Macroscopic, Histologic, and Immunomodulatory Response of Limb Wounds Following Intravenous Allogeneic Cord Blood-Derived Multipotent Mesenchymal Stromal Cell Therapy in Horses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recipient Animals
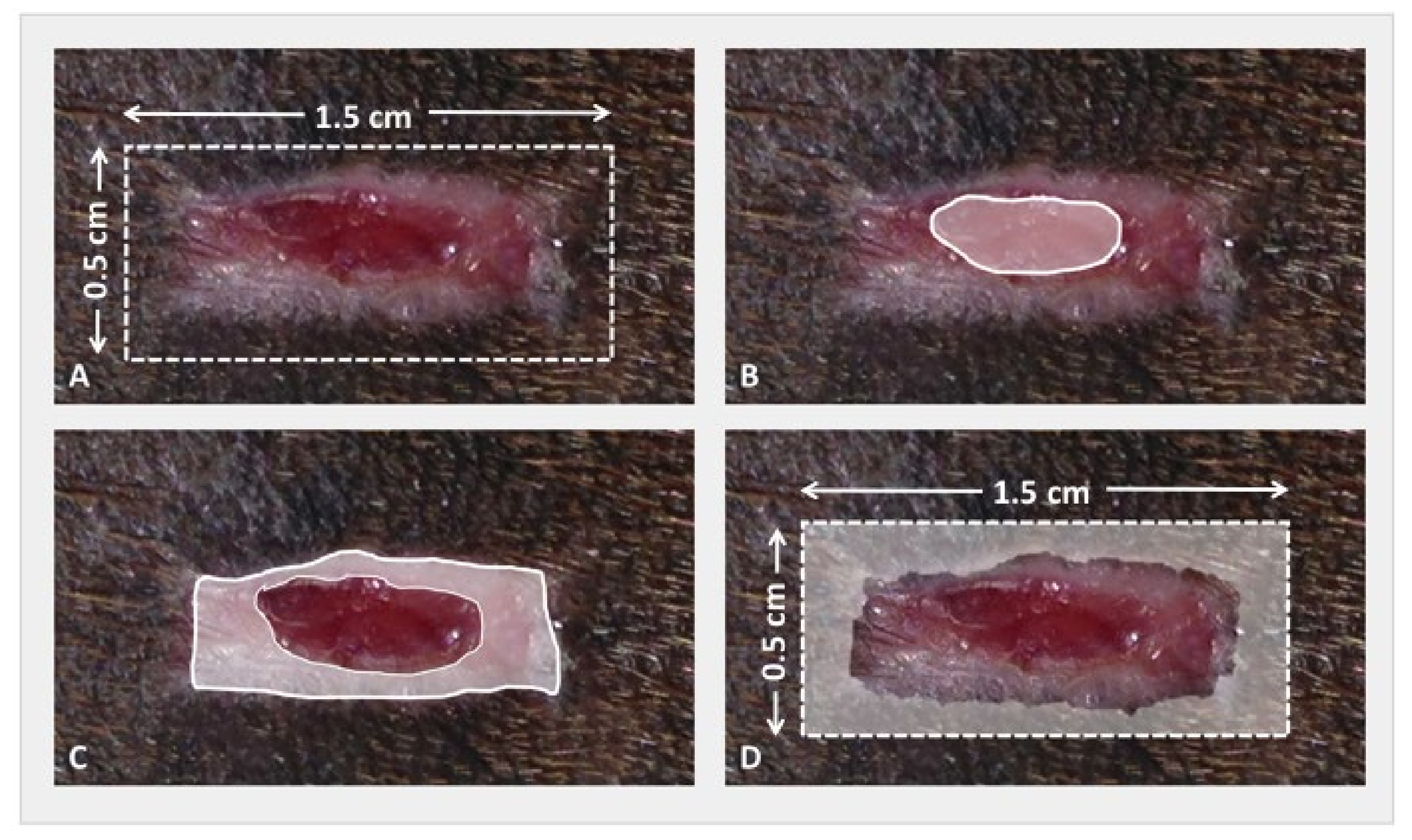
2.2. Wound Creation
2.3. Source of Allo-CB-MSCs, Administration, and Monitoring
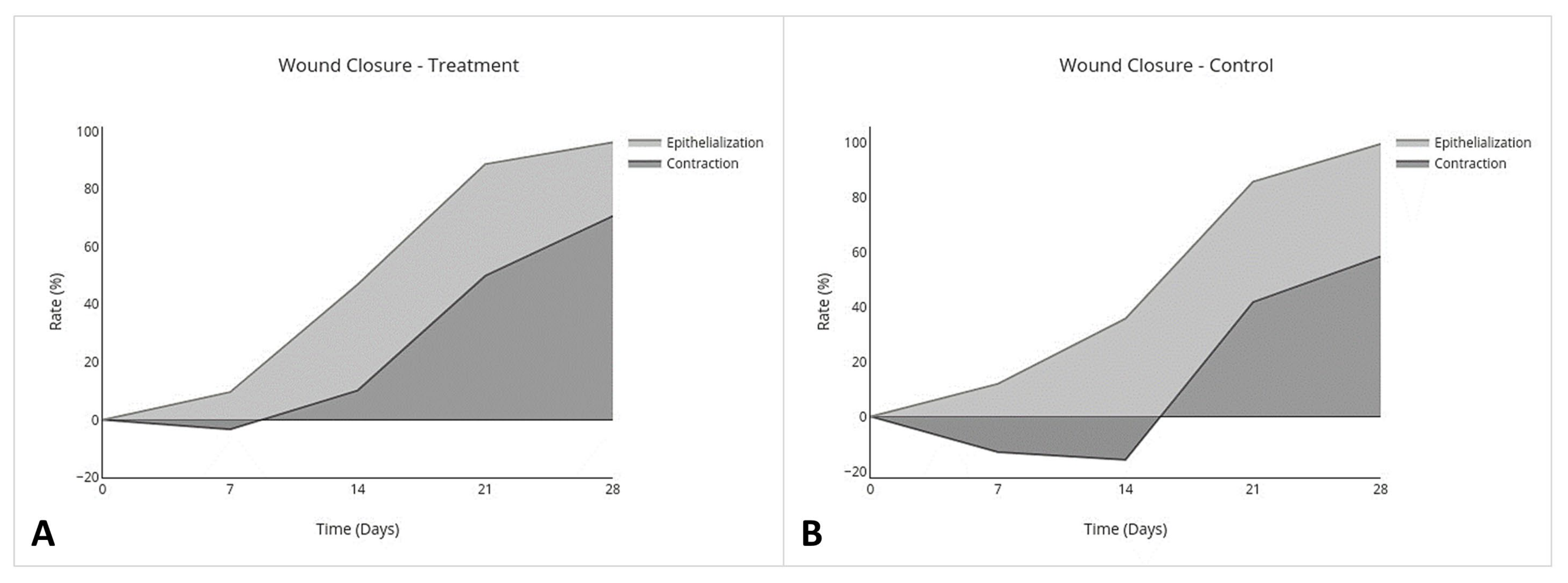
2.4. Wound Closure Analysis
- Percent Epithelialization(Area of the nonhaired epithelialized wound on day (x) mm2 − Area of open granulation tissue on day (x) mm2)/Area of the originally created wound on day 0 mm2 × 100.
- Percent ContractionArea of the originally created wound on day 0 mm2 − Area of the nonhaired epithelialized wound on day (x) mm2/Area of the originally created wound on day 0 mm2 × 100.
2.5. Biopsy Collection
2.6. Histologic Analysis
2.7. Cytokine Multiplex mRNA Assays
2.8. Statistical Analysis
3. Results
3.1. Allo-CB-MSC Administration and Monitoring
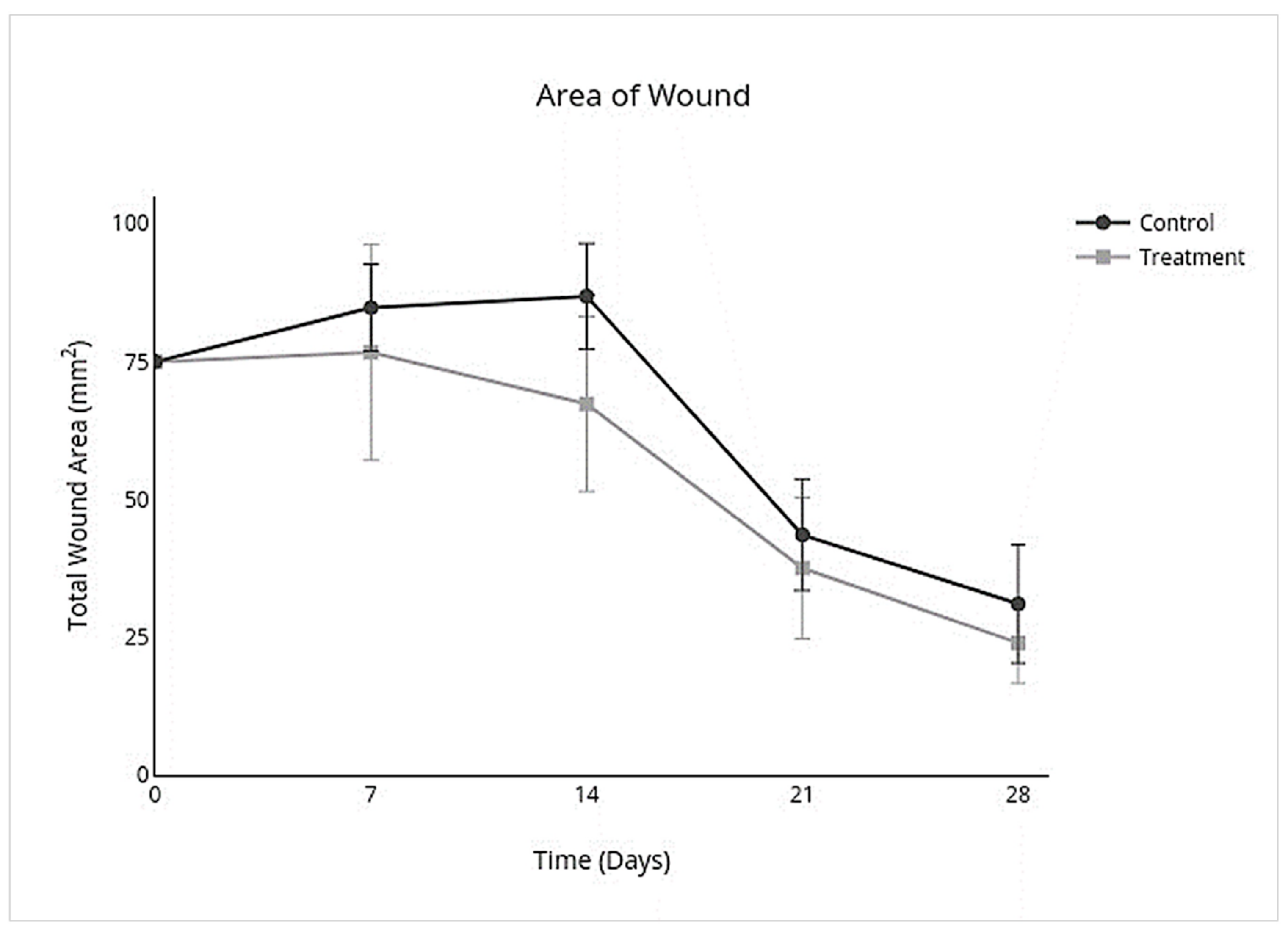
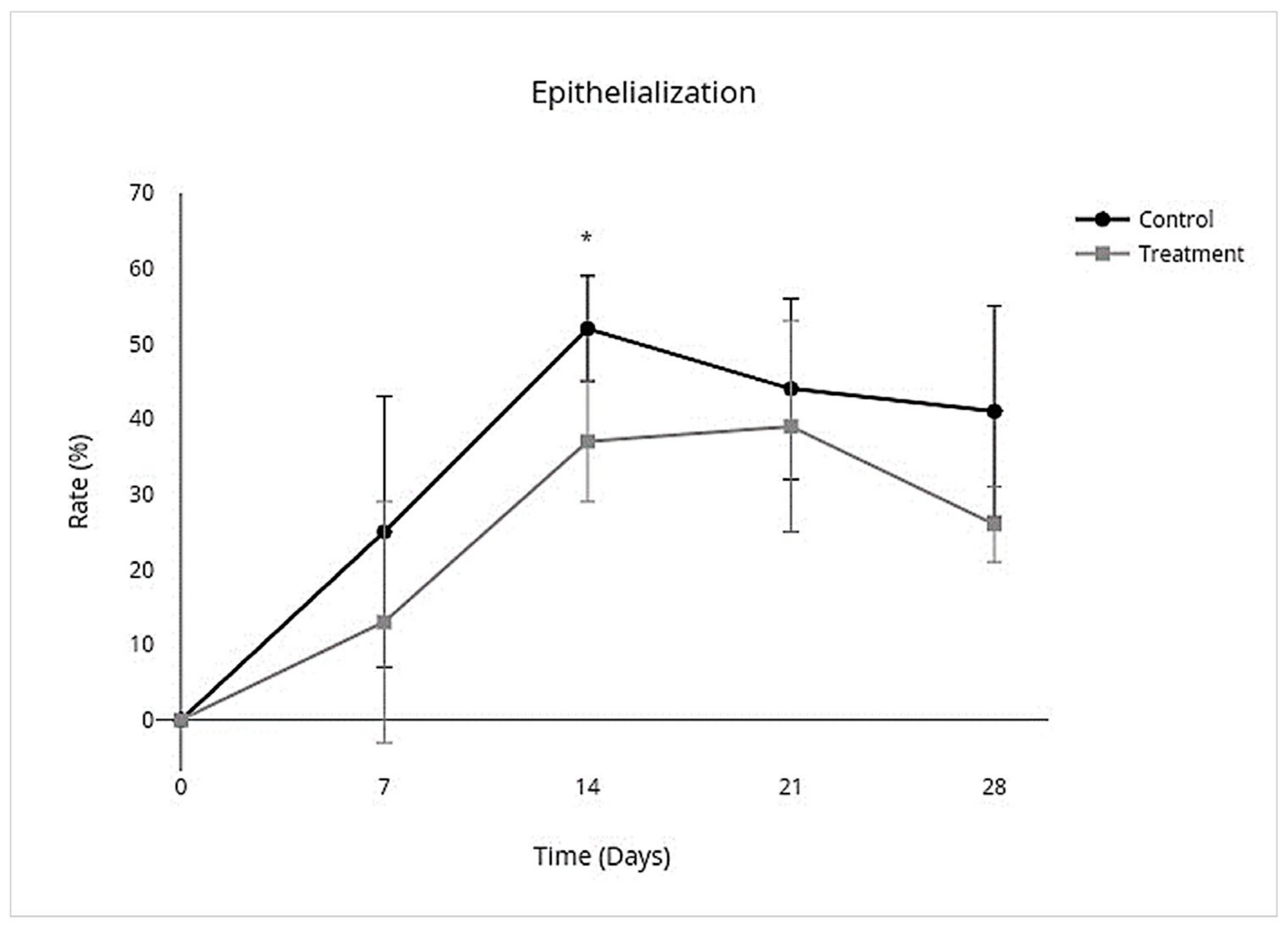
3.2. Wound Closure Analysis
3.3. Histologic Analysis
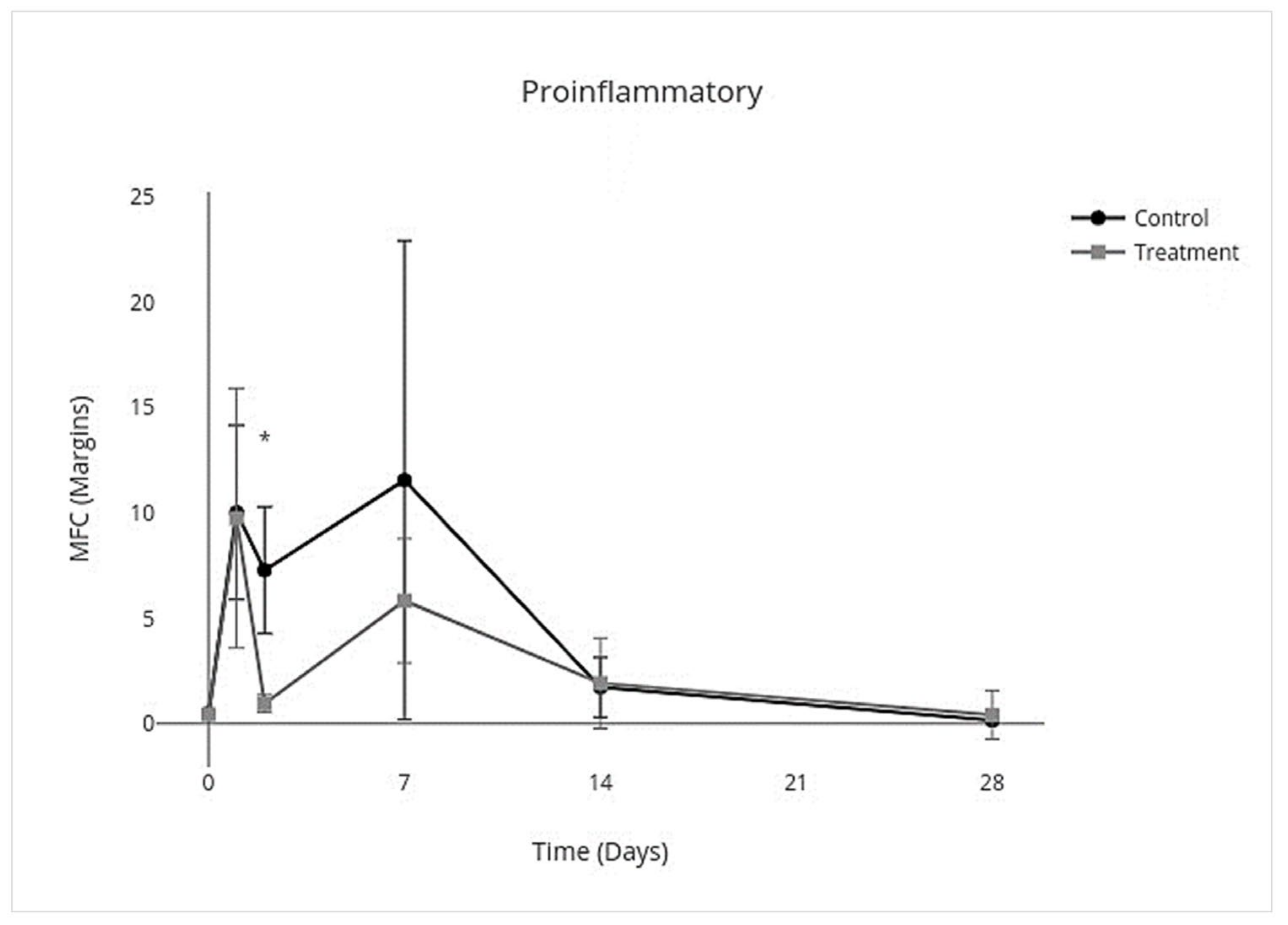
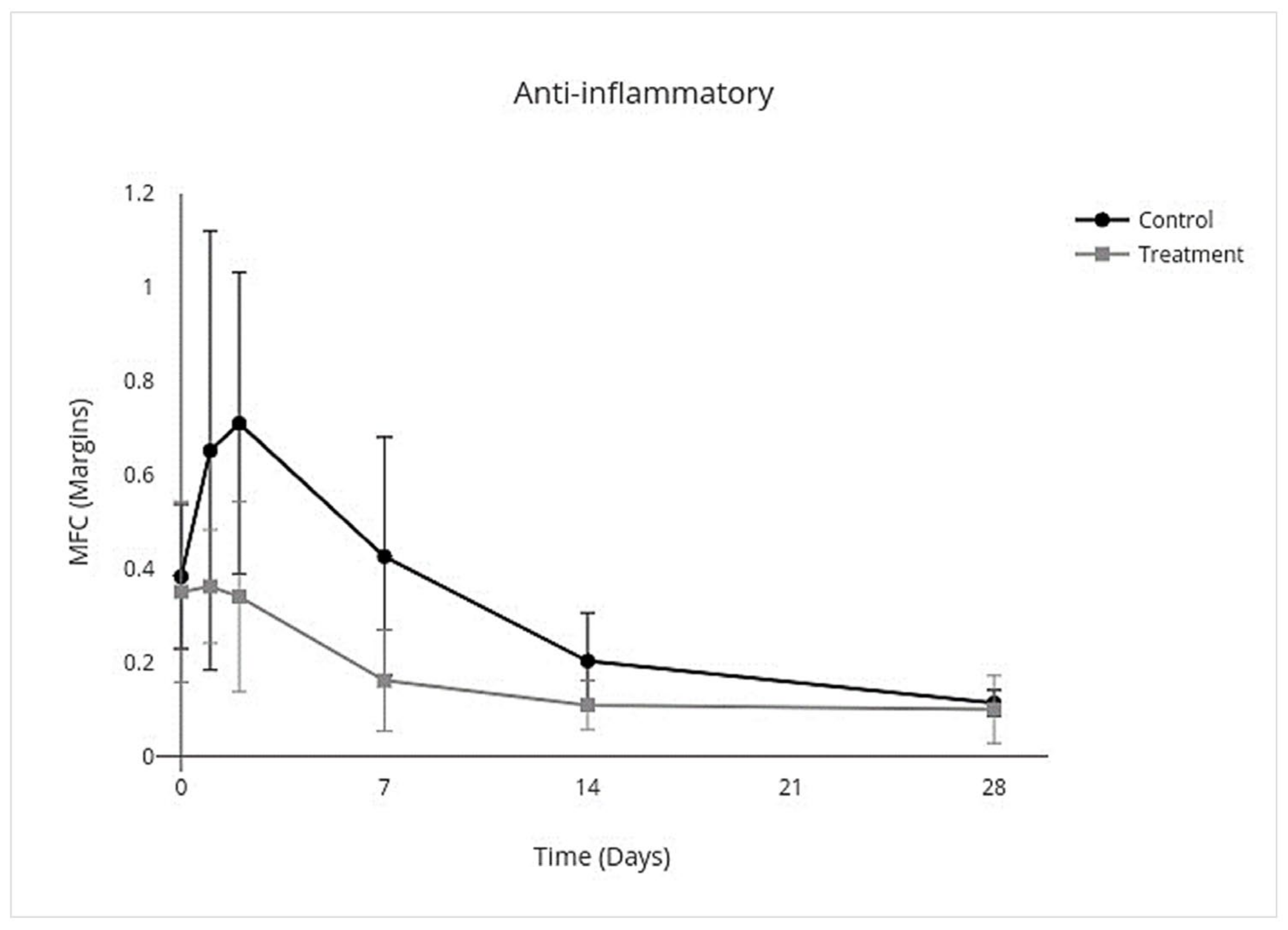
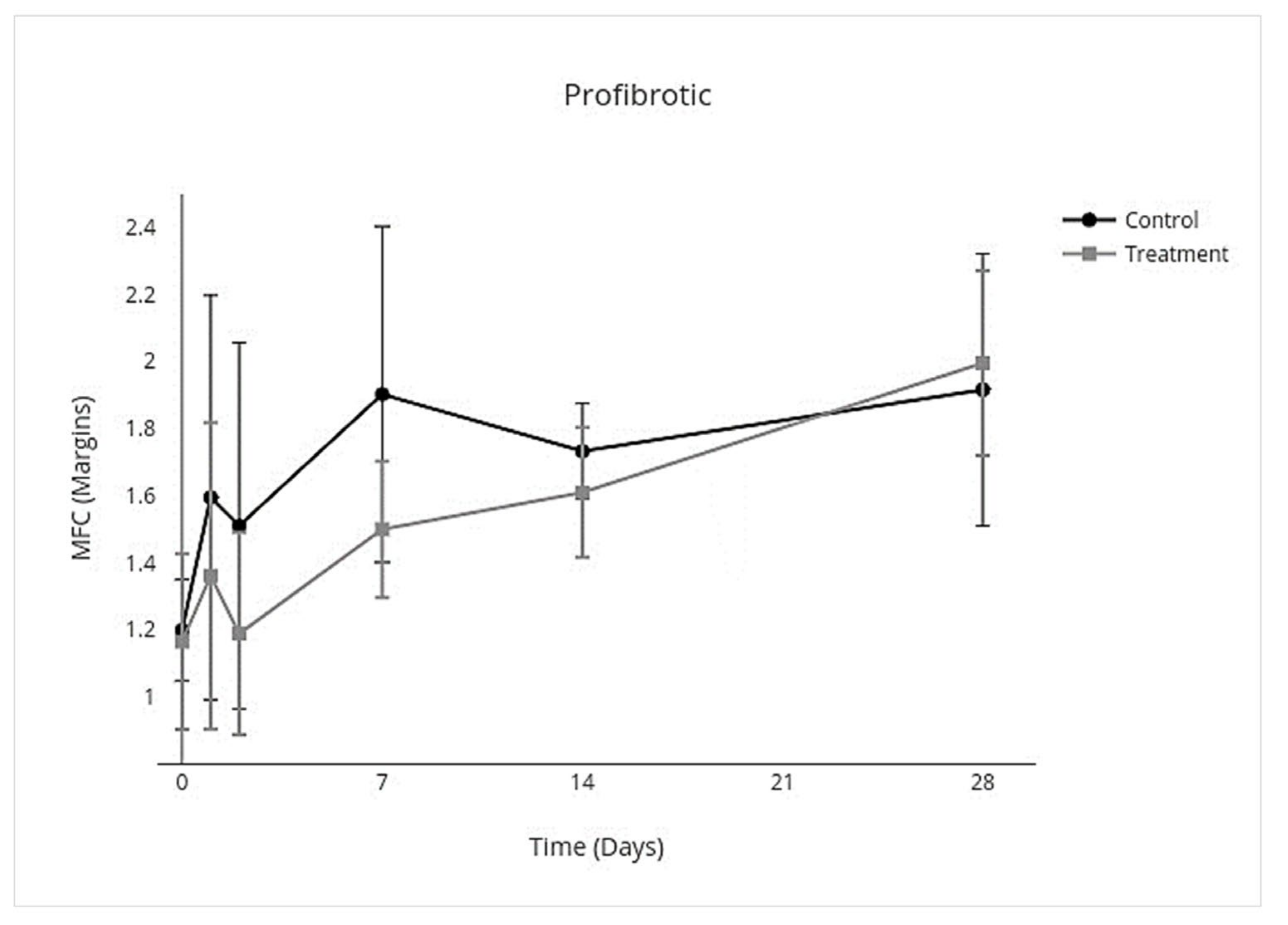
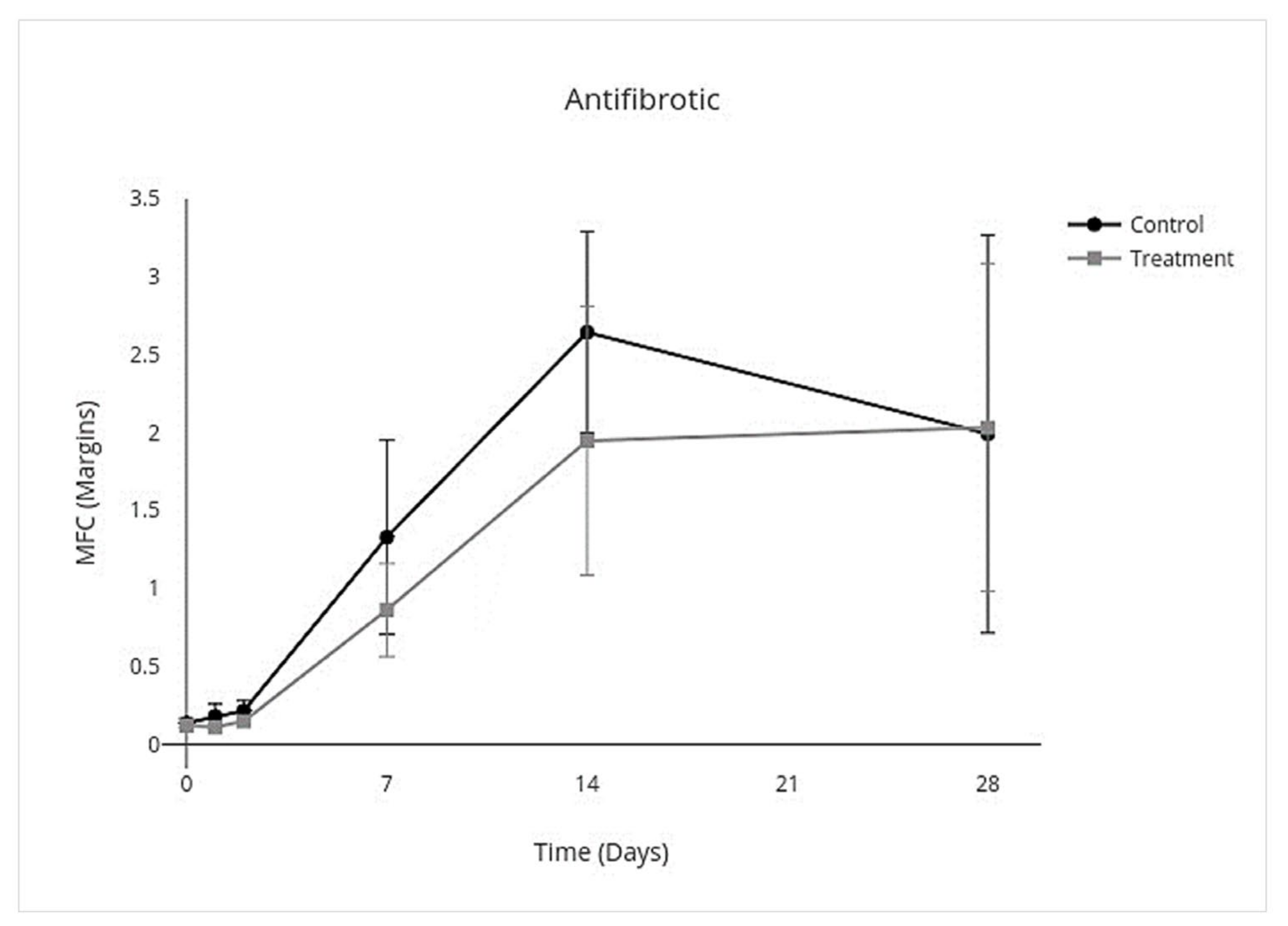
3.4. Cytokine Multiplex mRNA Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theoret, C.; Wilmink, J.M. Exuberant granulation tissue. In Equine Wound Management; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 369–384. [Google Scholar]
- Wilmink, J.M.; Stolk, P.W.T.; Van Weeran, P.R.; Barneveld, A. Differences in second-intention wound healing between horses and ponies: Macroscopic aspects. Equine Vet. J. 1999, 31, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Celeste, C.J.; Deschene, K.; Riley, C.B.; Theoret, C.L. Regional differences in wound oxygenation during normal healing in an equine model of cutaneous fibroproliferative disorder. Wound Repair Regen. 2011, 19, 89–97. [Google Scholar] [CrossRef]
- Theoret, C.L.; Moyana, T.N.; Barber, S.M.; Gordon, J.R. Preliminary observations on expression of transforming growth factors β1 and β3 in equine full-thickness skin wounds healing normally or with exuberant granulation tissue. Vet. Surg. 2002, 31, 266–273. [Google Scholar] [CrossRef]
- Theoret, C. Physiology of wound healing. In Equine Wound Management; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 1–13. [Google Scholar]
- Harper, D.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2014, 32, 445–450. [Google Scholar] [CrossRef]
- Monteiro, S.O.; Lepage, O.M.; Theoret, C.L. Effects of platelet-rich plasma on the repair of wounds on the distal aspect of the forelimb in horses. Am. J. Vet. Res. 2009, 70, 277–282. [Google Scholar] [CrossRef]
- Lepault, E.; Celeste, C.; Dore, M.; Martineau, D.; Theoret, C. Comparative study on microvascular occlusion and apoptosis in body and limb wounds in the horse. Wound Repair Regen. 2005, 13, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Theoret, C.L.; Barber, S.M.; Moyana, T.N.; Gordon, J.R. Expression of transforming growth factor β1, β3, and basic fibroblast growth factor in full-thickness skin wounds of equine limbs and thorax. Vet. Surg. 2001, 30, 269–277. [Google Scholar] [CrossRef]
- Lanci, A.; Merlo, B.; Mariella, J.; Castagnetti, C.; Iacono, E. Heterologous Wharton′s jelly derived mesenchymal stem cells application on a large chronic skin wound in a 6-month-old filly. Front. Vet. Sci. 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Textor, J.A.; Clark, K.C.; Walker, N.J.; Aristizobal, F.A.; Kol, A.; LeJeune, S.S.; Bledsoe, A.; Davidyan, A.; Gray, S.N.; Bohannon-Worsley, L.K.; et al. Allogeneic stem cells alter gene expression and improve healing of distal limb wounds in horses. Stem Cells Transl. Med. 2018, 7, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Liubaviciute, A.; Kaseta, V.; Vaitkuviene, A.; Mackiewicz, Z.; Biziuleviciene, G. Regenerative potential of partially differentiated mesenchymal stromal cells in a mouse model of a full-thickness wound. EXCLI J. 2018, 17, 871–888. [Google Scholar]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal stem cells home to sites of injury and inflammation. Adv. Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E.; Kean, J. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [Green Version]
- De Schauwer, C. Stem cell therapy in the horse: From laboratory to clinic. Vet. J. 2015, 203, 137. [Google Scholar] [CrossRef]
- Williams, L.B.; Tessier, L.; Koenig, J.B.; Koch, T.G. Post-thaw non-cultured and post-thaw cultured equine cord blood mesenchymal stromal cells equally suppress lymphocyte proliferation in vitro. PLoS ONE 2014, 9, e113615. [Google Scholar] [CrossRef]
- White, S.V.; Czisch, C.E.; Han, M.H.; Plant, C.D.; Harvey, A.R.; Plant, G.W. Intravenous transplantation of mesenchymal progenitors distribute solely to the lungs and improve outcomes in cervical spinal cord injury. Stem Cells 2016, 34, 1812–1825. [Google Scholar] [CrossRef] [Green Version]
- Kol, A.; Wood, J.A.; Carrade Holt, D.D.; Gillette, J.A.; Bohannon-Worsley, L.K.; Puchalski, S.M.; Walker, N.J.; Clark, K.C.; Watson, J.L.; Borjesson, D.L. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res. Ther. 2015, 6, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.B.; Co, C.; Koenig, J.B.; Tse, C.; Lindsay, E.; Koch, T.G. Response to intravenous allogeneic equine cord blood-derived mesenchymal stromal cells administered from chilled or frozen state in serum and protein-free media. Front. Vet. Sci. 2016, 3, 56. [Google Scholar] [CrossRef] [Green Version]
- Broeckx, S.; Borena, B.M.; Zimmerman, M.; Marien, T.; Serys, B.; Suls, M.; Duchateau, L.; Spaas, J.H. Intravenous application of allogeneic peripheral blood-derived mesenchymal stem cells: A safety assessment in 291 equine recipients. Curr. Stem Cell Res. Ther. 2014, 9, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.D.; Kol, A.; Walker, N.J.; Borjesson, D.L. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int. 2016, 2016, 5830103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanji, S.; Das, H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediat. Inflamm. 2017, 2017, 5217967. [Google Scholar] [CrossRef] [Green Version]
- Wilmink, J.M. Differences in wound healing between horses and ponies. In Equine Wound Management; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 14–29. [Google Scholar]
- Mund, S.J.K.; Kawamura, E.; Awang-Junaidi, A.H.; Campbell, J.; Wobeser, B.; MacPhee, D.J.; Honaramooz, A.; Barber, S. Homing and engraftment of intravenously administered equine cord blood-derived multipotent mesenchymal stromal cells to surgically created cutaneous wounds in horses: A pilot project. Cells 2020, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.J.; Perkins, N.R.; Dart, C.M.; Jeffcott, L.B.; Canfield, P. Effect of bandaging on second intention healing of wounds of the distal limb in horses. Aust. Vet. J. 2009, 87, 215–218. [Google Scholar] [CrossRef]
- Hanson, R.R. Medical Therapy in Equine Wound Management. Vet. Clin. N. Am. Equine Pract. 2018, 34, 591–603. [Google Scholar] [CrossRef]
- Huss, M.K.; Felt, S.A.; Pacharinsak, C. Influence of pain and analgesia on orthopedic and wound-healing models in rats and mice. Comp. Med. 2019, 69, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.G.; Thomsen, P.D.; Betts, D.H. Improved isolation protocol for equine cord blood-derived mesenchymal stromal cells. Cytotherapy 2009, 11, 443–447. [Google Scholar] [CrossRef]
- Tessier, L.; Bienzle, D.; Williams, L.B.; Koch, T.G. Phenotypic and immunomodulatory properties of equine cord blood-derived mesenchymal stromal cells. PLoS ONE 2015, 10, e0122954. [Google Scholar] [CrossRef]
- Yang, Y.; Honaramooz, A. Effects of medium and hypothermic temperatures on preservation of isolated porcine testis cells. Reprod. Fertil. Dev. 2010, 22, 523–532. [Google Scholar] [CrossRef]
- Correia, C.; Koshkin, A.; Carido, M.; Espinha, N.; Saric, T.; Lima, P.A.; Serra, M.; Alves, P.M. Effective hypothermic storage of human pluripotent stem cell-derived cardiomyocytes compatible with global distribution of cells for clinical applications and toxicology testing. Stem Cells Transl. Med. 2016, 5, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Freitas-Ribeiro, S.; Carvalho, A.F.; Costa, M.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Pirraco, R.P. Strategies for the hypothermic preservation of cell sheets of human adipose stem cells. PLoS ONE 2019, 14, e0222597. [Google Scholar] [CrossRef]
- Petrenko, Y.; Chudickova, M.; Vackova, I.; Groh, T.; Kosnarova, E.; Cejkova, J.; Turnovcova, K.; Petrenko, A.; Sykova, E.; Kubinova, S. Clinically relevant solution for the hypothermic storage and transportation of human multipotent mesenchymal stromal cells. Stem Cells Int. 2019, 2019, 5909524. [Google Scholar] [CrossRef] [Green Version]
- Yeh, D.D.; Nazarian, R.M.; Demetri, L.; Mesar, T.; Dijkink, S.; Larentzakis, A.; Velmahos, G.; Sadik, K.W. Histopathological assessment of OASIS Ultra on critical-sized wound healing: A pilot study. J. Cutan. Pathol. 2017, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Korchunjit, W.; Laikul, A.; Taylor, J.; Watchrarat, K.; Ritruechai, P.; Supokawej, A.; Wongtawan, T. Characterization and allogeneic transplantation of equine bone marrow–derived multipotent mesenchymal stromal cells collected from cadavers. J. Equine Vet. Sci. 2019, 73, 15–23. [Google Scholar] [CrossRef]
- Kawata, Y.; Tsuchiya, A.; Seino, S.; Watanabe, Y.; Kojima, Y.; Ikarashi, S.; Tominaga, K.; Yokoyama, J.; Yamagiwa, S.; Terai, S. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019, 376, 257–271. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Sharma, G.T. Equine mesenchymal stem cells: Properties, sources, characterization, and potential therapeutic applications. J. Equine Vet. Sci. 2019, 72, 16–27. [Google Scholar] [CrossRef]
- Huang, X.P.; Sun, Z.; Miyagi, Y.; Kinkaid, H.M.; Zhang, L.; Weisel, R.D.; Li, R.K. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 2010, 122, 2419–2429. [Google Scholar] [CrossRef] [Green Version]
- Tano, N.; Kaneko, M.; Ichihara, Y.; Ikebe, C.; Coppen, S.R.; Shiraishi, M.; Shintani, Y.; Yashiro, K.; Warrens, A.; Suzuki, K. Allogeneic mesenchymal stromal cells transplanted onto the heart surface achieve therapeutic myocardial repair despite immunologic responses in rats. J. Am. Heart Assoc. 2016, 5, e002815. [Google Scholar] [CrossRef] [Green Version]
- Pigott, J.H.; Ishihara, A.; Wellman, M.L.; Russell, D.S.; Bertone, A.L. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet. Comp. Orthop. Traumatol. 2013, 26, 453–460. [Google Scholar] [PubMed] [Green Version]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.B.; Koenig, J.B.; Black, B.; Gibson, T.W.G.; Sharif, S.; Koch, T.G. Equine allogeneic umbilical cord blood derived mesenchymal stromal cells reduce synovial fluid nucleated cell count and induce mild self-limiting inflammation when evaluated in an lipopolysaccharide induced synovitis model. Equine Vet. J. 2016, 48, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vitoria, A.; Albareda, J.; Prades, M.; Roca, M.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet. Res. 2018, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Mariën, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K.; et al. Equine allogeneic chondrogenic induced mesenchymal stem cells are an effective treatment for degenerative joint disease in horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Lutton, B.V.; Cho, P.S.; Hirsh, E.L.; Ferguson, K.K.; Teague, A.G.S.; Hanekamp, J.S.; Chi, N.; Goldman, S.N.; Messina, D.J.; Houser, S.; et al. Approaches to avoid immune responses induced by repeated subcutaneous injections of allogeneic umbilical cord tissue-derived cells. Transplantation 2010, 90, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Furlani, D.; Ugurlucan, M.; Ong, L.; Bieback, K.; Pittermann, E.; Westien, I.; Wang, W.; Yerebakan, C.; Li, W.; Gaebel, R.; et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 2009, 77, 370–376. [Google Scholar] [CrossRef]
- Monnet, X.; Teboul, J.L. Transpulmonary thermodilution: Advantages and limits. Crit. Care 2017, 21, 147. [Google Scholar] [CrossRef]
- Argueta, E.E.; Paniagua, D. Thermodilution cardiac output: A concept over 250 years in the making. Cardiol. Rev. 2019, 27, 138–144. [Google Scholar] [CrossRef]
- Taylor, M.; Bailes, J.; Elrifai, A.; Shih, S.-R.; Teeple, E.; Leavitt, M.; Baust, J.; Maroon, J. A new solution for life without blood: Asanguineous low-flow perfusion of a whole-body perfusate during 3 hours of cardiac arrest and profound hypothermia. Circulation 1995, 91, 431–444. [Google Scholar] [CrossRef]
- Fielding, C.L. Potassium homeostasis and derangements. In Equine Fluid Therapy; Fielding, C.L., Magdesian, K.G., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2015; pp. 27–44. [Google Scholar]
- Carvalho, A.É.S.; Sousa, M.R.R.; Alencar-Silva, T.; Carvalho, J.L.; Saldanha-Araujo, F. Mesenchymal stem cells immunomodulation: The road to IFN-γ licensing and the path ahead. Cytokine Growth Factor Rev. 2019, 47, 32–42. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Sole, A.; Spriet, M.; Galuppo, L.D.; Padgett, K.A.; Borjesson, D.L.; Wisner, E.R.; Brosnan, R.J.; Vidal, M.A. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using (99m) Tc-HMPAO. Equine Vet. J. 2012, 44, 594–599. [Google Scholar] [CrossRef]
- Sole, A.; Spriet, M.; Padgett, K.A.; Vaughan, B.; Galuppo, L.D.; Borjesson, D.L.; Wisner, E.R.; Vidal, M.A. Distribution and persistence of technetium-99 hexamethyl propylene amine oxime-labelled bone marrow-derived mesenchymal stem cells in experimentally induced tendon lesions after intratendinous injection and regional perfusion of the equine distal limb. Equine Vet. J. 2013, 45, 726–731. [Google Scholar] [CrossRef]
- Trela, J.M.; Spriet, M.; Padgett, K.A.; Galuppo, L.D.; Vaughan, B.; Vidal, M.A. Scintigraphic comparison of intra-arterial injection and distal intravenous regional limb perfusion for administration of mesenchymal stem cells to the equine foot. Equine Vet. J. 2014, 46, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Becerra, P.; Valdés Vázquez, M.A.; Dudhia, J.; Fiske-Jackson, A.R.; Neves, F.; Hartman, N.G.; Smith, R.K.W. Distribution of injected technetium99m-labeled mesenchymal stem cells in horses with naturally occurring tendinopathy. J. Orthop. Res. 2013, 31, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. The MSC adhesion cascade—Insights into homing and transendothelial migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [Green Version]
- Ezquer, F.E.; Ezquer, M.E.; Vicencio, J.M.; Calligaris, S.D. Two complementary strategies to improve cell engraftment in mesenchymal stem cell-based therapy: Increasing transplanted cell resistance and increasing tissue receptivity. Cell Adhes. Migr. 2017, 11, 110–119. [Google Scholar] [CrossRef]
- Guest, D.J.; Smith, M.R.W.; Allen, W.R. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Vet. J. 2010, 42, 636–642. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Yamada, A.L.M.; Golim, M.A.; Álvarez, L.E.C.; Hussni, C.A.; Alves, A.L.G. Evaluation of mesenchymal stem cell migration after equine tendonitis therapy. Equine Vet. J. 2014, 46, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Spriet, M.; Trela, J.M.; Galuppo, L.D. Ultrasound-guided injection of the median artery in the standing sedated horse. Equine Vet. J. 2015, 47, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xiong, Y.-Y.; Li, Q.; Hu, M.-J.; Huang, P.-S.; Xu, J.-Y.; Tian, X.-Q.; Jin, C.; Liu, J.-D.; Qian, L.; et al. Optimization of timing and times for administration of atorvastatin-oretreated mesenchymal stem cells in a preclinical model of acute myocardial infarction. Stem Cells Transl. Med. 2019, 8, 1068–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, O.E.; Mahrous, K.F.; Shafey, H.I. Mitochondrial DNA genetic variations among four horse populations in Egypt. J. Genet. Eng. Biotechnol. 2017, 15, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.M.; Vogel, C. Quantifying gene expression: The importance of being subtle. Mol. Syst. Biol. 2016, 12, 885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, M.G.; Gallagher, E.P. A targeted gene expression platform allows for rapid analysis of chemical-induced antioxidant mRNA expression in zebrafish larvae. PLoS ONE 2017, 12, e0171025. [Google Scholar] [CrossRef] [PubMed]
- Flagella, M.; Bui, S.; Zheng, Z.; Nguyen, C.T.; Zhang, A.; Pastor, L.; Ma, Y.; Yang, W.; Crawford, K.L.; McMaster, G.K.; et al. A multiplex branched DNA assay for parallel quantitative gene expression profiling. Anal. Biochem. 2006, 352, 50–60. [Google Scholar] [CrossRef] [PubMed]




| Repair Grade | Inflammation Grade | ||||||
|---|---|---|---|---|---|---|---|
| Score | Epithelialization | Score | Collagen | Score | Vascularization | Score | Inflammatory Response |
| 0 | No epithelium present | 0 | Absent | 0 | No activated endothelium | 0 | No inflammation present |
| 1 | <50% covering by epithelium | 1 | Minimal granulation tissue | 1 | Activated endothelium | 1 | Hemorrhage—no inflammatory cells |
| 2 | >50% covering by epithelium | 2 | Granulation tissue | 2 | Vascular proliferation with vertical orientation | 2 | Macrophage dominated |
| 3 | Bridging of excision | 3 | Fibroplasia with minimal collagen | 3 | Declining vascular proliferation | 3 | Admixed neutrophils/macro-phages |
| 4 | Keratinization | 4 | Return to normal dermal collagen | 4 | Return to normal vascular | 4 | Neutrophil dominated |
| Total = Repair Score | Total = Inflammation Score | ||||||
| Horse | Group | Subgroup | Symptoms Summary | Response to Decreased Infusion Rate |
|---|---|---|---|---|
| 8 | Treatment | 2 | Reaction following 28 mLs of infusion. Normothermic, mild tachycardia (48 bpm), moderate tachypnea (64 bpm), mild restlessness | Discontinued infusion for five minutes. Vital parameters returned to normal. Continued infusion. No alterations in vital parameters. |
| 12 | Treatment | 2 | Reaction following 20 mLs of infusion. Normothermic, mild tachycardia (48 bpm), moderate tachypnea (42 bpm), mild restlessness, mild colic symptoms (lip curling) | Continued infusion at a slower rate. Symptoms resolved within fifteen minutes. |
| 7 | Treatment | 3 | Reaction following 28 mLs of infusion. Normothermic, moderate tachycardia (60 bpm), moderate tachypnea (40 bpm), moderate restlessness, moderate colic symptoms (stretching, looking at flank) | Discontinued infusion for ten minutes. Heart rate and respiratory rate returned to normal. Continued infusion slowly. Colic symptoms resolved 15 min after infusion was completed. |
| 11 | Control | 3 | Reaction following 12 mLs of infusion. Normothermic, marked tachycardia (72 bpm), moderate tachypnea (40 bpm), marked restlessness, diffuse muscle fasciculations | Discontinued infusion for fifteen minutes. Muscle fasciculations resolved. Continued infusion slowly. Heart rate fluctuated between 48 and 60 bpm during infusion. Restlessness resolved shortly after complete infusion. |
| Non-Haired Wound Area (mm2) | Epithelialization (%) | Contraction (%) | ||||
|---|---|---|---|---|---|---|
| 95% CI | p Value | 95% CI | p Value | 95% CI | p Value | |
| Overall Treatment effect | −24.0 to 3.5 | 0.1 * | −0.21 to −0.023 | p = 0.02 * | −0.046 to 0.31 | p = 0.1 * |
| Individual Day Treatment Effect | 95% CI | p Value | 95% CI | p Value | 95% CI | p Value |
| Day 7 | −35 to 19 | 0.9 | −0.419 to 0.176 | 0.8 | −0.22 to 0.42 | 0.9 |
| Day 14 | −43.0 to 4.0 | 0.1 | −0.28 to −0.019 | 0.02 ** | −0.054 to 0.57 | 0.1 |
| Day 21 | −27 to 15 | 0.9 | −0.28 to 0.18 | >0.9 | −0.20 to 0.36 | 0.9 |
| Day 28 | −24.0 to 9.4 | 0.8 | −0.34 to 0.041 | 0.2 * | −0.13 to 0.32 | 0.7 |
| Histologic Repair Score | Histologic Inflammation Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Horse | Day 1 | Day 2 | Day 7 | Day 14 | Day 28 | Day 1 | Day 2 | Day 7 | Day 14 | Day 28 |
| Treatment | 2 | 3 | 4 | 8 | 12 | N/A | 5 | 5 | 4 | 3 | N/A |
| 3 | 3 | 4 | 9 | 12 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 6 | 3 | 4 | 8 | 12 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 7 | 3 | 4 | 8 | 11 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 8 | 3 | 4 | 8 | 11 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 12 | 3 | 4 | 8 | 12 | 15 | 5 | 5 | 4 | 3 | 1 | |
| Control | 1 | 3 | 4 | 8 | 13 | 15 | 5 | 5 | 4 | 1 | 1 |
| 4 | 3 | 4 | 8 | 11 | 15 | 5 | 5 | 4 | 4 | 1 | |
| 5 | 3 | 4 | 8 | 11 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 9 | 3 | 4 | 8 | 12 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 10 | 3 | 4 | 8 | 12 | 15 | 5 | 5 | 4 | 3 | 1 | |
| 11 | 3 | 4 | 8 | 11 | 15 | 5 | 5 | 4 | 3 | 1 | |
| Proinflammatory | Anti-Inflammatory | Inflammation Resolving | Profibrotic | Antifibrotic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | p Value | 95% CI | p Value | 95% CI | p Value | 95% CI | p Value | 95% CI | p Value | |
| Overall Treatment effect | −3.8 to −0.17 | 0.03 * | −0.33 to −0.025 | 0.02 * | −0.56 to −0.023 | 0.03 * | −0.43 to 0.086 | 0.2 * | −0.72 to 0.29 | 0.4 |
| Individual Day Treatment Effect | 95% CI | p Value | 95% CI | p Value | 95% CI | p Value | 95% CI | p Value | 95% CI | p Value |
| Day 0 | −0.33 to 0.27 | >0.9 | −0.36 to 0.30 | >0.9 | −0.67 to 0.51 | >0.9 | −0.44 to 0.37 | >0.9 | −0.081 to 0.049 | - |
| Day 1 | −10.0 to 9.7 | >0.9 | −0.94 to 0.36 | 0.8 | −1.4 to 0.48 | 0.8 | −1.3 to 0.78 | >0.9 | −0.18 to 0.041 | - |
| Day 2 | −10.0 to −2.3 | <0.001 ** | −0.88 to 0.14 | 0.3 | −1.4 to 0.18 | 0.2 | −1.2 to 0.52 | 0.9 | −0.17 to 0.030 | - |
| Day 7 | −22 to 10 | 0.9 | −0.64 to 0.12 | 0.3 | −1.1 to 0.15 | 0.2 | −1.1 to 0.32 | 0.6 | −1.4 to 0.46 | - |
| Day 14 | −3.3 to 3.7 | >0.9 | −0.25 to 0.061 | 0.5 | −0.46 to 0.18 | 0.8 | −0.45 to 0.20 | 0.9 | −2.1 to 0.75 | - |
| Day 28 | −1.3 to 1.8 | >0.9 | −0.12 to 0.090 | >0.9 | −0.47 to 0.47 | >0.9 | −0.58 to 0.74 | >0.9 | −2.2 to 2.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mund, S.J.K.; MacPhee, D.J.; Campbell, J.; Honaramooz, A.; Wobeser, B.; Barber, S.M. Macroscopic, Histologic, and Immunomodulatory Response of Limb Wounds Following Intravenous Allogeneic Cord Blood-Derived Multipotent Mesenchymal Stromal Cell Therapy in Horses. Cells 2021, 10, 2972. https://doi.org/10.3390/cells10112972
Mund SJK, MacPhee DJ, Campbell J, Honaramooz A, Wobeser B, Barber SM. Macroscopic, Histologic, and Immunomodulatory Response of Limb Wounds Following Intravenous Allogeneic Cord Blood-Derived Multipotent Mesenchymal Stromal Cell Therapy in Horses. Cells. 2021; 10(11):2972. https://doi.org/10.3390/cells10112972
Chicago/Turabian StyleMund, Suzanne J. K., Daniel J. MacPhee, John Campbell, Ali Honaramooz, Bruce Wobeser, and Spencer M. Barber. 2021. "Macroscopic, Histologic, and Immunomodulatory Response of Limb Wounds Following Intravenous Allogeneic Cord Blood-Derived Multipotent Mesenchymal Stromal Cell Therapy in Horses" Cells 10, no. 11: 2972. https://doi.org/10.3390/cells10112972
APA StyleMund, S. J. K., MacPhee, D. J., Campbell, J., Honaramooz, A., Wobeser, B., & Barber, S. M. (2021). Macroscopic, Histologic, and Immunomodulatory Response of Limb Wounds Following Intravenous Allogeneic Cord Blood-Derived Multipotent Mesenchymal Stromal Cell Therapy in Horses. Cells, 10(11), 2972. https://doi.org/10.3390/cells10112972







