1274. [Google Scholar] [CrossRef]
- Bousselamti, A.; El Hasbaoui, B.; Echahdi, H.; Krouile, Y. Psychomotor regression due to vitamin B12 deficiency. Pan Afr. Med. J. 2018, 30, 152. [Google Scholar] [CrossRef]
- Guez, S.; Chiarelli, G.; Menni, F.; Salera, S.; Principi, N.; Esposito, S. Severe vitamin B12 deficiency in an exclusively breastfed 5-month-old Italian infant born to a mother receiving multivitamin supplementation during pregnancy. BMC Pediatr. 2012, 12, 85. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).