Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases
Abstract
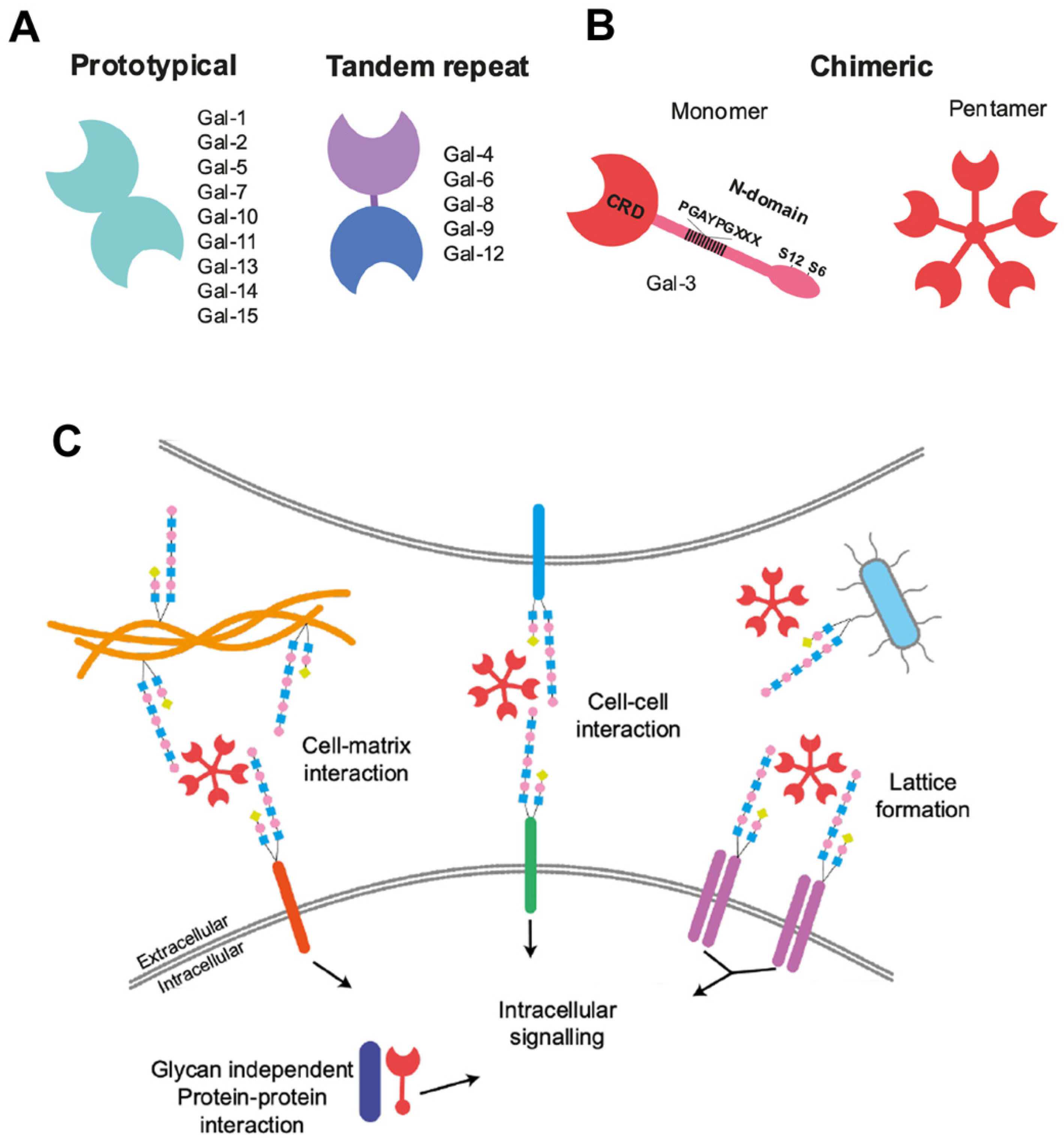
:1. Introduction
2. Galectin-3 Regulates Adult Subventricular Zone Cell Migration
3. Galectin-3 Functions in Gliogenesis and Gliomagenesis
3.1. Gal-3 Regulates Postnatal Gliogenesis
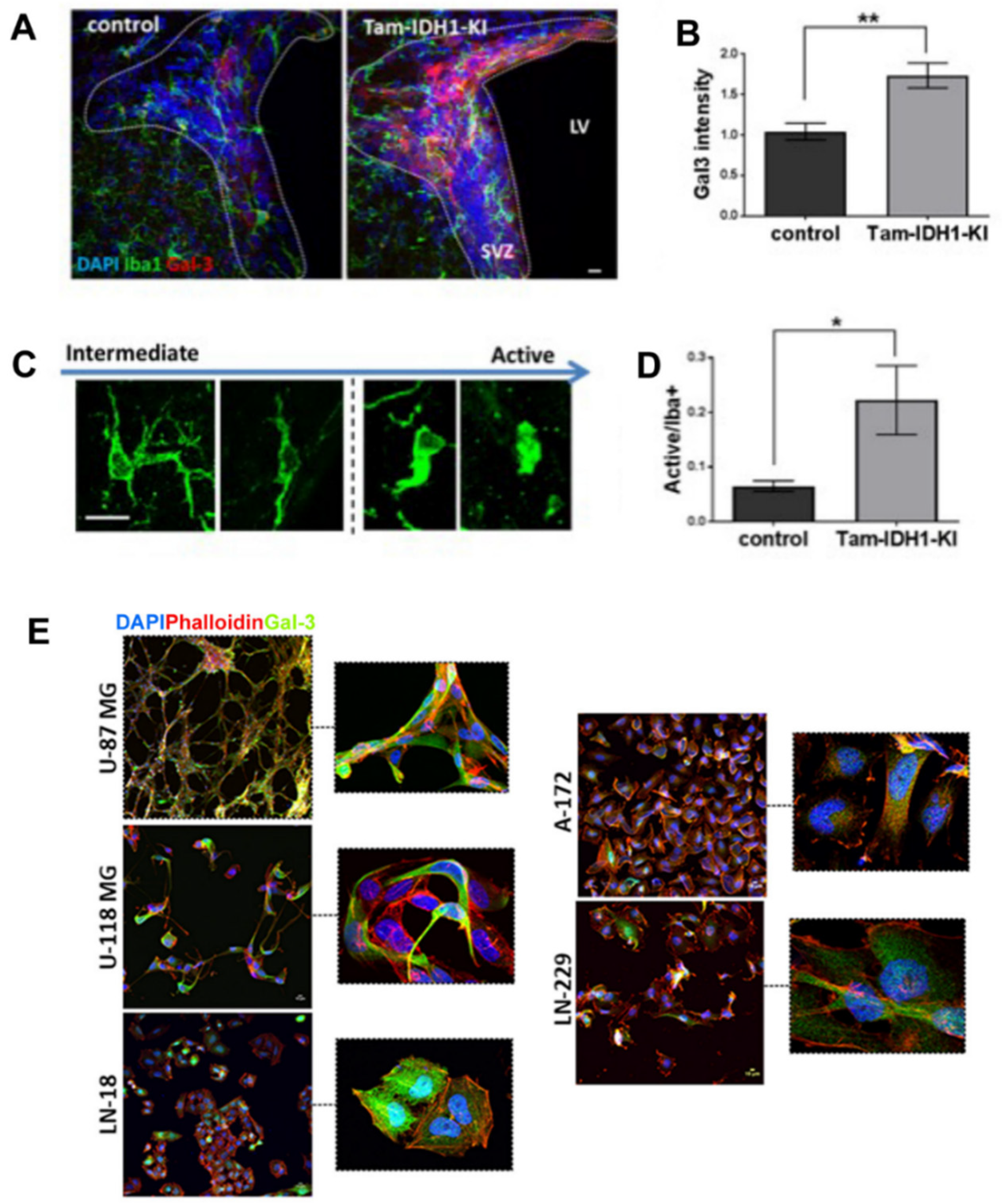
3.2. Gal-3 a Tumorigenesis Marker with Functional Relevance in Gliomagenesis
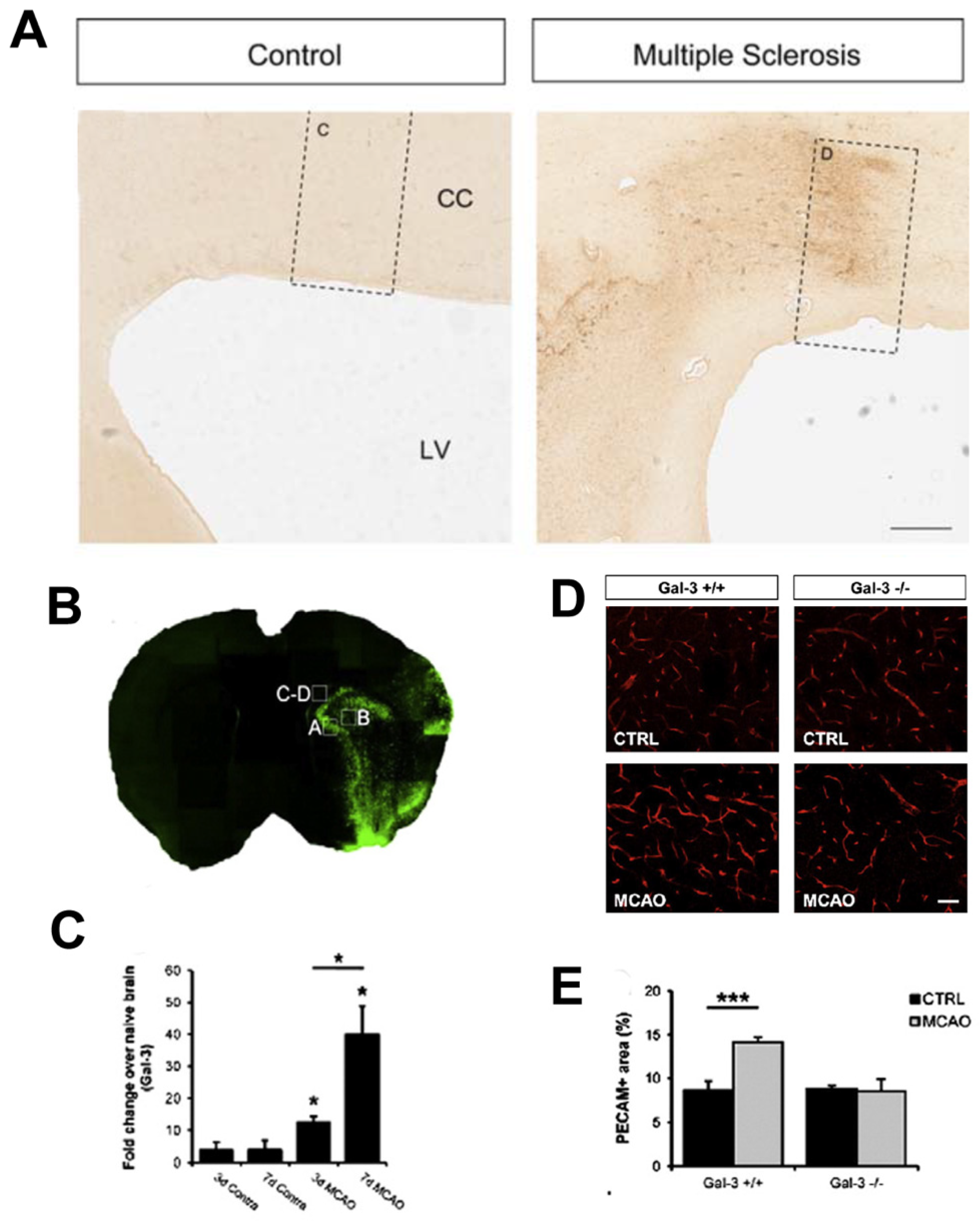
4. Galectin-3 Actions in Multiple Sclerosis and Stroke
4.1. MS, Gal-3 and the SVZ
4.2. Mgat5-Mediated Sugar Branching Affects MS
4.3. Gal-3 Expression following Demyelination in Humans
4.4. Animal Models of MS and Gal-3
4.5. Gal-3 Microglial, Inflammation and Molecular Mechanisms in Demyelination
4.6. Gal-3 Affects Oligodendrocyte Differentiation
4.7. Galectin-3 Functions in Adult Stroke
4.8. Gal-3 Functions in Neonatal Hypoxia Ischemia
5. Galectin-3 Can Exacerbate Alzheimer’s Disease and Diabetes
5.1. Gal-3 Elicits Alzheimer’s Pathology and Symptoms
5.2. The Links between AD, Diabetes, Gal-3 and Insulin
6. Galectin-3 Relevance in COVID-19 Brain Pathology
6.1. Galectins Mediate Viral Infection
6.2. Targeting Gal-3 in COVID-19
6.3. Does Gal-3 Block Pathogen Entry through the SVZ?
7. Galectin-1 Modulates Neurogenesis in the Healthy and Injured Brain
7.1. Gal-1 Functions in the SVZ
7.2. Gal-1 Function in the Diseased Brain
7.3. Gal-1 Regulates Hippocampal Neurogenesis
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef]
- Starossom, S.C.; Mascanfroni, I.D.; Imitola, J.; Cao, L.; Raddassi, K.; Hernandez, S.F.; Bassil, R.; Croci, D.O.; Cerliani, J.P.; Delacour, D.; et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 2012, 37, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, S.; Lu, M.; Bandyopadhyay, G.; Oh, D.; Imamura, T.; Johnson, A.M.; Sears, D.; Shen, Z.; Cui, B.; et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell 2016, 167, 973–984.e12. [Google Scholar] [CrossRef] [Green Version]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Galectins. In Essentials of Glycobiology, 3rd; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 469–480. [Google Scholar]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular functions of galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Tan, Y.; Zheng, Y.; Xu, D.; Sun, Z.; Yang, H.; Yin, Q. Galectin-3: A key player in microglia-mediated neuroinflammation and Alzheimer’s disease. Cell. Biosci. 2021, 11, 78. [Google Scholar] [CrossRef]
- Al-Dalahmah, O.; Campos Soares, L.; Nicholson, J.; Draijer, S.; Mundim, M.; Lu, V.M.; Sun, B.; Tyler, T.; Adorjan, I.; O’Neill, E.; et al. Galectin-3 modulates postnatal subventricular zone gliogenesis. Glia 2020, 68, 435–450. [Google Scholar]
- Walther, M.; Kuklinski, S.; Pesheva, P.; Guntinas-Lichius, O.; Angelov, D.N.; Neiss, W.F.; Asou, H.; Probstmeier, R. Galectin-3 is upregulated in microglial cells in response to ischemic brain lesions, but not to facial nerve axotomy. J. Neurosci. Res. 2000, 61, 430–435. [Google Scholar] [CrossRef]
- Akazawa, C.; Nakamura, Y.; Sango, K.; Horie, H.; Kohsaka, S. Distribution of the galectin-1 mRNA in the rat nervous system: Its transient upregulation in rat facial motor neurons after facial nerve axotomy. Neuroscience 2004, 125, 171–178. [Google Scholar] [CrossRef]
- Young, C.C.; Al-Dalahmah, O.; Lewis, N.J.; Brooks, K.J.; Jenkins, M.M.; Poirier, F.; Buchan, A.M.; Szele, F.G. Blocked angiogenesis in Galectin-3 null mice does not alter cellular and behavioral recovery after middle cerebral artery occlusion stroke. Neurobiol. Dis. 2014, 63, 155–164. [Google Scholar] [CrossRef]
- Boza-Serrano, A.; Ruiz, R.; Sanchez-Varo, R.; Garcia-Revilla, J.; Yang, Y.; Jimenez-Ferrer, I.; Paulus, A.; Wennstrom, M.; Vilalta, A.; Allendorf, D.; et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019, 138, 251–273. [Google Scholar] [CrossRef] [Green Version]
- Burguillos, M.A.; Svensson, M.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E.; Santiago, M.; Viceconte, N.; Oliva-Martin, M.J.; Osman, A.M.; et al. Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef] [Green Version]
- Van den Brule, F.A.; Buicu, C.; Sobel, M.E.; Liu, F.T.; Castronovo, V. Galectin-3, a laminin binding protein, fails to modulate adhesion of human melanoma cells to laminin. Neoplasma 1995, 42, 215–219. [Google Scholar]
- Kuwabara, I.; Liu, F.T. Galectin-3 promotes adhesion of human neutrophils to laminin. J. Immunol. 1996, 156, 3939–3944. [Google Scholar]
- Hikita, C.; Vijayakumar, S.; Takito, J.; Erdjument-Bromage, H.; Tempst, P.; Al-Awqati, Q. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J. Cell Biol. 2000, 151, 1235–1246. [Google Scholar]
- Ochieng, J.; Warfield, P.; Green-Jarvis, B.; Fentie, I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J. Cell. Biochem. 1999, 75, 505–514. [Google Scholar] [CrossRef]
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791. [Google Scholar] [CrossRef]
- Probstmeier, R.; Montag, D.; Schachner, M. Galectin-3, a beta-galactoside-binding animal lectin, binds to neural recognition molecules. J. Neurochem. 1995, 64, 2465–2472. [Google Scholar] [CrossRef]
- Partridge, E.A.; Le Roy, C.; Di Guglielmo, G.M.; Pawling, J.; Cheung, P.; Granovsky, M.; Nabi, I.R.; Wrana, J.L.; Dennis, J.W. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 2004, 306, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhou, L.; Liao, W.; Chen, H.; Du, Z.; Shao, C.; Wang, P.; Ding, K. HH1-1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr. Polym. 2019, 204, 111–123. [Google Scholar]
- Comte, I.; Kim, Y.; Young, C.C.; van der Harg, J.M.; Hockberger, P.; Bolam, P.J.; Poirier, F.; Szele, F.G. Galectin-3 maintains cell motility from the subventricular zone to the olfactory bulb. J. Cell Sci. 2011, 124 Pt 14, 2438–2447. [Google Scholar] [CrossRef] [Green Version]
- Markowska, A.I.; Jefferies, K.C.; Panjwani, N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 2011, 286, 29913–29921. [Google Scholar]
- Medzihradszky, K.F.; Kaasik, K.; Chalkley, R.J. Tissue-Specific Glycosylation at the Glycopeptide Level. Mol. Cell Proteom. 2015, 14, 2103–2110. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Lee, H.W.; Gu Kang, H.; La, S.H.; Choi, I.J.; Ro, J.Y.; Bresalier, R.S.; Song, J.; Chun, K.H. Ablation of galectin-3 induces p27(KIP1)-dependent premature senescence without oncogenic stress. Cell Death Differ. 2014, 21, 1769–1779. [Google Scholar]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737–6742. [Google Scholar]
- Shimura, T.; Takenaka, Y.; Tsutsumi, S.; Hogan, V.; Kikuchi, A.; Raz, A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004, 64, 6363–6367. [Google Scholar] [CrossRef] [Green Version]
- Al-Dalahmah, O.; Nicholson, J.; Draijer, S.; Soares, L.C.; Szele, F.G. Galectin-3 diminishes Wnt signaling in the postnatal subventricular zone. Stem Cells 2020, 38, 1149–1158. [Google Scholar]
- Banfer, S.; Schneider, D.; Dewes, J.; Strauss, M.T.; Freibert, S.A.; Heimerl, T.; Maier, U.G.; Elsasser, H.P.; Jungmann, R.; Jacob, R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. USA 2018, 115, E4396–E4405. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87.e8. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Srejovic, I.; Selakovic, D.; Jovicic, N.; Jakovljevic, V.; Lukic, M.L.; Rosic, G. Galectin-3: Roles in Neurodevelopment, Neuroinflammation, and Behavior. Biomolecules 2020, 10, 798. [Google Scholar]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [Green Version]
- Goings, G.E.; Kozlowski, D.A.; Szele, F.G. Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia 2006, 54, 329–342. [Google Scholar] [CrossRef]
- Bardella, C.; Al-Shammari, A.R.; Soares, L.; Tomlinson, I.; O’Neill, E.; Szele, F.G. The role of inflammation in subventricular zone cancer. Prog. Neurobiol. 2018, 170, 37–52. [Google Scholar]
- Altman, J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969, 137, 433–457. [Google Scholar] [CrossRef]
- Altman, J. Are new neurons formed in the brains of adult mammals? Science 1962, 135, 1127–1128. [Google Scholar]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar]
- Fuentealba, L.C.; Obernier, K.; Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell 2012, 10, 698–708. [Google Scholar]
- Doetsch, F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [CrossRef]
- Gheusi, G.; Lledo, P.M. Adult neurogenesis in the olfactory system shapes odor memory and perception. Prog. Brain. Res. 2014, 208, 157–175. [Google Scholar]
- Kalamakis, G.; Brune, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catala-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e14. [Google Scholar] [CrossRef] [Green Version]
- Dulken, B.W.; Leeman, D.S.; Boutet, S.C.; Hebestreit, K.; Brunet, A. Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep. 2017, 18, 777–790. [Google Scholar]
- Mizrak, D.; Levitin, H.M.; Delgado, A.C.; Crotet, V.; Yuan, J.; Chaker, Z.; Silva-Vargas, V.; Sims, P.A.; Doetsch, F. Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages. Cell Rep. 2019, 26, 394–406.e5. [Google Scholar]
- Hochgerner, H.; Zeisel, A.; Lonnerberg, P.; Linnarsson, S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 2018, 21, 290–299. [Google Scholar]
- Shin, J.; Berg, D.A.; Zhu, Y.; Shin, J.Y.; Song, J.; Bonaguidi, M.A.; Enikolopov, G.; Nauen, D.W.; Christian, K.M.; Ming, G.L.; et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell 2015, 17, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Valladares, M.; Moreno-Cugnon, L.; Silva, T.M.; Garces, J.P.; Saenz-Antonanzas, A.; Alvarez-Satta, M.; Matheu, A. CD8(+) T cells are increased in the subventricular zone with physiological and pathological aging. Aging Cell 2020, e13198. [Google Scholar] [CrossRef]
- Dulken, B.W.; Buckley, M.T.; Navarro Negredo, P.; Saligrama, N.; Cayrol, R.; Leeman, D.S.; George, B.M.; Boutet, S.C.; Hebestreit, K.; Pluvinage, J.V.; et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 2019, 571, 205–210. [Google Scholar]
- James, R.E.; Hillis, J.; Adorjan, I.; Gration, B.; Mundim, M.V.; Iqbal, A.J.; Majumdar, M.M.; Yates, R.L.; Richards, M.M.; Goings, G.E.; et al. Loss of galectin-3 decreases the number of immune cells in the subventricular zone and restores proliferation in a viral model of multiple sclerosis. Glia 2016, 64, 105–121. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar]
- Ernst, A.; Alkass, K.; Bernard, S.; SalSehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisen, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar]
- Paredes, M.F.; James, D.; Gil-Perotin, S.; Kim, H.; Cotter, J.A.; Ng, C.; Sandoval, K.; Rowitch, D.H.; Xu, D.; McQuillen, P.S.; et al. Extensive migration of young neurons into the infant human frontal lobe. Science 2016, 354, aaf7073. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Young, C.C.; van der Harg, J.M.; Lewis, N.J.; Brooks, K.J.; Buchan, A.M.; Szele, F.G. Ependymal ciliary dysfunction and reactive astrocytosis in a reorganized subventricular zone after stroke. Cereb. Cortex 2013, 23, 647–659. [Google Scholar]
- Hillis, J.M.; Davies, J.; Mundim, M.V.; Al-Dalahmah, O.; Szele, F.G. Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. J. Neuroinflammation 2016, 13, 190. [Google Scholar] [CrossRef] [Green Version]
- Zywitza, V.; Misios, A.; Bunatyan, L.; Willnow, T.E.; Rajewsky, N. Single-Cell Transcriptomics Characterizes Cell Types in the Subventricular Zone and Uncovers Molecular Defects Impairing Adult Neurogenesis. Cell Rep. 2018, 25, 2457–2469.e8. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Comte, I.; Szabo, G.; Hockberger, P.; Szele, F.G. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS ONE 2009, 4, e8122. [Google Scholar] [CrossRef]
- Levison, S.W.; Goldman, J.E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 1993, 10, 201–212. [Google Scholar]
- Sirko, S.; Irmler, M.; Gascon, S.; Bek, S.; Schneider, S.; Dimou, L.; Obermann, J.; De Souza Paiva, D.; Poirier, F.; Beckers, J.; et al. Astrocyte reactivity after brain injury-: The role of galectins 1 and 3. Glia 2015, 63, 2340–2361. [Google Scholar] [CrossRef] [Green Version]
- Lalancette-Hebert, M.; Swarup, V.; Beaulieu, J.M.; Bohacek, I.; Abdelhamid, E.; Weng, Y.C.; Sato, S.; Kriz, J. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. 2012, 32, 10383–10395. [Google Scholar] [PubMed] [Green Version]
- Siew, J.J.; Chen, H.M.; Chen, H.Y.; Chen, H.L.; Chen, C.M.; Soong, B.W.; Wu, Y.R.; Chang, C.P.; Chan, Y.C.; Lin, C.H.; et al. Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’s disease. Nat. Commun. 2019, 10, 3473. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Sun, G.; Li, W.; Yang, S.; Ye, P.; Zhao, C.; Yu, R.T.; Gage, F.H.; Evans, R.M.; Shi, Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 2010, 12, 31–40, sup pp. 1–9. [Google Scholar] [CrossRef] [Green Version]
- Piccin, D.; Morshead, C.M. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells 2011, 29, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Mirzadeh, Z.; Sakaguchi, M.; Yamashita, T.; Nikolcheva, T.; Gotoh, Y.; Peltz, G.; Gong, L.; Kawase, T.; Alvarez-Buylla, A.; et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 2007, 25, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Fischer, B.; Hurtado-Chong, A.; Draganova, K.; Cantu, C.; Zemke, M.; Sommer, L.; Butt, A.; Raineteau, O. Persistent Wnt/beta-Catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells 2014, 32, 1301–1312. [Google Scholar]
- Marinaro, C.; Pannese, M.; Weinandy, F.; Sessa, A.; Bergamaschi, A.; Taketo, M.M.; Broccoli, V.; Comi, G.; Gotz, M.; Martino, G.; et al. Wnt signaling has opposing roles in the developing and the adult brain that are modulated by Hipk1. Cereb. Cortex 2012, 22, 2415–2427. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Shimura, T.; Yajima, T.; Kubo, N.; Araki, K.; Tsutsumi, S.; Suzuki, H.; Kuwano, H.; Raz, A. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of beta-catenin. Int. J. Cancer J. Int. Du Cancer 2011, 129, 2775–2786. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Mazurek, N.; Liu, C.; Sun, Y.; Ding, Q.Q.; Liu, K.; Hung, M.C.; Bresalier, R.S. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009, 69, 1343–1349. [Google Scholar]
- Zhang, D.; Chen, Z.G.; Liu, S.H.; Dong, Z.Q.; Dalin, M.; Bao, S.S.; Hu, Y.W.; Wei, F.C. Galectin-3 gene silencing inhibits migration and invasion of human tongue cancer cells in vitro via downregulating beta-catenin. Acta Pharm. Sin. 2013, 34, 176–184. [Google Scholar]
- Colak, D.; Mori, T.; Brill, M.S.; Pfeifer, A.; Falk, S.; Deng, C.; Monteiro, R.; Mummery, C.; Sommer, L.; Gotz, M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J. Neurosci. 2008, 28, 434–446. [Google Scholar]
- Lim, D.A.; Tramontin, A.D.; Trevejo, J.M.; Herrera, D.G.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 2000, 28, 713–726. [Google Scholar] [PubMed] [Green Version]
- Gajera, C.R.; Emich, H.; Lioubinski, O.; Christ, A.; Beckervordersandforth-Bonk, R.; Yoshikawa, K.; Bachmann, S.; Christensen, E.I.; Gotz, M.; Kempermann, G.; et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J. Cell Sci. 2010, 123 Pt 11, 1922–1930. [Google Scholar]
- Mercier, F.; Douet, V. Bone morphogenetic protein-4 inhibits adult neurogenesis and is regulated by fractone-associated heparan sulfates in the subventricular zone. J. Chem. Neuroanat. 2014, 57–58, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Bonaguidi, M.A.; McGuire, T.; Hu, M.; Kan, L.; Samanta, J.; Kessler, J.A. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development 2005, 132, 5503–5514. [Google Scholar] [PubMed] [Green Version]
- Ciceroni, C.; Mosillo, P.; Mastrantoni, E.; Sale, P.; Ricci-Vitiani, L.; Biagioni, F.; Stocchi, F.; Nicoletti, F.; Melchiorri, D. mGLU3 metabotropic glutamate receptors modulate the differentiation of SVZ-derived neural stem cells towards the astrocytic lineage. Glia 2010, 58, 813–822. [Google Scholar] [CrossRef]
- Gomes, W.A.; Mehler, M.F.; Kessler, J.A. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev. Biol. 2003, 255, 164–177. [Google Scholar]
- Grinspan, J.B.; Edell, E.; Carpio, D.F.; Beesley, J.S.; Lavy, L.; Pleasure, D.; Golden, J.A. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J. Neurobiol. 2000, 43, 1–17. [Google Scholar] [CrossRef]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog. Neurobiol. 2013, 109, 28–41. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal. New Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Sowers, J.L.; Johnson, K.M.; Conrad, C.; Patterson, J.T.; Sowers, L.C. The role of inflammation in brain cancer. Adv. Exp. Med. Biol. 2014, 816, 75–105. [Google Scholar]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, D.; Sims-Mourtada, J. Wound healing and cancer stem cells: Inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis 2015, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.B.; Yoon, H.J.; Chang, C.Y.; Koh, H.S.; Jeon, S.H.; Park, E.J. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J. Immunol. 2010, 185, 7037–7046. [Google Scholar] [CrossRef] [Green Version]
- Neder, L.; Marie, S.K.; Carlotti, C.G., Jr.; Gabbai, A.A.; Rosemberg, S.; Malheiros, S.M.; Siqueira, R.P.; Oba-Shinjo, S.M.; Uno, M.; Aguiar, P.H.; et al. Galectin-3 as an immunohistochemical tool to distinguish pilocytic astrocytomas from diffuse astrocytomas, and glioblastomas from anaplastic oligodendrogliomas. Brain Pathol. 2004, 14, 399–405. [Google Scholar] [CrossRef]
- Strik, H.M.; Deininger, M.H.; Frank, B.; Schluesener, H.J.; Meyermann, R. Galectin-3: Cellular Distribution and Correlation with WHO-grade in Human Gliomas. J. Neuro-Oncol. 2001, 53, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.H.; Trautmann, K.; Meyermann, R.; Schluesener, H.J. Galectin-3 labeling correlates positively in tumor cells and negatively in endothelial cells with malignancy and poor prognosis in oligodendroglioma patients. Anticancer Res. 2002, 22, 1585–1592. [Google Scholar] [PubMed]
- Chen, A.; Jiang, Y.; Li, Z.; Wu, L.; Santiago, U.; Zou, H.; Cai, C.; Sharma, V.; Guan, Y.; McCarl, L.H.; et al. Chitinase-3-like 1 protein complexes modulate macrophage-mediated immune suppression in glioblastoma. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1(NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Fukumori, T.; Takenaka, Y.; Oka, N.; Yoshii, T.; Hogan, V.; Inohara, H.; Kanayama, H.-O.; Kim, H.-R.C.; Raz, A. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004, 64, 3376–3379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Sakai, T.; Sano, N.; Fukui, K. Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem. J. 2004, 380, 31–41. [Google Scholar]
- Yu, F.; Finley, R.L.; Raz, A.; Kim, H.-R.C. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria A role for synexin in galectin-3 translocation. J. Biol. Chem. 2002, 277, 15819–15827. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-R.C.; Lin, H.-M.; Biliran, H.; Raz, A. Cell Cycle Arrest and Inhibition of Anoikis by Galectin-3 in Human Breast Epithelial Cells. Cancer Res. 1999, 59, 4148–4154. [Google Scholar]
- Zhao, Q.; Barclay, M.; Hilkens, J.; Guo, X.; Barrow, H.; Rhodes, J.M.; Yu, L.-G. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 2010, 9, 154. [Google Scholar]
- Seguin, L.; Kato, S.; Franovic, A.; Camargo, M.F.; Lesperance, J.; Elliott, K.C.; Yebra, M.; Mielgo, A.; Lowy, A.M.; Husain, H.; et al. An integrin β₃-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014, 16, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Seguin, L.; Desgrosellier, J.S.; Weis, S.M.; Cheresh, D.A. Integrins and cancer: Regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015, 25, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Seguin, L.; Camargo, M.F.; Wettersten, H.I.; Kato, S.; Desgrosellier, J.S.; von Schalscha, T.; Elliott, K.C.; Cosset, E.; Lesperance, J.; Weis, S.M.; et al. Galectin-3, a Druggable Vulnerability for KRAS-Addicted Cancers. Cancer Discov. 2017, 7, 1464–1479. [Google Scholar]
- Steed, T.C.; Treiber, J.M.; Patel, K.; Ramakrishnan, V.; Merk, A.; Smith, A.R.; Carter, B.S.; Dale, A.M.; Chow, L.M.; Chen, C.C. Differential localization of glioblastoma subtype: Implications on glioblastoma pathogenesis. Oncotarget 2016, 7, 24899–24907. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Alvarez-Buylla, A.; Berger, M.S. Neural Stem Cells and the Origin of Gliomas. New Engl. J. Med. 2005, 353, 811–822. [Google Scholar] [CrossRef]
- Lim, D.A.; Cha, S.; Mayo, M.C.; Chen, M.-H.; Keles, E.; VandenBerg, S.; Berger, M.S. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-Oncology 2007, 9, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Fan, X.; Ma, J.; Ma, W.; Wang, R.; Jiang, T. Anatomical Involvement of the Subventricular Zone Predicts Poor Survival Outcome in Low-Grade Astrocytomas. PLoS ONE 2016, 11, e0154539. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Fan, X.; Ma, J.; Qiu, X.; Jiang, T. Association of MRI-classified subventricular regions with survival outcomes in patients with anaplastic glioma. Clin. Radiol. 2017, 72, 426.e1–426.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chaichana, K.L.; Kleinberg, L.; Ye, X.; Quinones-Hinojosa, A.; Redmond, K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother. Oncol. 2015, 116, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafri, N.F.; Clarke, J.L.; Weinberg, V.; Barani, I.J.; Cha, S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro. Oncol. 2013, 15, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Mistry, A.M.; Hale, A.T.; Chambless, L.B.; Weaver, K.D.; Thompson, R.C.; Ihrie, R.A. Influence of glioblastoma contact with the lateral ventricle on survival: A meta-analysis. J. Neurooncol. 2017, 131, 125–133. [Google Scholar]
- Vergani, F.; Martino, J.; Goze, C.; Rigau, V.; Duffau, H. World Health Organization Grade II gliomas and subventricular zone: Anatomic, genetic, and clinical considerations. Neurosurgery 2011, 68, 1293–1298, discussion 1298–1299. [Google Scholar]
- Young, G.S.; Macklin, E.A.; Setayesh, K.; Lawson, J.D.; Wen, P.Y.; Norden, A.D.; Drappatz, J.; Kesari, S. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J. Neurooncol. 2011, 104, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeberg, S.; König, L.; Bostel, T.; Harrabi, S.; Welzel, T.; Debus, J.; Combs, S.E. Glioblastoma Recurrence Patterns After Radiation Therapy With Regard to the Subventricular Zone. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Nair, V.; Jalali, R. Stem cell niche irradiation in glioblastoma: Providing a ray of hope? CNS Oncol. 2014, 3, 367–376. [Google Scholar] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [PubMed] [Green Version]
- Seguin, L.; Odouard, S.; Corlazzoli, F.; Haddad, S.A.; Moindrot, L.; Calvo Tardon, M.; Yebra, M.; Koval, A.; Marinari, E.; Bes, V.; et al. Macropinocytosis requires Gal-3 in a subset of patient-derived glioblastoma stem cells. Commun. Biol. 2021, 4, 718. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Harazono, Y.; Gao, X.; Hogan, V.; Raz, A. Galectin-3 Inhibits Osteoblast Differentiation through Notch Signaling. Neoplasia 2014, 16, 939–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.G.; Kim, D.-H.; Kim, S.-J.; Cho, Y.; Jung, J.; Jang, W.; Chun, K.-H. Galectin-3 supports stemness in ovarian cancer stem cells by activation of the Notch1 intracellular domain. Oncotarget 2016, 7, 68229–68241. [Google Scholar]
- Kuo, H.Y.; Hsu, H.T.; Chen, Y.C.; Chang, Y.W.; Liu, F.T.; Wu, C.W. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology 2016, 26, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazzoni, R.; Bentivegna, A. Role of Notch Signaling Pathway in Glioblastoma Pathogenesis. Cancers 2019, 11, 292. [Google Scholar] [CrossRef] [Green Version]
- Stockhausen, M.-T.; Kristoffersen, K.; Poulsen, H.S. The functional role of Notch signaling in human gliomas. Neuro. Oncol. 2010, 12, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Zong, H.; Ma, C.; Ming, X.; Shang, M.; Li, K.; He, X.; Du, H.; Cao, L. Epidermal growth factor receptor in glioblastoma. Oncol. Lett. 2017, 14, 512–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, S.N.; Sheldon, H.; Pereira, J.X.; Paluch, C.; Bridges, E.M.; El-Cheikh, M.C.; Harris, A.L.; Bernardes, E.S. Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget 2017, 8, 49484–49501. [Google Scholar] [CrossRef] [Green Version]
- Xi, G.; Best, B.; Mania-Farnell, B.; James, C.D.; Tomita, T. Therapeutic Potential for Bone Morphogenetic Protein 4 in Human Malignant Glioma. Neoplasia 2017, 19, 261–270. [Google Scholar]
- Sachdeva, R.; Wu, M.; Johnson, K.; Kim, H.; Celebre, A.; Shahzad, U.; Graham, M.S.; Kessler, J.A.; Chuang, J.H.; Karamchandani, J.; et al. BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma. Sci. Rep. 2019, 9, 14569. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, H.; Zeng, Z.; Yao, H.; Jiang, W.; Qu, H. Wnt/β-catenin signaling cascade: A promising target for glioma therapy. J. Cell. Physiol. 2019, 234, 2217–2228. [Google Scholar]
- Shimura, T.; Takenaka, Y.; Fukumori, T.; Tsutsumi, S.; Okada, K.; Hogan, V.; Kikuchi, A.; Kuwano, H.; Raz, A. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005, 65, 3535–3537. [Google Scholar] [CrossRef] [Green Version]
- Bardella, C.; Al-Dalahmah, O.; Krell, D.; Brazauskas, P.; Al-Qahtani, K.; Tomkova, M.; Adam, J.; Serres, S.; Lockstone, H.; Freeman-Mills, L.; et al. Expression of Idh1R132H in the Murine Subventricular Zone Stem Cell Niche Recapitulates Features of Early Gliomagenesis. Cancer Cell 2016, 30, 578–594. [Google Scholar]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Swoger, J.; Greene, S.; Saito, T.; Hurh, J.; Sweeley, C.; Leestma, J.; Mkrdichian, E.; Cerullo, L.; Nishikawa, A.; et al. β1,6-N-Acetylglucosamine-bearing N-Glycans in Human Gliomas: Implications for a Role in Regulating Invasivity. Cancer Res. 2000, 60, 134–142. [Google Scholar]
- Padhiar, A.A.; Fan, J.; Tang, Y.; Yu, J.; Wang, S.; Liu, L.; Niang, B.; Annani-Akollor, M.E.; Wang, L.; Wang, Q.; et al. Upregulated β1-6 branch N-glycan marks early gliomagenesis but exhibited biphasic expression in the progression of astrocytic glioma. Am. J. Cancer Res. 2015, 5, 1101–1116. [Google Scholar]
- Hassani, Z.; Saleh, A.; Turpault, S.; Khiati, S.; Morelle, W.; Vignon, J.; Hugnot, J.P.; Uro-Coste, E.; Legrand, P.; Delaforge, M.; et al. Phostine PST3.1a Targets MGAT5 and Inhibits Glioblastoma-Initiating Cell Invasiveness and Proliferation. Mol. Cancer Res. 2017, 15, 1376–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Song, X.; Huang, Q.; Xu, T.; Yun, D.; Wang, Y.; Hu, L.; Yan, Y.; Chen, H.; Lu, D.; et al. LGALS3 Promotes Treatment Resistance in Glioblastoma and Is Associated with Tumor Risk and Prognosis. Cancer Epidemiol. Biomark. Prev. 2019, 28, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar]
- Stegmayr, J.; Zetterberg, F.; Carlsson, M.C.; Huang, X.; Sharma, G.; Kahl-Knutson, B.; Schambye, H.; Nilsson, U.J.; Oredsson, S.; Leffler, H. Extracellular and intracellular small-molecule galectin-3 inhibitors. Sci. Rep. 2019, 9, 2186. [Google Scholar] [CrossRef] [Green Version]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Denic, A.; Johnson, A.J.; Bieber, A.J.; Warrington, A.E.; Rodriguez, M.; Pirko, I. The relevance of animal models in multiple sclerosis research. Pathophysiology 2011, 18, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.L.; Roth, P.T.; Stratton, J.A.; Chuang, B.H.; Danne, J.; Ellis, S.L.; Ng, S.W.; Kilpatrick, T.J.; Merson, T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014, 34, 14128–14146. [Google Scholar]
- Nait-Oumesmar, B.; Decker, L.; Lachapelle, F.; Avellana-Adalid, V.; Bachelin, C.; Van Evercooren, A.B. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999, 11, 4357–4366. [Google Scholar] [CrossRef]
- Nait-Oumesmar, B.; Picard-Riera, N.; Kerninon, C.; Decker, L.; Seilhean, D.; Hoglinger, G.U.; Hirsch, E.C.; Reynolds, R.; Baron-Van Evercooren, A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 4694–4699. [Google Scholar]
- Picard-Riera, N.; Decker, L.; Delarasse, C.; Goude, K.; Nait-Oumesmar, B.; Liblau, R.; Pham-Dinh, D.; Evercooren, A.B. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13211–13216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, F.; Wojcik, J.; Rodegher, M.; Radaelli, M.; Moiola, L.; Ghezzi, A.; Capra, R.; Brambilla, P.; Sorosina, M.; Giacalone, G.; et al. MGAT5 and disease severity in progressive multiple sclerosis. J. Neuroimmunol. 2011, 230, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Grigorian, A.; Pawling, J.; Chen, I.J.; Gao, G.; Mozaffar, T.; McKerlie, C.; Demetriou, M. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J. Biol. Chem. 2007, 282, 33725–33734. [Google Scholar] [CrossRef] [Green Version]
- Stancic, M.; van Horssen, J.; Thijssen, V.L.; Gabius, H.J.; van der Valk, P.; Hoekstra, D.; Baron, W. Increased expression of distinct galectins in multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 2011, 37, 654–671. [Google Scholar] [CrossRef]
- Haines, J.D.; Vidaurre, O.G.; Zhang, F.; Riffo-Campos, A.L.; Castillo, J.; Casanova, B.; Casaccia, P.; Lopez-Rodas, G. Multiple sclerosis patient-derived CSF induces transcriptional changes in proliferating oligodendrocyte progenitors. Mult. Scler. 2015, 21, 1655–1669. [Google Scholar]
- Dawson, J.D. The histology of disseminated sclerosis. R. Soc. Edin. 1916, 50, 517–740. [Google Scholar] [CrossRef] [Green Version]
- Reichert, F.; Rotshenker, S. Galectin-3/MAC-2 in experimental allergic encephalomyelitis. Exp. Neurol. 1999, 160, 508–514. [Google Scholar]
- Goings, G.E.; Greisman, A.; James, R.E.; Abram, L.K.; Begolka, W.S.; Miller, S.D.; Szele, F.G. Hematopoietic cell activation in the subventricular zone after Theiler’s virus infection. J. Neuroinflammation 2008, 5, 44–66. [Google Scholar] [CrossRef] [Green Version]
- Hoyos, H.C.; Rinaldi, M.; Mendez-Huergo, S.P.; Marder, M.; Rabinovich, G.A.; Pasquini, J.M.; Pasquini, L.A. Galectin-3 controls the response of microglial cells to limit cuprizone-induced demyelination. Neurobiol. Dis. 2014, 62, 441–455. [Google Scholar] [CrossRef]
- Jiang, H.R.; Al Rasebi, Z.; Mensah-Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.T.; Liew, F.Y.; Lukic, M.L. Galectin-3 Deficiency Reduces the Severity of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef]
- Rotshenker, S.; Reichert, F.; Gitik, M.; Haklai, R.; Elad-Sfadia, G.; Kloog, Y. Galectin-3/MAC-2, Ras and PI3K activate complement receptor-3 and scavenger receptor-AI/II mediated myelin phagocytosis in microglia. Glia 2008, 56, 1607–1613. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, D.H.; Block, M.L.; Lorenzl, S.; Yang, L.; Kim, Y.J.; Sugama, S.; Cho, B.P.; Hwang, O.; Browne, S.E.; et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007, 21, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Hoyos, H.C.; Marder, M.; Ulrich, R.; Gudi, V.; Stangel, M.; Rabinovich, G.A.; Pasquini, L.A.; Pasquini, J.M. The Role of Galectin-3: From Oligodendroglial Differentiation and Myelination to Demyelination and Remyelination Processes in a Cuprizone-Induced Demyelination Model. Adv. Exp. Med. Biol. 2016, 949, 311–332. [Google Scholar] [PubMed]
- Espinosa-Oliva, A.M.; Garcia-Miranda, P.; Alonso-Bellido, I.M.; Carvajal, A.E.; Gonzalez-Rodriguez, M.; Carrillo-Jimenez, A.; Temblador, A.J.; Felices-Navarro, M.; Garcia-Dominguez, I.; Roca-Ceballos, M.A.; et al. Galectin-3 Deletion Reduces LPS and Acute Colitis-Induced Pro-Inflammatory Microglial Activation in the Ventral Mesencephalon. Front. Pharmacol. 2021, 12, 706439. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihara, H.; Shimizu, F.; Kitagawa, T.; Yamanaka, N.; Akada, J.; Kuramitsu, Y.; Sano, Y.; Takeshita, Y.; Maeda, T.; Abe, M.; et al. Identification of galectin-3 as a possible antibody target for secondary progressive multiple sclerosis. Mult. Scler. 2017, 23, 382–394. [Google Scholar] [PubMed]
- Pasquini, L.A.; Millet, V.; Hoyos, H.C.; Giannoni, J.P.; Croci, D.O.; Marder, M.; Liu, F.T.; Rabinovich, G.A.; Pasquini, J.M. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011, 18, 1746–1756. [Google Scholar] [CrossRef]
- Young, C.C.; Brooks, K.J.; Buchan, A.M.; Szele, F.G. Cellular and molecular determinants of stroke-induced changes in subventricular zone cell migration. Antioxid. Redox Signal 2011, 14, 1877–1888. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Wang, Z.H.; Zhang, N.; Liu, S.D.; Zhao, J.J.; Liu, S.Y. Serum Galectin-3 level, not Galectin-1, is associated with the clinical feature and outcome in patients with acute ischemic stroke. Oncotarget 2017, 8, 109752–109761. [Google Scholar] [CrossRef] [Green Version]
- He, X.W.; Li, W.L.; Li, C.; Liu, P.; Shen, Y.G.; Zhu, M.; Jin, X.P. Serum levels of galectin-1, galectin-3, and galectin-9 are associated with large artery atherosclerotic stroke. Sci. Rep. 2017, 7, 40994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, H.; Plate, K.H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009, 117, 481–496. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.J.; Zhou, L.; Zheng, Y.H.; Ding, Y.S. The serum galectin-3 levels are associated with the severity and prognosis of ischemic stroke. Aging 2021, 13, 7454–7464. [Google Scholar] [CrossRef]
- Fenton-Navarro, B.; Garduno Rios, D.; Torner, L.; Letechipia-Vallejo, G.; Cervantes, M. Melatonin Decreases Circulating Levels of Galectin-3 and Cytokines, Motor Activity, and Anxiety Following Acute Global Cerebral Ischemia in Male Rats. Arch. Med. Res. 2021, 52, 505–513. [Google Scholar] [PubMed]
- Wesley, U.V.; Sutton, I.C.; Cunningham, K.; Jaeger, J.W.; Phan, A.Q.; Hatcher, J.F.; Dempsey, R.J. Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation. J. Cereb. Blood Flow Metab. 2021, 41, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Doverhag, C.; Hedtjarn, M.; Poirier, F.; Mallard, C.; Hagberg, H.; Karlsson, A.; Savman, K. Galectin-3 contributes to neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 2010, 38, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lalancette-Hebert, M.; Gowing, G.; Simard, A.; Weng, Y.C.; Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007, 27, 2596–2605. [Google Scholar]
- Yan, Y.P.; Lang, B.T.; Vemuganti, R.; Dempsey, R.J. Galectin-3 mediates post-ischemic tissue remodeling. Brain. Res. 2009, 1288, 116–124. [Google Scholar] [CrossRef]
- Savman, K.; Wang, W.; Rafati, A.H.; Svedin, P.; Nair, S.; Golubinskaya, V.; Ardalan, M.; Brown, K.L.; Karlsson-Bengtsson, A.; Mallard, C. Galectin-3 Modulates Microglia Inflammation in vitro but Not Neonatal Brain Injury in vivo under Inflammatory Conditions. Dev. Neurosci. 2021, 43, 296–311. [Google Scholar] [CrossRef]
- Da Rosa, M.M.; de Aguiar Ferreira, M.; de Oliveira Lima, C.A.; Santos Mendonca, A.C.; Silva, Y.M.; Sharjeel, M.; de Melo Rego, M.J.B.; Pereira, M.C.; da Rocha Pitta, M.G. Alzheimer’s disease: Is there a role for galectins? Eur. J. Pharm. 2021, 909, 174437. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, S.; Lin, F.; Chu, W.; Yue, S. Elevated Galectin-3 Levels in the Serum of Patients With Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2013, 30, 729–732. [Google Scholar]
- Yazar, T.; Olgun Yazar, H.; Cihan, M. Evaluation of serum galectin-3 levels at Alzheimer patients by stages: A preliminary report. Acta Neurol. Belg. 2021, 121, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.M.; Baeesa, S.S. Investigation of Gal-3 Expression Pattern in Serum and Cerebrospinal Fluid of Patients Suffering From Neurodegenerative Disorders. Front. Neurosci. 2018, 12, 430. [Google Scholar] [PubMed] [Green Version]
- Tao, C.C.; Cheng, K.M.; Ma, Y.L.; Hsu, W.L.; Chen, Y.C.; Fuh, J.L.; Lee, W.J.; Chao, C.C.; Lee, E.H.Y. Galectin-3 promotes Abeta oligomerization and Abeta toxicity in a mouse model of Alzheimer’s disease. Cell Death Differ. 2020, 27, 192–209. [Google Scholar] [PubMed]
- Seki, T.; Kanagawa, M.; Kobayashi, K.; Kowa, H.; Yahata, N.; Maruyama, K.; Iwata, N.; Inoue, H.; Toda, T. Galectin 3-binding protein suppresses amyloid-beta production by modulating beta-cleavage of amyloid precursor protein. J. Biol. Chem. 2020, 295, 3678–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Miller, S.M.; Sahay, A. Functions of adult-born neurons in hippocampal memory interference and indexing. Nat. Neurosci. 2019, 22, 1565–1575. [Google Scholar] [PubMed]
- Chen, Y.C.; Ma, Y.L.; Lin, C.H.; Cheng, S.J.; Hsu, W.L.; Lee, E.H. Galectin-3 Negatively Regulates Hippocampus-Dependent Memory Formation through Inhibition of Integrin Signaling and Galectin-3 Phosphorylation. Front. Mol. Neurosci. 2017, 10, 217. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Torres-Aleman, I. The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 2012, 13, 225–239. [Google Scholar] [CrossRef]
- Ziegler, A.N.; Levison, S.W.; Wood, T.L. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat. Rev. Endocrinol. 2015, 11, 161–170. [Google Scholar] [PubMed] [Green Version]
- Bonds, J.A.; Shetti, A.; Stephen, T.K.L.; Bonini, M.G.; Minshall, R.D.; Lazarov, O. Deficits in hippocampal neurogenesis in obesity-dependent and -independent type-2 diabetes mellitus mouse models. Sci. Rep. 2020, 10, 16368. [Google Scholar] [PubMed]
- Karlsen, A.E.; Storling, Z.M.; Sparre, T.; Larsen, M.R.; Mahmood, A.; Storling, J.; Roepstorff, P.; Wrzesinski, K.; Larsen, P.M.; Fey, S.; et al. Immune-mediated beta-cell destruction in vitro and in vivo-A pivotal role for galectin-3. Biochem. Biophys. Res. Commun. 2006, 344, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Darrow, A.L.; Shohet, R.V.; Maresh, J.G. Transcriptional analysis of the endothelial response to diabetes reveals a role for galectin-3. Physiol. Genom. 2011, 43, 1144–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, M.; Dominguez, L.J. Type 2 diabetes mellitus and Alzheimer’s disease. World J. Diabetes 2014, 5, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehina, E.M.F.; Taylor, S.; Boghozian, R.; White, E.; Choi, S.E.; Cheema, M.S.; Korbelin, J.; Brown, C.E. Invasion of phagocytic Galectin 3 expressing macrophages in the diabetic brain disrupts vascular repair. Sci. Adv. 2021, 7, eabg2712. [Google Scholar] [CrossRef] [PubMed]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar]
- Wang, W.H.; Lin, C.Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.H.; Liu, F.T.; Wang, S.F. The role of galectins in virus infection—A systemic literature review. J. Microbiol. Immunol. Infect. 2019, 53, 925–935. [Google Scholar] [CrossRef]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, C.; Xia, H.; Haller, S.L.; Azar, S.R.; Liu, Y.; Liu, J.; Muruato, A.E.; Chen, R.; Rossi, S.L.; Wakamiya, M.; et al. A Zika virus envelope mutation preceding the 2015 epidemic enhances virulence and fitness for transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 20190–20197. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Chen, Y.H.; Wang, S.W.; Huang, Y.J.; Leu, C.H.; Yeh, N.C.; Chu, C.Y.; Lin, C.C.; Shieh, G.S.; Chen, Y.L.; et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J. Virol. 2011, 85, 10010–10020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenza, M.P.; Oyenarte, I.; Diercks, T.; Quintana, J.I.; Gimeno, A.; Coelho, H.; Diniz, A.; Peccati, F.; Delgado, S.; Bosch, A.; et al. Structural Characterization of N-Linked Glycans in the Receptor Binding Domain of the SARS-CoV-2 Spike Protein and their Interactions with Human Lectins. Angew. Chem. Int. Ed. Engl. 2020, 59, 23763–23771. [Google Scholar] [PubMed]
- Casalino, L.; Gaieb, Z.; Goldsmith, J.A.; Hjorth, C.K.; Dommer, A.C.; Harbison, A.M.; Fogarty, C.A.; Barros, E.P.; Taylor, B.C.; McLellan, J.S.; et al. Beyond Shielding: The Roles of Glycans in the SARS-CoV-2 Spike Protein. ACS Cent. Sci. 2020, 6, 1722–1734. [Google Scholar] [PubMed]
- Shajahan, A.; Supekar, N.T.; Gleinich, A.S.; Azadi, P. Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology 2020, 30, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutmann, C.; Takov, K.; Burnap, S.A.; Singh, B.; Ali, H.; Theofilatos, K.; Reed, E.; Hasman, M.; Nabeebaccus, A.; Fish, M.; et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat. Commun. 2021, 12, 3406. [Google Scholar]
- McCullough, P.A.; Olobatoke, A.; Vanhecke, T.E. Galectin-3: A novel blood test for the evaluation and management of patients with heart failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar] [PubMed]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Oberg, C.T.; Leffler, H.; Nilsson, U.J. Inhibition of galectins with small molecules. Chimia 2011, 65, 18–23. [Google Scholar] [CrossRef]
- Strik, H.M.; Kolodziej, M.; Oertel, W.; Basecke, J. Glycobiology in Malignant Gliomas: Expression and Functions of Galectins and Possible Therapeutic Options. Curr. Pharm. Biotechnol. 2011, 13, 2299–2307. [Google Scholar]
- McCullough, P.A. Practical experience using galectin-3 in heart failure. Clin. Chem. Lab. Med. 2014, 52, 1425–1431. [Google Scholar] [CrossRef]
- Caniglia, J.L.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. A potential role for Galectin-3 inhibitors in the treatment of COVID-19. PeerJ 2020, 8, e9392. [Google Scholar] [PubMed]
- Uchino, Y.; Woodward, A.M.; Mauris, J.; Peterson, K.; Verma, P.; Nilsson, U.J.; Rajaiya, J.; Argueso, P. Galectin-3 is an amplifier of the interleukin-1beta-mediated inflammatory response in corneal keratinocytes. Immunology 2018, 154, 490–499. [Google Scholar] [PubMed] [Green Version]
- Cerliani, J.P.; Stowell, S.R.; Mascanfroni, I.D.; Arthur, C.M.; Cummings, R.D.; Rabinovich, G.A. Expanding the Universe of Cytokines and Pattern Recognition Receptors: Galectins and Glycans in Innate Immunity. J. Clin. Immunol. 2011, 31, 10–21. [Google Scholar] [PubMed]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Toit, A. Endocytosis: Bend it like galectin 3. Nat. Rev. Mol. Cell Biol. 2014, 15, 230–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; Sun, D.; Rajashankar, K.R.; Qian, Z.; Holmes, K.V.; Li, F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 10696–10701. [Google Scholar] [PubMed] [Green Version]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020. [Google Scholar]
- Sun, X.L. The Role of Cell Surface Sialic Acids for SARS-CoV-2 Infection. Glycobiology 2020, 143, 3104–3120. [Google Scholar]
- Szele, F.G.; Chesselet, M.F. Cortical lesions induce an increase in cell number and PSA-NCAM expression in the subventricular zone of adult rats. J. Comp. Neurol. 1996, 368, 439–454. [Google Scholar]
- Ramirez Hernandez, E.; Sanchez-Maldonado, C.; Mayoral Chavez, M.A.; Hernandez-Zimbron, L.F.; Patricio Martinez, A.; Zenteno, E.; Limon Perez de Leon, I.D. The therapeutic potential of galectin-1 and galectin-3 in the treatment of neurodegenerative diseases. Expert Rev. Neurother. 2020, 20, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Shingo, T.; Shimazaki, T.; Okano, H.J.; Shiwa, M.; Ishibashi, S.; Oguro, H.; Ninomiya, M.; Kadoya, T.; Horie, H.; et al. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7112–7117. [Google Scholar] [PubMed] [Green Version]
- Whitney, P.L.; Powell, J.T.; Sanford, G.L. Oxidation and chemical modification of lung beta-galactoside-specific lectin. Biochem. J. 1986, 238, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morshead, C.M.; Reynolds, B.A.; Craig, C.G.; McBurney, M.W.; Staines, W.A.; Morassutti, D.; Weiss, S.; van der Kooy, D. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron 1994, 13, 1071–1082. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Sakaguchi, M.; Morishita, T.; Ito, M.; Poirier, F.; Sawamoto, K.; Okano, H. Galectin-1 is expressed in early-type neural progenitor cells and down-regulates neurogenesis in the adult hippocampus. Mol. Brain 2011, 4, 7. [Google Scholar] [PubMed] [Green Version]
- Sakaguchi, M.; Imaizumi, Y.; Shingo, T.; Tada, H.; Hayama, K.; Yamada, O.; Morishita, T.; Kadoya, T.; Uchiyama, N.; Shimazaki, T.; et al. Regulation of adult neural progenitor cells by Galectin-1/beta1 Integrin interaction. J. Neurochem. 2010, 113, 1516–1524. [Google Scholar]
- Ishibashi, S.; Kuroiwa, T.; Sakaguchi, M.; Sun, L.; Kadoya, T.; Okano, H.; Mizusawa, H. Galectin-1 regulates neurogenesis in the subventricular zone and promotes functional recovery after stroke. Exp. Neurol. 2007, 207, 302–313. [Google Scholar]
- Han, H.; Xia, Y.; Wang, S.; Zhao, B.; Sun, Z.; Yuan, L. Synergistic effects of galectin-1 and reactive astrocytes on functional recovery after contusive spinal cord injury. Arch. Orthop. Trauma Surg. 2011, 131, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Yamane, J.; Nakamura, M.; Iwanami, A.; Sakaguchi, M.; Katoh, H.; Yamada, M.; Momoshima, S.; Miyao, S.; Ishii, K.; Tamaoki, N.; et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J. Neurosci. Res. 2010, 88, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, K.; Nomaru, H.; Ifuku, M.; Yutsudo, N.; Dan, Y.; Miura, T.; Tsuchimoto, D.; Sakumi, K.; Kadoya, T.; Horie, H.; et al. Galectin-1 promotes basal and kainate-induced proliferation of neural progenitors in the dentate gyrus of adult mouse hippocampus. Cell Death Differ. 2009, 16, 417–427. [Google Scholar] [PubMed] [Green Version]
- Sakaguchi, M.; Arruda-Carvalho, M.; Kang, N.H.; Imaizumi, Y.; Poirier, F.; Okano, H.; Frankland, P.W. Impaired spatial and contextual memory formation in galectin-1 deficient mice. Mol. Brain 2011, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Akers, K.G.; Martinez-Canabal, A.; Restivo, L.; Yiu, A.P.; De Cristofaro, A.; Hsiang, H.L.; Wheeler, A.L.; Guskjolen, A.; Niibori, Y.; Shoji, H.; et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014, 344, 598–602. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, L.C.; Al-Dalahmah, O.; Hillis, J.; Young, C.C.; Asbed, I.; Sakaguchi, M.; O’Neill, E.; Szele, F.G. Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells 2021, 10, 3047. https://doi.org/10.3390/cells10113047
Soares LC, Al-Dalahmah O, Hillis J, Young CC, Asbed I, Sakaguchi M, O’Neill E, Szele FG. Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells. 2021; 10(11):3047. https://doi.org/10.3390/cells10113047
Chicago/Turabian StyleSoares, Luana C., Osama Al-Dalahmah, James Hillis, Christopher C. Young, Isaiah Asbed, Masanori Sakaguchi, Eric O’Neill, and Francis G. Szele. 2021. "Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases" Cells 10, no. 11: 3047. https://doi.org/10.3390/cells10113047
APA StyleSoares, L. C., Al-Dalahmah, O., Hillis, J., Young, C. C., Asbed, I., Sakaguchi, M., O’Neill, E., & Szele, F. G. (2021). Novel Galectin-3 Roles in Neurogenesis, Inflammation and Neurological Diseases. Cells, 10(11), 3047. https://doi.org/10.3390/cells10113047





