Role of Deep Learning in Predicting Aging-Related Diseases: A Scoping Review
Abstract
:1. Introduction
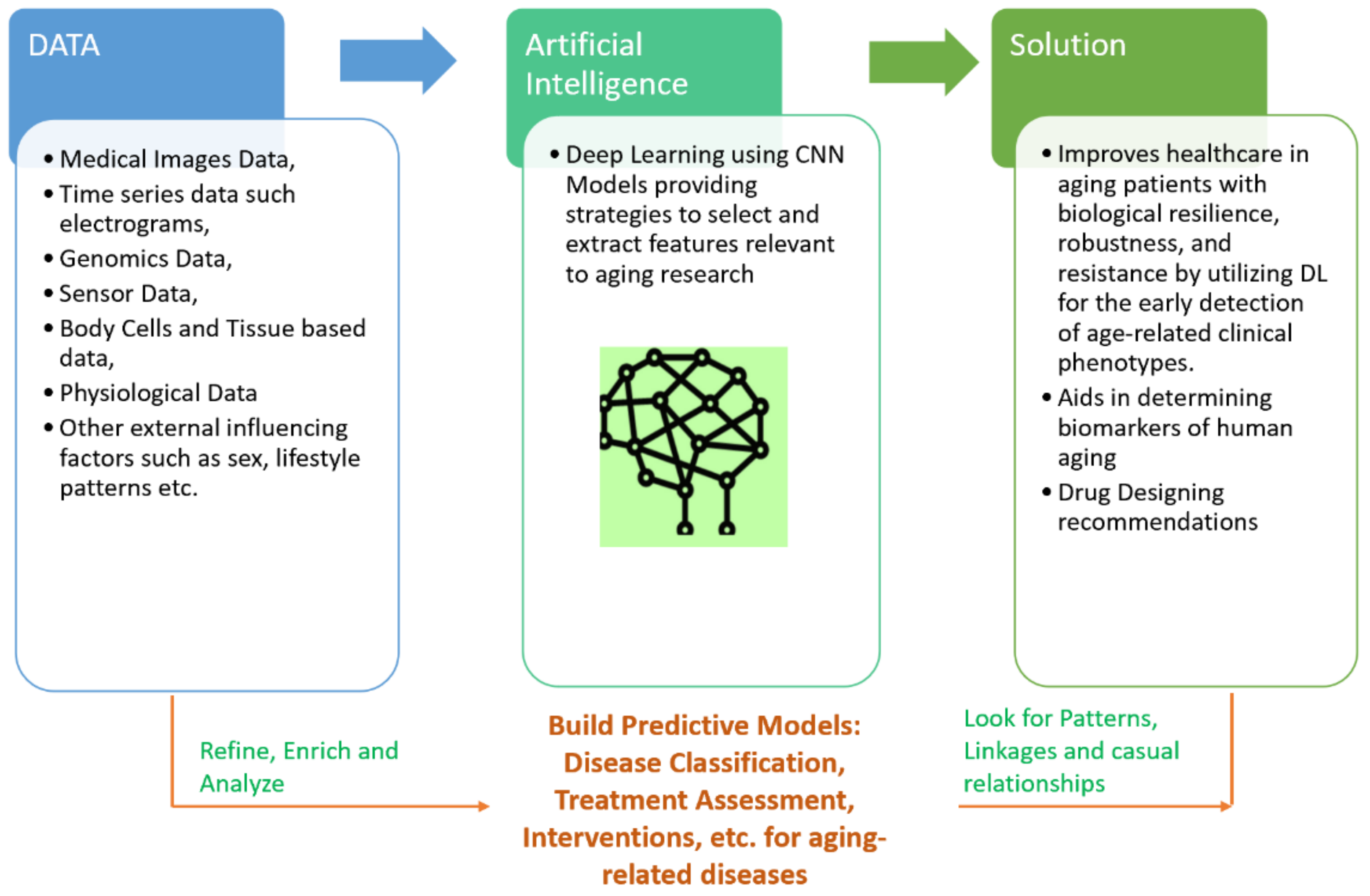
1.1. Conceptual Modelling with DL to Solve Aging-Related Diseases
1.2. Background on DL Models
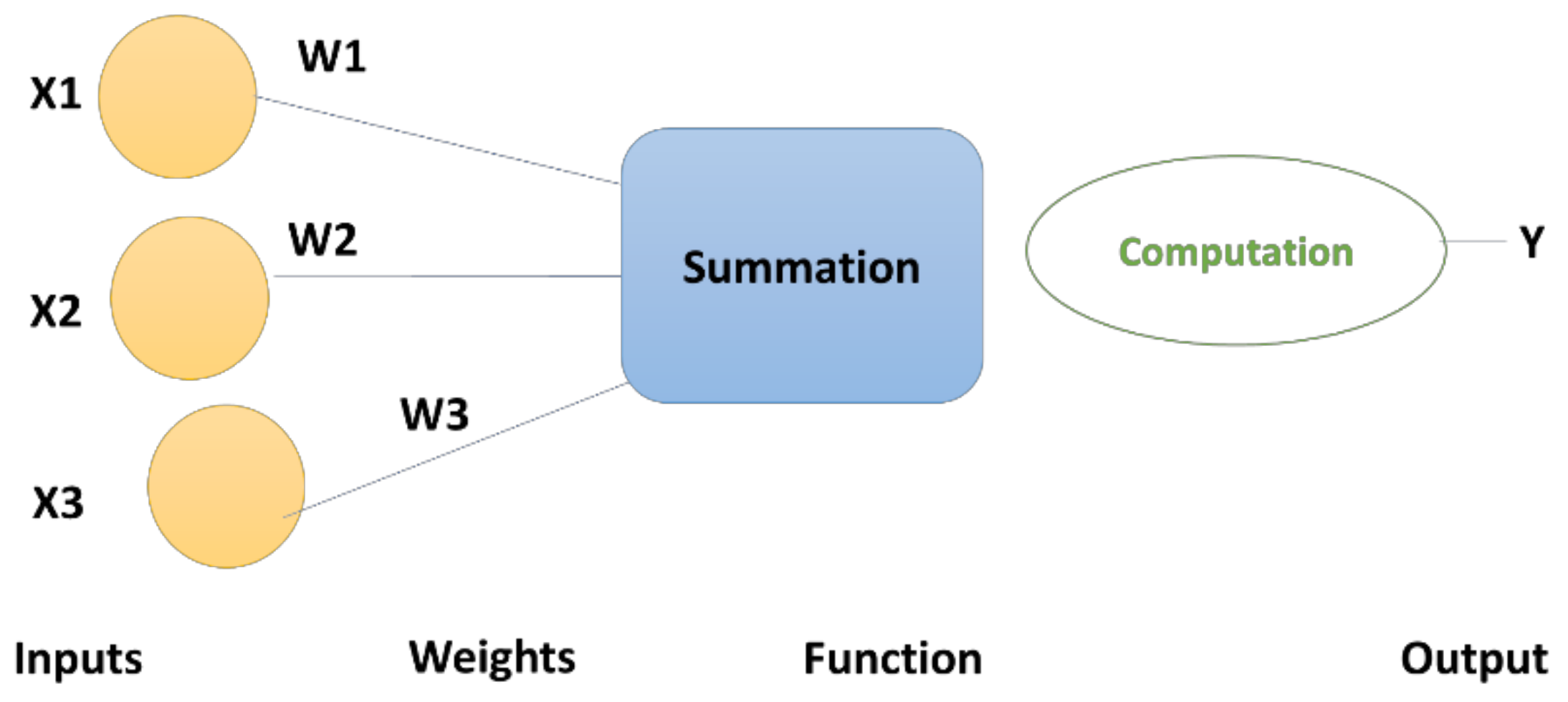
- DNN
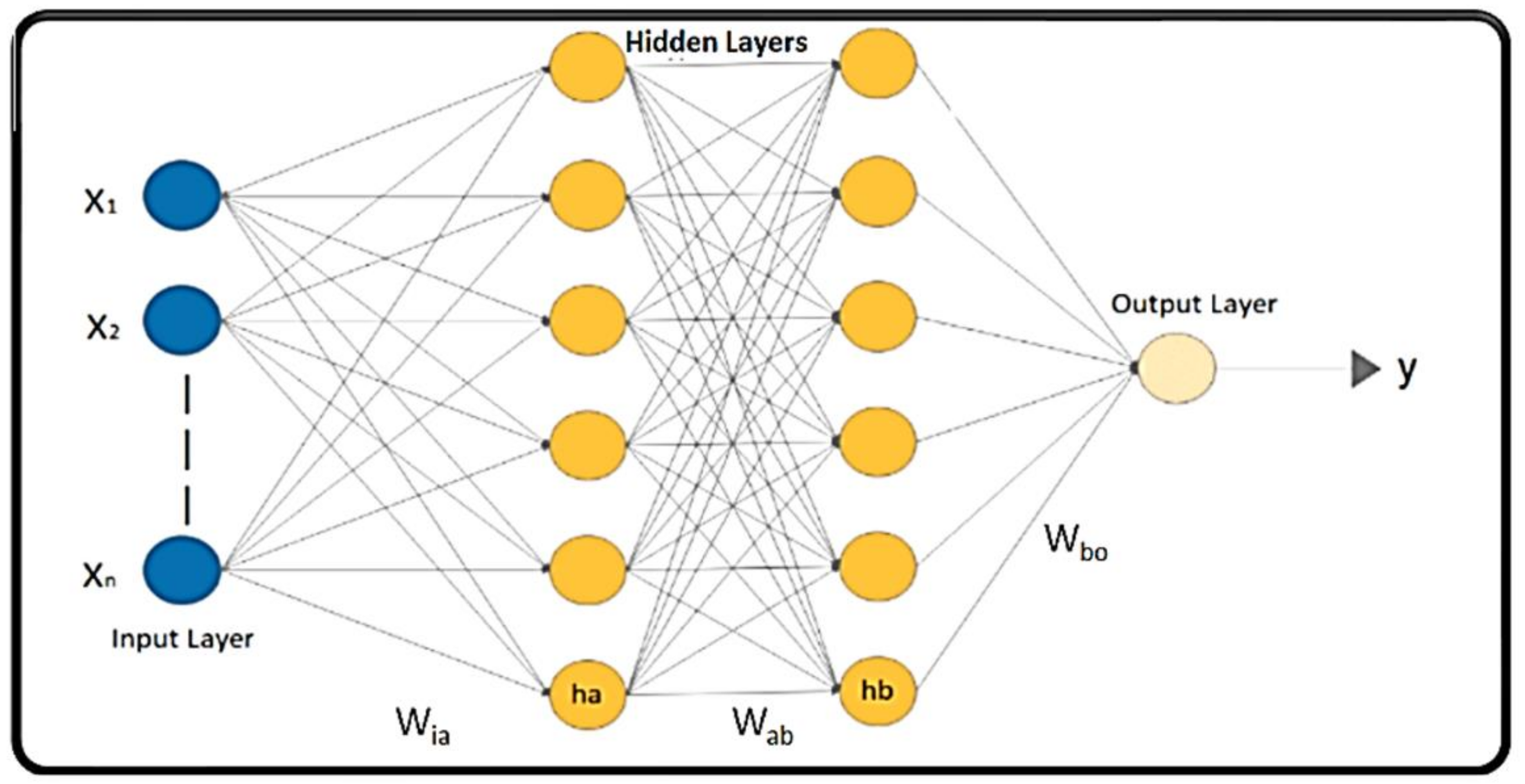
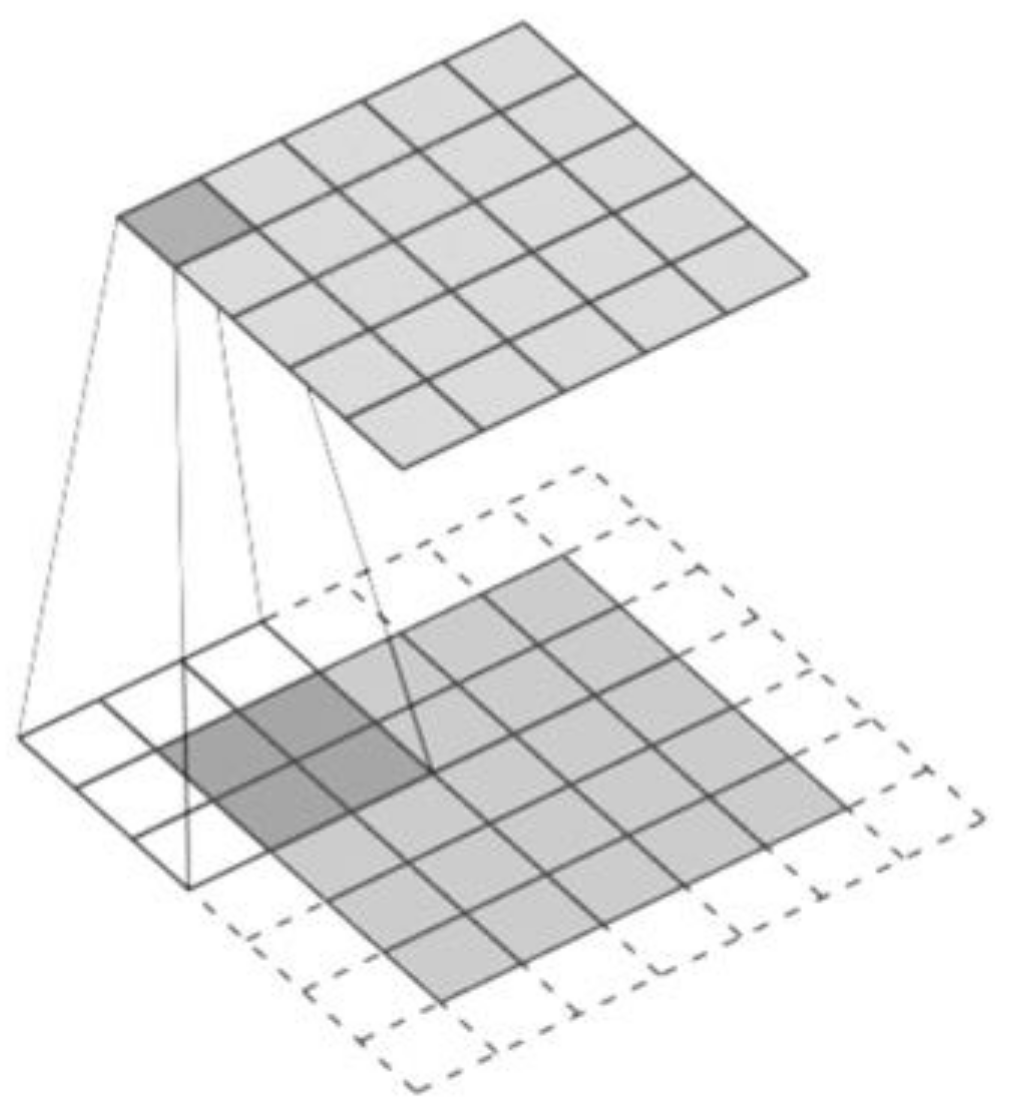
- CNN
- AE
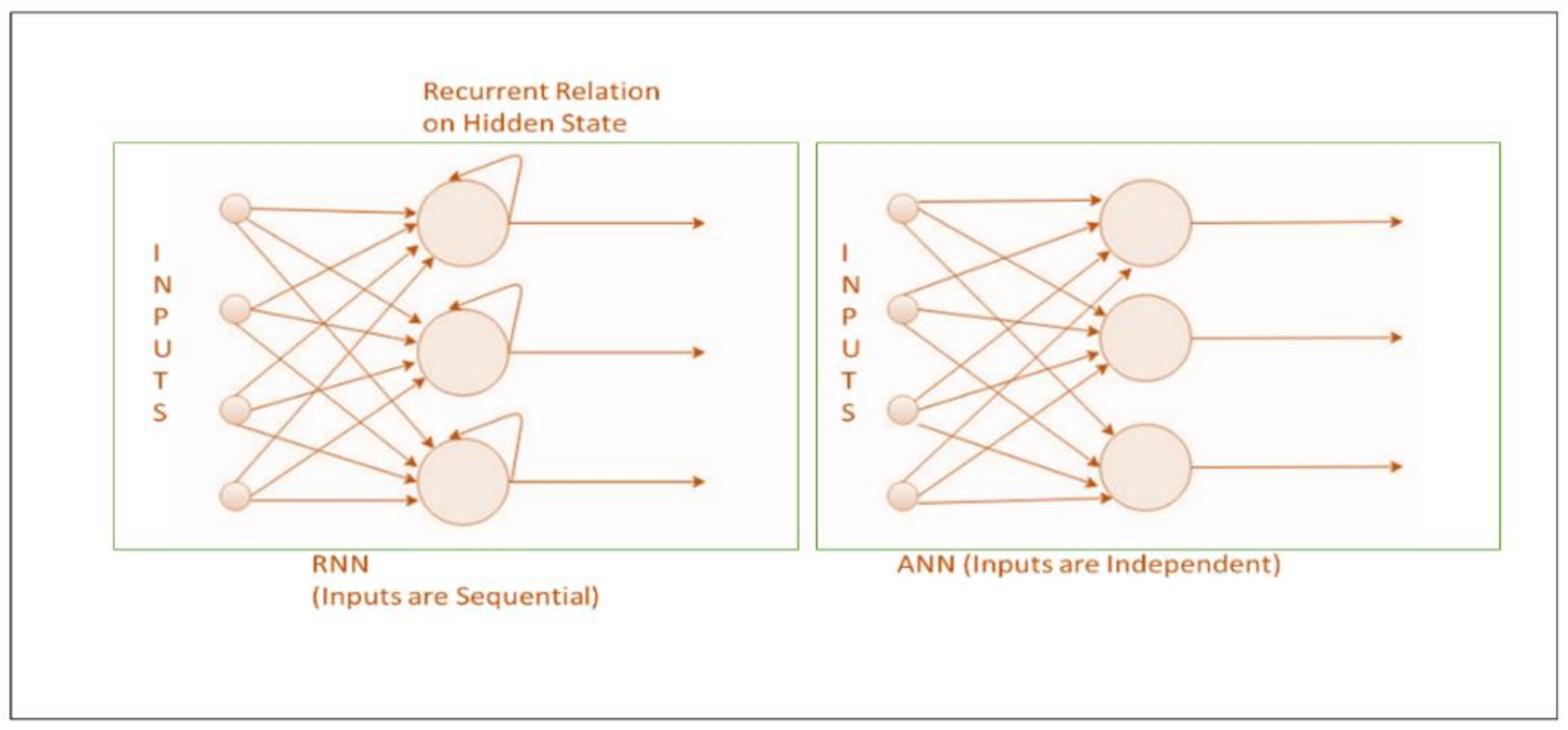
- RNN
- DBN
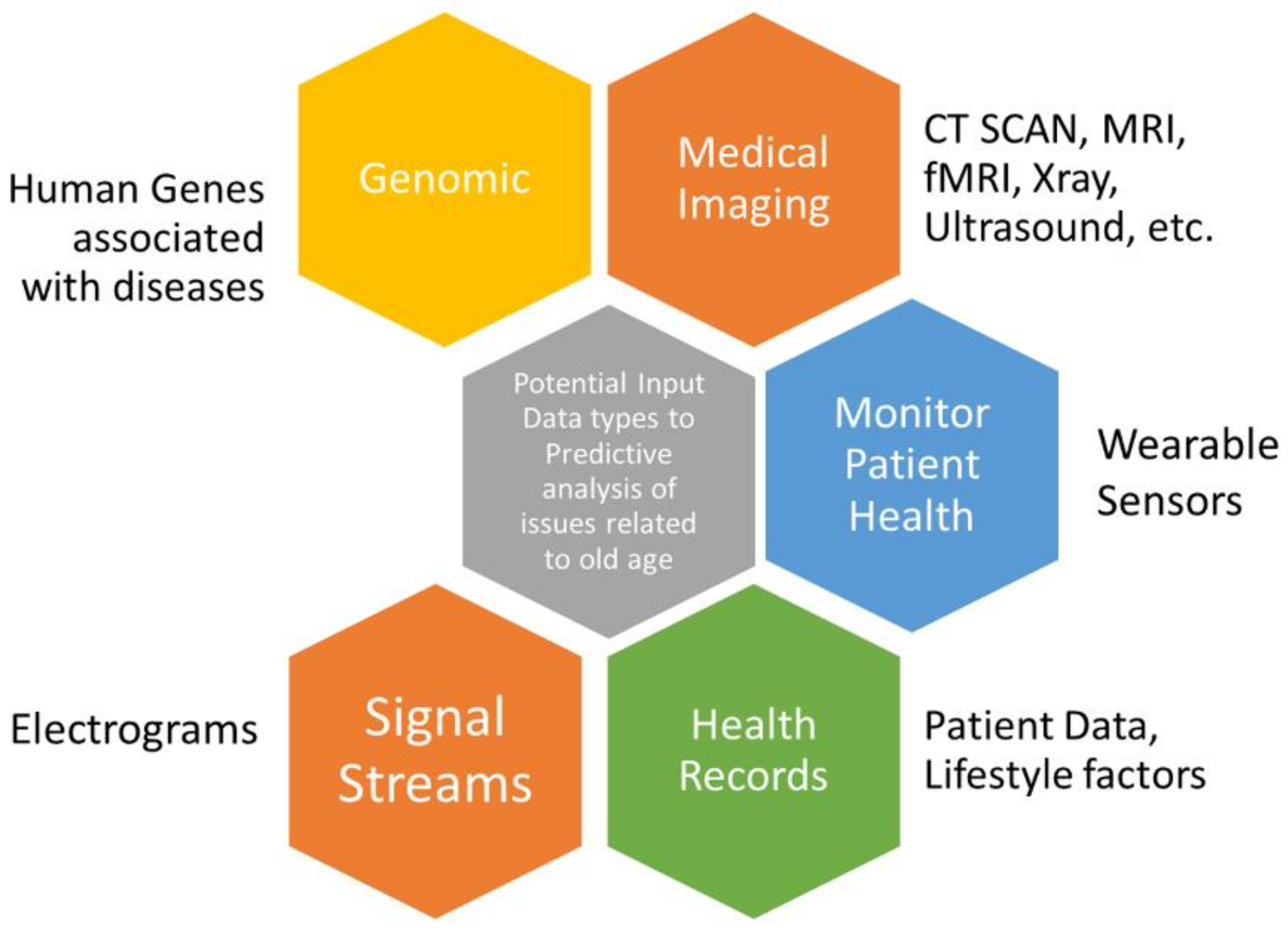
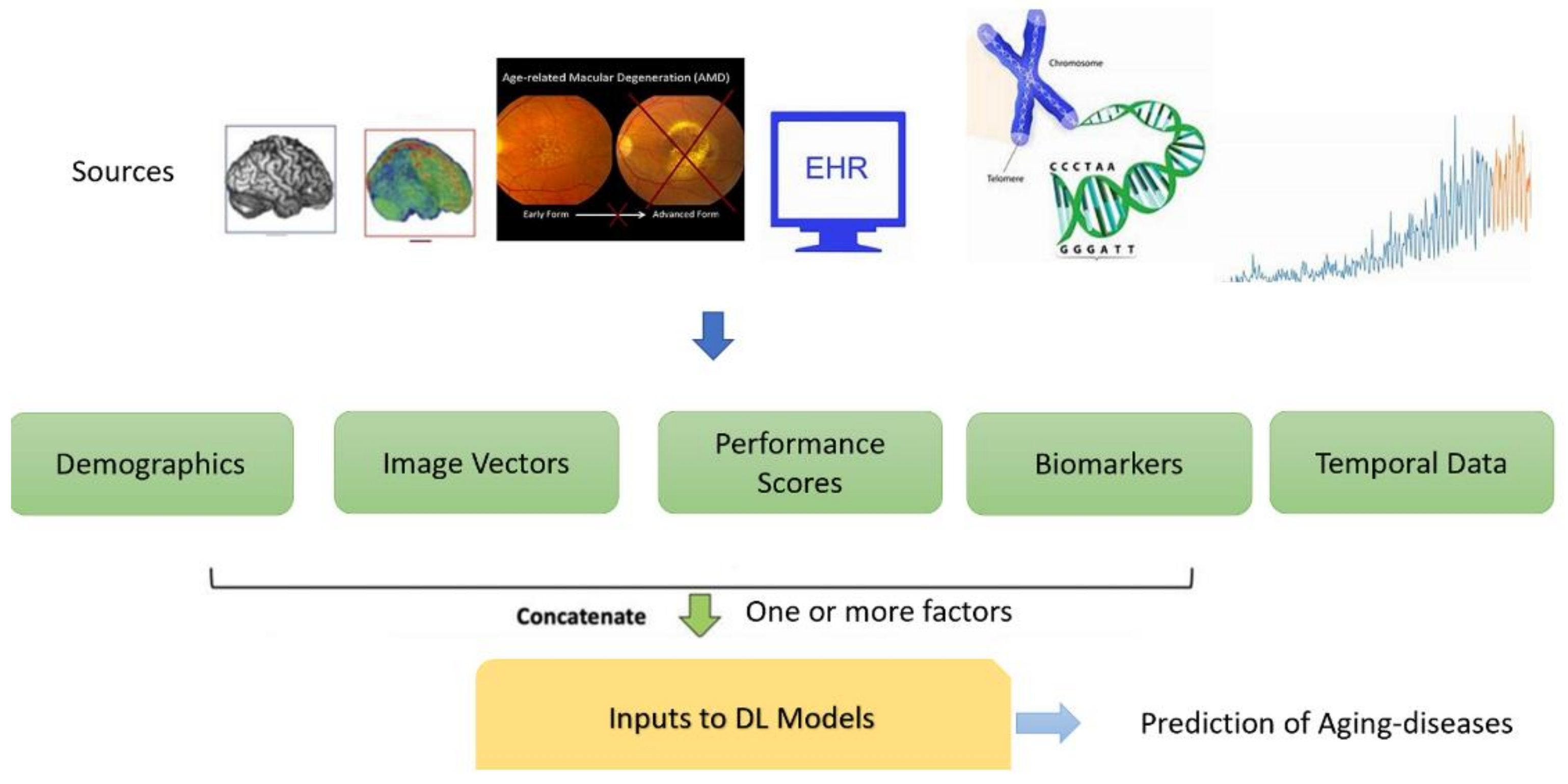
1.3. Data Types Pertaining to Age-Related Diseases
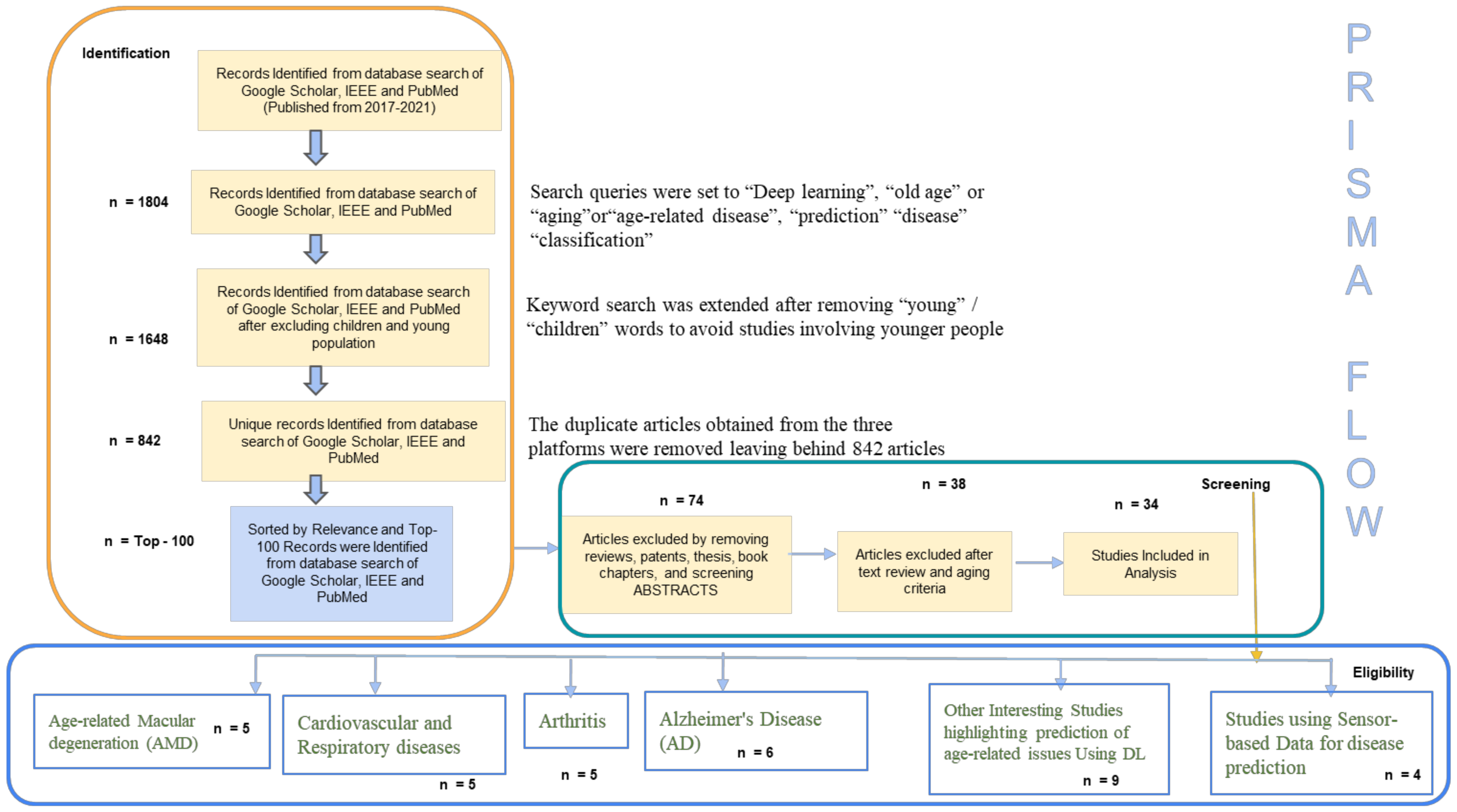
2. Methods
- Identification
- Screening
- Eligibility
3. Results
- Age-related macular degeneration (AMD)
- Cardiovascular and Respiratory Disorders in Aging People
- Aging People and Arthritis
- Alzheimer’s Disease (AD): A Common Disease in Aging People
- Prediction over Spectrum of Age-Related Issues
4. Discussion
- DL learns the important patterns or relationships in large amounts of healthcare data and allows clinicians to perform model-based analysis integrated with their observations; leading to smart care achievable from such big data.
- Remarkably, DL has achieved human-level performance in disease classification, learning over patterns/objects contained in medical images.
- When DL is applied over the training data, it becomes more precise with multi-stream architecture and subsequently provides more accurate insights into care processes and diagnostics of aging diseases.
- DL helps in the detection of clinically relevant features by learning patterns in medical imaging data beyond as perceived by a human observer/clinician.
- DL approaches are now leading to lower costs and improved and faster outcomes in monitoring the health of aging people.
- DL provides end-to-end learning models for heterogenous, uncertain and complex medical data.
- DL provides clinicians with the support they need to understand medical environments.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
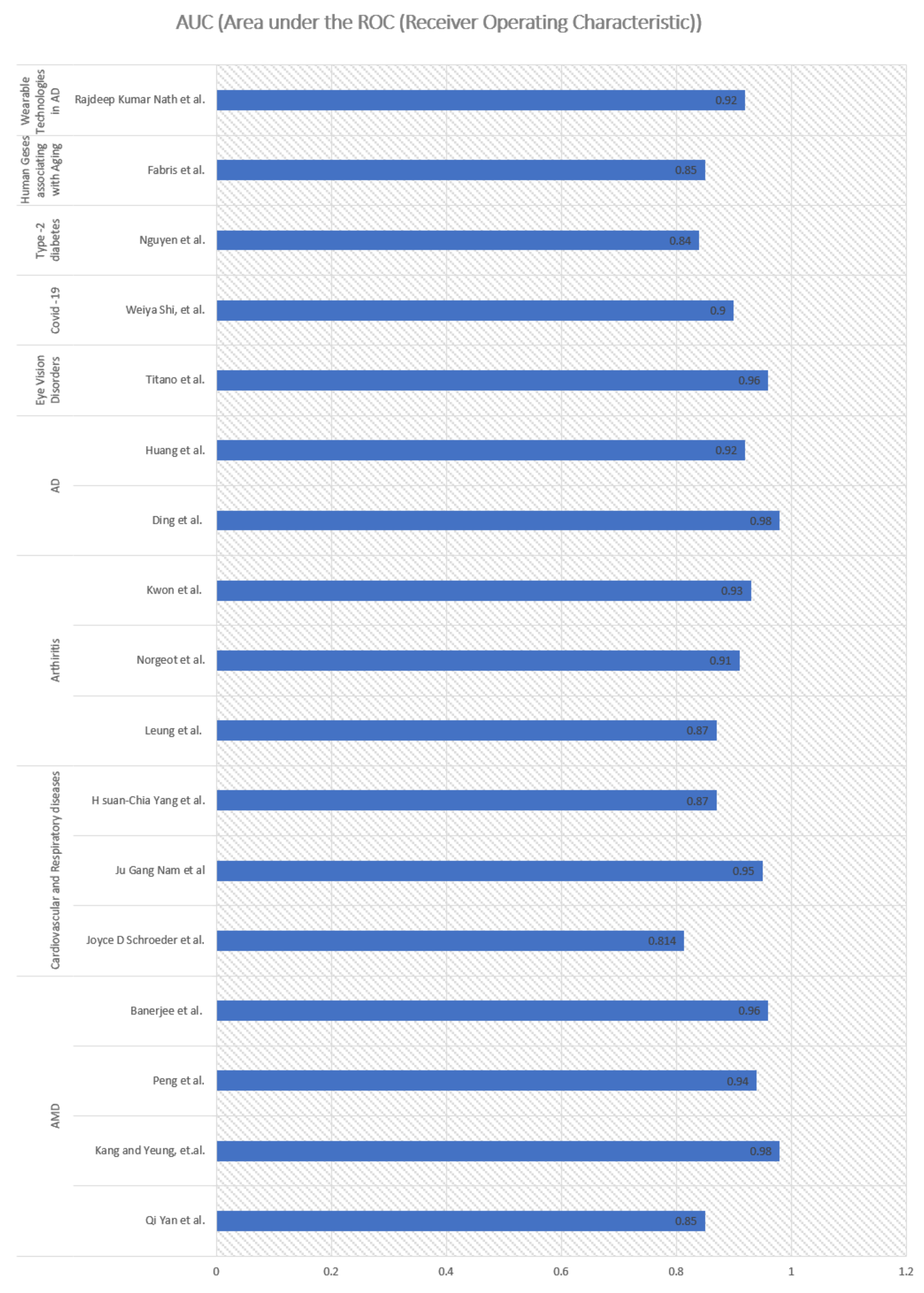
| Qi Yan et al. | In this study, both genotypes and fundus images are used to predict age-related macular degeneration (AMD) with a deep CNN. | The study used 31,262 fundus images and 52 AMD-associated genetic variants from 1,351 subjects of Age-Related Eye Disease Study over a period of 12 years. The subjects were between 55 and 80 years old at the baseline. The Inception-v3 CNN architecture was used to extract image features. Pre-trained weights were used to train the network using the ImageNet database. Three-level severity labels—no AMD, early or intermediate AMD; and late AMD were used in the study. | The study first time jointly used genotypes and fundus images in a prediction mode, enhancing the predictive performance of detecting severity of AMD. | AUC value of 0.85 (95% confidence interval 0.83–0.86). | [15] |
| Chuan and Yeung et al. | The study focuses on disease classification and to detect treatment-requiring retinal vascular diseases including diabetic macular edema (DME), neovascular age-related macular degeneration (named), myopic choroidal neovascularization (mCNV), branch and central retinal vein occlusion (BRVO/CRVO) in elderly people using multimodal imaging. | The study was conducted over the enrolled participants with multimodal ophthalmic imaging data from 3 hospitals in Taiwan from 2013 to 2019. EfficientNetB4 [106] was used as the convolutional neural network (CNN) as the multimodal classification model. | First study to use multimodal DL–based architecture for detecting multiple retinal vascular diseases using multiple image modalities, including retinal fundus photography, OCT, and FA/ICGA, for predicting neovascular retinal diseases. Developed model does not depend on a fixed image distribution for different modalities. The model highlighted areas with haemorrhage in retinal fundus images of subjects | High AUCs for detecting mCNV, DME, nAMD, BRVO, and CRVO were found to be 0.996, 0.995, 0.990, 0.959, and 0.988, respectively. | [67] |
| Peng et al. | The study deals with classification of patient-based age-related macular degeneration (AMD) severity from colour fundus photographs. | DeepSeeNet was developed consisting of three sub-networks by detecting AMD risk factors including pigmentary abnormalities for human eyes and then calculating a patient-based AMD severity score utilizing the age-Related Eye Disease Study (AREDS) simplified severity-scale. DL model was trained on 58,402 images and tested on 900 images obtained from the 4549 participants from AREDS. DeepSeeNet consists of three constituent parts that contribute to its output: (a) a sub-network, Drusen-Net (D-Net), which detects drusen in three size categories (small/none, medium, and large); (b) a sub-network, Pigment-Net (P-Net), which detects the presence or absence of pigmentary abnormalities (hypopigmentation or hyperpigmentation); and (c) a sub-network, Late AMD-Net (LA-Net), which detects the presence or absence of late AMD (neovascular AMD or central GA) DeepSeeNet is publicly available online: https://github.com/ncbi-nlp/DeepSeeNet accessed on 17 October 2021). AMD possibility increases exponentially with age: as estimated by meta-analysis at 6% at 80 years and 20% at 90 years. | The study proposed a model for better clinical decision support, mimicking the human grading process by first detecting individual risk factors of drusen and pigmentary abnormalities in each eye and then combining values from both eyes to develop an AMD score for the patient. This study shows that DeepSeeNet performed patient-based AMD severity classification with a higher level of accuracy than as predicted by group of human retinal specialists. | High AUC of >0.94 in detection of severity of AMDs. | [68] |
| Burlina et al. | The study highlights how DL algorithms could better characterize age-related macular degeneration from fundus images, assessing the severity of Age-Related Eye Disease. | The study utilized the AREDS data set from National Institutes of Health, including samples from 13 November 1992 to 30 November 2005, dealing with 67,401 colour fundus images from the 4613 study participants. To perform classification, deep convolutional neural networks (DCNNs) of ResNet-50 network was used. It consisted of many computational layers that performed convolutions and the related nonlinear activation operations. | DL outlines fundus and OCT image analysis, which further helps in refining retinal diagnostics in AMDs | Predicted retinal diagnostics in AMDs MAE of 3.5% to 5.3%. | [69] |
| Banerjee et al. | The study proposed hybrid sequential prediction model called “Deep Sequence”, integrating imaging demographic, and visual features with DL to predict the risk of exudation within AMD suffering eyes where eyes may convert from dry to wet when patients who progress to AMD. This may cause loss of vision too. | The study was conducted over the clinical trial dataset that includes 671 AMD fellow eyes with 13,954 observations of patients up to 85 years of age. It analysed longitudinal OCT imaging features and demographic information in an RNN model to predict the exudative event in eyes with AMD within 3–21 months span. | The importance of this study lies in integrating advanced imaging with sequential deep learning, RNN model, for making predictions about AMD disease progression. | Predicted exudation within AMD in the short-term (within 3 months) with 0.96 ± 0.02 AUC, and long-term (within 21 months) with 0.97 ± 0.02 AUC. | [16] |
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Pengbo Zhang and Fen Xu | The study analyses and explore the application value of DL for the prediction of possible complications of coronary heart disease, and its effect on improvement of nursing and care. | DL was applied to data of 182 patients (age from 48 to 80 years old, average age: (65.27 ± 7.34) years old), collected from health records, including their previous medical history, clinical diagnosis, examination results, abnormal indicators, living habits and other information. | High-risk patients with coronary heart disease indicate relation with old age, medical history, characteristics such as lack of cognition and unhealthy lifestyle. DL Application could effectively predict the risk of related complications of heart diseases in a more accurate way. | The proposed model attained a high Accuracy of 87.5%. | [70] |
| Goallec et al. | Heart disease is one of the primary causes of death after age 65 and, with the world population aging. This study gain insights from DL models aiding in predicting heart age. | The study involved training of magnetic resonance videos MRI videos with 3D CNN, images with 2D CNN, time series ECG with 1D CNN over 45,000 heart MRI and electrocardiograms [ECG] from the UK Biobank within the range 45–81 years. | The study reported biomarkers, clinical phenotypes, diseases, environmental and socioeconomic biomarkers associated with accelerated heart aging. The study also highlighted the aorta, the mitral valve, and the interventricular septum as key anatomical features driving heart age prediction. | MRI-based anatomical features predicted age better than ECG-based electro-physiological features (RMSE = 2.89 ± 0.02 years vs. 6.09 ± 0.0.02 years). | [71] |
| Joyce D. Schroeder et al. | The study aims to predict Chronic obstructive pulmonary disease (COPD) using DL methods. | The study uses 6749 two-view chest radiograph exams (2012–2017) involving mean age as near to 60 years, also discussing COPD case of 62-year-old female. The frontal and lateral images are fed as inputs to two parallel convolutional neural networks (CNN) with pulmonary function tests (PFT) annotation. | A CNN Model trained on chest radiographs for quantitative prediction of COPD performs better than state-of-the-art algorithms of Natural Language Processing (NLP) in the field, attaining good accuracy. | AUC of 0.814 for prediction of obstructive lung disease. | [72] |
| Ju Gang Nam et al. | Detecting 10 common abnormalities (pneumothorax, mediastinal widening, pneumoperitoneum, nodule/mass, consolidation, pleural effusion, linear atelectasis, fibrosis, calcification and cardiomegaly) to evaluate its impact in predictive diagnostic and judging the timeliness of reporting. | The proposed approach used a ResNet34-based deep CNN over samples with mean ± SD age 57.6 ± 17.9 years on the chest radiographs. | The proposed model advanced the reporting time for critical and urgent cases, aiding better health in elderly people. | The study successfully detected 10 common abnormalities in two external validation datasets with high AUCs, ranging from 0.895 to 1.00. The training data of were curated by radiologists mostly without CT reference, intended to resemble radiologists’ performance, resulting in better results. | [73] |
| H. Suan-Chia Yang et al. | To predict a patient’s risk of developing lung cancer, using DL approaches | The analysis included 11,617 patients with lung cancer and 1,423,154 control patients with mean age 66 years. A total of 9261 cases of lung cancers were identified in subjects with age >= 55. CNNs have been applied to radiographic images of chest and to facilitate detection and low-dose computed tomography classification of pulmonary nodules in lungs. Xception architecture which includes a 126-layer CNN-based neural network with a moderate number of parameters, was used for feature extraction. | The study involved time-related sequential information from the medical histories to evaluate lung cancer risk in patients rather than relying on does not rely on smoking status, socioeconomic status, or BMI. | AUCs of 0.87 in patients with age ≥ 55. | [74] |
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Kalweit et al. | The study investigated use of DL for the prediction of rheumatoid arthritis. | An adaptive recurrent neural network (AdaptiveNet), a dynamic and recurrent deep neural network architecture, designed for chronological clinical data was trained on the data collected from over 9500 patients from the Swiss Quality Management (SCQM) database. | Disease prediction was improved over patients having longer disease duration, age > 50 or having antibody positivity. Also, when compared to the ML models of linear regression, random forest and support vector machines, AdaptiveNet showed an increased performance. | Accuracy of 75.6% was achieved for disease prediction. | [75] |
| Leung et al. | A multitask DL model was applied over knee radiographs to accurately classify patients with high-risk osteoarthritis in both patients who underwent knee replacement (TKR) and the control patients who did not. | The proposed model used a transfer learning approach based on the ResNet34 architecture to evaluate 728 participants (with mean age, 64 years ± 8). | Accurately predicted osteoarthritis in patients who would be requiring knee replacement in 9 years. | AUC of 0.87 (95% confidence interval [CI]: 0.85, 0.90). | [76] |
| Norgeot et al. | Longitudinal DL models were developed for predicting disease activity of rheumatoid arthritis | A fully dense DL architecture was applied over the data of 820 patients that were extracted from the EHRs of 2 different hospitals: a university hospital (UH). rheumatology clinic (University of California, San Francisco) and a rheumatology clinic from a safety-net hospital (SNH) (Zuckerberg San Francisco General Hospital) (mean age, 57–60 ± 15 years). | Models developed for predicting rheumatoid arthritis disease from EHR data, is informative with quantifiable outcomes in the outpatient setting. | AUC of 0.91 (95% CI, 0.86–0.96) | [77] |
| Hirano et al. | The study predicted radiographic finger joint destruction in rheumatoid arthritis (RA). | The network of the CNN consists of two convolution layers, two pooling layers and three fully connected layers was applied over the data of 216 radiographs of 108 patients with RA, for joint evaluation. Finger joints, such as PIP, IP and MCP joints, were detected by the model and scoring scheme was used by CNN to judge the differences between joints. Radiographs in the testing dataset were used to evaluate the trained CNN model by comparing scores assigned by the model and by clinicians. Interquartile range of (53.5, 72.6) years old was taken for this study. | Processing radiographic images and determine joint destruction with the trained convolutional neural network model is promising. The authors claimed to apply CNN for the first time to detect joint destruction in RA, (particularly joint space narrowing and bone erosion of the fingers). | Accuracy reached 49.3–65.4% for Joint space narrowing and 70.6–74.1% for detecting erosion of joints. | [78] |
| Kwon et al. | The study aimed at developing an automated classification model for Knee osteoarthritis (KOA) based on features extracted from DL model. | Radiographic image features extracted from a DL network, namely, Inception-ResNet-v2, were further exploited using a support vector machine for KOA multi-classification. | The proposed model outperformed a common DL approach that is based on using only radiographic images as the input data. | Highest AUC of 0.93 was achieved. | [98] |
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Qiao et al. | The study proposes DL classification framework with multivariate data-driven based feature extraction for automatic diagnosis of AD. | 34 participants with mean age 68.64 ± 9.85 years, were taken as sample from memory outpatient clinic at the Huashan Hospital of Fudan University. A total of 34 participants with mean age 65.55 ± 8.98 years, were invited by public advertisement to take part in the study. The proposed method was based on a three-level hierarchical partner matching independent component analysis (3LHPM-ICA) and Granger causality (GC) to determine effective connectivity features playing role in AD diagnosis. | Identified brain features that can serve as important biomarkers for AD. | Accuracy of 95.59% in diagnosing AD | [80] |
| Qureshi et al. | The study performed automatic assessment of dementia severity using a DL framework applied to resting-state functional magnetic resonance imaging (rs-fMRI) data. | The demographics included the participants with mean age of 73. The 3D-CNN-based DL classification framework is used in this study to assess dementia. | The research supported automatic classification of AD into two groups of disease severity (very mild and mild vs. moderate and severe) enabling important contributions for clinical practice. | Accuracy of >90% was achieved for the disease classification. | [81] |
| Choi and Jin et al. | The study aims to develop an automatic image interpretation system based on a deep convolutional neural network (CNN) to predict future cognitive decline in mild cognitive impairment (MCI) patients using flurodeoxyglucose and florbetapir positron emission tomography (PET) | Deep CNN was trained using 3-dimensional PET volumes of AD. The data used in this study included subjects recruited in Alzheimer’s Disease Neuroimaging Initiative-II (ADNI-2) with available baseline data on FDG and AV-45 PET (http://adni.loni.usc.edu, accessed on 17 October 2021) with a mean age of 73 years. | Importance of DL as a practical tool for developing predictive neuroimaging biomarker. | Accuracy of 84.2% to predict cognitive decline in AD. | [82] |
| Ding et al. | A DL algorithm is used for an early prediction tool for Alzheimer’s disease providing therapeutic intervention by using biochemical and imaging tests. | PET imaging studies from 1002 patients, from Alzheimer’s Disease Neuroimaging Initiative (ADNI)-1, ADNI2, and ADNI-GO (Grand Opportunities) studies was considered. The average age of the patients was 76 years (range, 55–93 years) for men and 75 years (range, 55–96 years) for women. Convolutional neural network architecture, Inception v3, was used in this study stacking over 11 modules where each module consists of pooling layers and convolutional filters with rectified linear units as activation function over the two-dimensional images of 16 horizontal sections of the brain. | A DL algorithm developed for successful early prediction of Alzheimer’s disease by using fluorine 18 fluorodeoxyglucose PET of the brain. | AUC of 98% for predicting AD. | [83] |
| Lu et al. | The study is aimed at identifying the stage of Alzheimer’s Disease (AD) patients through the use of mobility data and DL models. | The study applied a state-of-the-art architecture deep convolutional neural network, Inception-ResNet-V2, to pre-train brain images involving elderly people of age > 65. | Modelling to classify AD, showed outstanding characteristics as a progression biomarker. Interestingly, the study involved weighted brain structural image and information on participant sex. | Accuracy of AD classification found to be >90%. | [84] |
| Huang et al. | The research proposes a practical brain imaging-based AD diagnostic classifier using DL/transfer learning on MRI dataset of unprecedented size and diversity. | The data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. The study chose 2861 T1-MR images, including AD subjects with a mean age of 76.13 ± 7.50. The study proposed a multi-modality CNN-based classifier for AD diagnosis and prognosis. | The work distinguishes between AD or potential AD patients from cognitively unimpaired (labelled as CN) subjects accurately and automatically using medical images to facilitate a fast-preclinical diagnosis. | AUC of 92.01% to differentiate between AD and Controls. | [85] |
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Titano et al. | The study applied a novel DL architecture to a clinically heterogeneous set of three-dimensional optical coherence tomography (OCT) scans from patients referred to a major eye, London, United Kingdom. | 3D U-Net architecture was used to determine referral recommendation to clinicians for disease prediction involving OCT scans of majority people of age > 50, on a range of sight-threatening retinal diseases. Data was collected about raw retinal OCT scans around the macula). Deep segmentation network was trained with manually segmented OCT scans to determine tissue map images. Deep classification network, was then trained over the tissue maps with diagnoses of disease with predicted diagnosis probabilities providing recommendations to the clinicians. | The study presented a novel framework that analyses clinical 3D OCT scans and makes referral suggestions to a standard comparable to clinical experts. | AUC of >0.96 with a taxonomy of different diagnostic labels suggested to clinicians. | [89] |
| Betancur et al. | The study applied DL for prediction of obstructive disease from myocardial perfusion imaging (MPI). Obstructive disease was defined as ≥70% narrowing of coronary arteries. | The study comprised of 1638 patients from 2008 to 2015, at 9 national and international (Canada, Switzerland, Israel) sites with mean age of 65.2 ± 11.0 in obstructive disease. The feature maps comprised of 3 fully connected layers transformed the image features into the final scores. | The potential of DL for prediction of obstructive diseases from MPI, as compared with the standard quantitative analysis of total perfusion deficit (TPD). | AUC disease prediction by DL was higher (0.80 per patient) than current standard method (0.78 per patient). | [90] |
| Weiya Shi et al. | The study applied DL model based on quantitative computed tomography (CT) images to predict the severity of current global pandemic COVID-19. | 196 patients with confirmed COVID-19 those were enrolled from 20 January to 10 February 2020, Shanghai Public Health Clinical Centre, Fudan University, China, were considered for analysis. CT images help in studying prognostic and diagnostic characteristics of COVID-19, the quantification of these with DL is useful to predict he severity of disease. The median age of the severe group considered in this study was 65 years. Methodology utilized the V-shaped neural network (V-Net) which is a convolutional neural network (CNN) and combined it with transfer learning to segment the infected regions on images. | DL over CT model containing radiological features serve as an efficient tool for prediction of COVID-19 severity. CT suggests disease progression and it was found that with other age-related diseases, CT has prognostic value for predicting mortality. | A high AUC of 0.900 was achieved for predicting COVID severity. | [91] |
| Varadarajan et al. | DL is applied to extract novel features such as refractive error from retinal fundus imaging in a novel way. | Retinal fundus images used in this study were obtained from the UK Biobank and the Age-Related Eye Disease Study (AREDS) clinical trials. Refractive error is one of the most common causes of visual impairment in age related issues worldwide. A DL model residual network (ResNet) consisting of three residual blocks, an attention layer to learn the most predictive eye features, and two fully connected layers was trained to predict the refractive error from fundus images. Poor quality images were excluded to be fed into DL model. | Successfully found features that predict refractive error in retinal fundus in patients. | MAE of 0.91 D (95% CI: 0.89–0.93) was obtained on the AREDS clinical validation data set. | [92] |
| Nguyen et al. | The study applied DL model combined with generalized linear model improve the prediction of the onset of type 2 diabetes mellitus (T2DM) | The dataset consists of identified electronic health records for 9948 patients, of which 1904 have been diagnosed with T2DM of age-range up to 84 years. | Using DL over EHRs aids better in predicting the onset of diabetes and useful in improving the quality and efficiency of medical care | AUC of 84% is achieved by proposed method. | [19] |
| Jonsson et al. | Aging has a significant structural impact on the brain that correlates with decreased mental and physical fitness. The current study focusses on determining brain age to predict neurodegenerative diseases from a T1-weighted MRI. | The proposed method uses a 3D CNN trained on MRIs to predict brain age. CNN is validated on 104 images from the IXI dataset left out during training (validation set) and tested on 12,395 images from the UK Biobank dataset. CNN derives on five residual blocks, each followed by a max pooling layer of stride 2 × 2 × 2 and kernel size 3 × 3 × 3, and one fully connected block. | T1-weighted MRIs are useful to predict brain age associating them with neuro degenerative diseases and achieved promising results. | MAE of 3.996 was achieved with CNN. | [17] |
| Fabris et al. | The study was aimed at predicting whether human genes are associated with several age-related diseases at the same time using Deep Neural Network (DNN) methods. | DNN with three fully connected layers followed by an output layer to predict whether each s gene is associated with age-related diseases (27 classes of diseases). Human protein coding genes were obtained from NCBI BioMart v. 87, and age-related disease gene associations were the subset of the Genetic Association Database. | Popular genes were identified that are strongly linked to aging-related diseases. Use of DNN improved the predictive performance. | AUC > 85% was achieved with DNN for predicting age-related diseases. | [48] |
| Basheer et al. | The study aims towards early prediction of dementia using CNN (convolutional neural networks), a DL method is over neuroimaging data | 150 subjects aging from 60 to 96 years old. The study fed images in a type of image retrieval system, using the CNN method and the proposed technique of using CapNet. The proposed model uses the modified capsule network technique for classifying the dementia groups into demented and non-demented category. | Age is the most dependent feature for Dementia. The proposed model can handle hierarchical modelling problems and is computationally more efficient with better accuracy as compared with the other models. | Accuracy of 92.39% for predicting dementia. | [93] |
| Cheon et al. | The study proposes use of deep neural networks to detect stroke patient mortality using medical service and health behaviour data | Subjects collected from 2013 to 2016 by the Korea Centres for Disease Control and Prevention with mean age of 66 years. The study proposed DNN/scaled principal component analysis (PCA) to automatically generate features from the data and identify risk factors for stroke. Feed-forward neural networks were trained using a standard backpropagation. | Early detection of patients at high risk of stroke who would need additional attention before disease exacerbation. | AUC of 83.48% to predict stroke patient mortality. | [94] |
| Authors | Application | Material and Methods | Important Findings | Performance | Reference |
|---|---|---|---|---|---|
| Papagiannaki et al. | Use of DL in physiological monitoring of older people using wearable sensors for improving their health status and quality of life. | The study involved convolutional neural network architectures (CNNs) in recognizing movement patterns of older people. Three CNN layers were used and of which each convolutional layer is followed by a normalization layer and a rectified linear unit (ReLU) activation unit. The recordings were obtained from older people aged 70–92 who participated in the FrailSafe project available online: https://frailsafe-project.eu/ (accessed on 17 October 2021). | The study proposed DL-driven activity recognition scheme for older people involving feature extraction with classification demonstrating advantages of DL techniques. | More than 80% Accuracy over different smart sensor locations in physiological monitoring of old people. | [95] |
| J. Prince et al. | The study proposes detection and assessment of Parkinson’s disease (PD) using wearable sensors, highlighting the suitability of DL over the collected data. | Analyses was conducted over the sample of 1815 participants over the age of 45 years. | The study indicated similarity between classification of manually extracted feature set, and the features learnt by a CNN in diagonisis of PD. The study also indicated the suitability of DL for assessing PD when large population is available | DL approaches capable of PD disease classification, performed better than traditional methods. | [86] |
| Mishkhal et al. | The purpose of this study is to identify daily activities that consider a key to falling cases for older adults at care homes. | A deep activity CNNet was used to determine features recorded from daily activities sensors data and to associate them with highest falling risk activities in testing data. People aged 66–86 years, participated in the experiments. | DL techniques aid in effectively assessing fall risk based on wearable sensors looking for a way to keep the elder people’s lives safe and better. | Achieved an Accuracy of >96% in different settings. | [87] |
| Rajdeep Kumar Nath et al. | To distinguish between stressed and non-stressed state in older adults using wearable technologies and application of DL to improving the quality of life in aging. Chronic stress is one of the risk factors in contributing to the development of Alzheimer’s Disease (AD) also in older people. | A finger-tip sensor was used during the Trier Social Stress Test, to reliably induce stress in humans (within social setting) to record signals. DL model of recurrent neural network using Long Short-Term Memory (LSTM) was applied over the collected data to classify older people into stressed or non-stressed state. Data of 19 healthy older adults (11 female and 8 male) with mean age 73.15 ± 5.79 were used for this study. | DL-based LSTM classifier attained better performance than traditional ML models in distinguishing between stressed and non-stressed state. An efficient system for unobtrusive wearable device system for physiological stress detection in older adults was devised aiding in prevention of cognitive decline in older adults. | High AUC score of 0.90 was achieved with DL using LSTM. | [88] |
| Lu et al. | The study is aimed at identifying the stage of Alzheimer’s Disease (AD) patients through the use of mobility data and DL models. | The study applied a state-of-the-art architecture deep convolutional neural network, Inception-ResNet-V2, to pre-train brain images involving elderly people of age > 65. | Modelling to classify AD, showed outstanding characteristics as a progression biomarker. Interestingly, the study involved weighted brain structural image and information on participant sex. | Accuracy of AD classification found to be >90%. | [84] |
| Huang et al. | The research proposes a practical brain imaging-based AD diagnostic classifier using DL/transfer learning on MRI dataset of unprecedented size and diversity. | The data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. The study chose 2861 T1-MR images, including AD subjects with a mean age of 76.13 ± 7.50. The study proposed a multi-modality CNN-based classifier for AD diagnosis and prognosis. | The work distinguishes between AD or potential AD patients from cognitively unimpaired (labelled as CN) subjects accurately and automatically using medical images to facilitate a fast-preclinical diagnosis. | AUC of 92.01% to differentiate between AD and Controls. | [85] |
References
- Rose, M.R.; Flatt, T.; Graves, J.L.; Greer, L.F.; Martinez, D.E.; Matos, M.; Mueller, L.D.; Shmookler Reis, R.J.; Shahrestani, P. What is aging? Front. Genet. 2012, 3, 134. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Erin McNemar, M. Deep Learning, Predictive Analytics Helps Identify Chronic Diseases. Available online: https://healthitanalytics.com/news/deep-learning-predictive-analytics-helps-identify-chronic-diseases (accessed on 17 October 2021).
- Cao, C.; Liu, F.; Tan, H.; Song, D.; Shu, W.; Li, W.; Zhou, Y.; Bo, X.; Xie, Z. Deep Learning and Its Applications in Biomedicine. Genomics. Proteom. Bioinform. 2018, 16, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Nho, K.; Saykin, A.J. Deep Learning in Alzheimer’s Disease: Diagnostic Classification and Prognostic Prediction Using Neuroimaging Data. Front. Aging Neurosci. 2019, 11, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shoukry, S.; Rassem, T.H.; Makbol, N.M. Alzheimer’s diseases detection by using deep learning algorithms: A mini-review. IEEE Access 2020, 8, 77131–77141. [Google Scholar] [CrossRef]
- Yue, T.; Wang, H. Deep Learning for Genomics: A Concise Overview. arXiv 2018, arXiv:1802.00810. [Google Scholar]
- Kieu, S.T.H.; Bade, A.; Hijazi, M.H.A.; Kolivand, H. A Survey of Deep Learning for Lung Disease Detection on Medical Images: State-of-the-Art, Taxonomy, Issues and Future Directions. J. Imaging 2020, 6, 131. [Google Scholar] [CrossRef]
- Wang, H.; Pujos-Guillot, E.; Comte, B.; de Miranda, J.L.; Spiwok, V.; Chorbev, I.; Castiglione, F.; Tieri, P.; Watterson, S.; McAllister, R.; et al. Deep learning in systems medicine. Brief. Bioinform. 2021, 22, 1543–1559. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Hao, S.; Peng, X.; Hu, L. Deep learning for sensor-based activity recognition: A survey. Pattern Recognit. Lett. 2019, 119, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based. Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Erkan. Aging Problems. Available online: https://www.themost10.com/common-problems-of-aging/ (accessed on 17 October 2021).
- Lee, C.S.; Latimer, C.S.; Henriksen, J.C.; Blazes, M.; Larson, E.B.; Crane, P.K.; Keene, C.D.; Lee, A.Y. Application of deep learning to understand resilience to Alzheimer’s disease pathology. Brain Pathol. 2021, 31, e12974. [Google Scholar] [CrossRef]
- Putin, E.; Mamoshina, P.; Aliper, A.; Korzinkin, M.; Moskalev, A.; Kolosov, A.; Ostrovskiy, A.; Cantor, C.; Vijg, J.; Zhavoronkov, A. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging 2016, 8, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Weeks, D.E.; Xin, H.; Swaroop, A.; Chew, E.Y.; Huang, H.; Ding, Y.; Chen, W. Deep-learning-based prediction of late age-related macular degeneration progression. Nat. Mach. Intell. 2020, 2, 141–150. [Google Scholar] [CrossRef]
- Banerjee, I.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Osborne, A.; Rosenfeld, P.J.; Gregori, G.; Durbin, M.; Rubin, D. Prediction of age-related macular degeneration disease using a sequential deep learning approach on longitudinal SD-OCT imaging biomarkers. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Jonsson, B.A.; Bjornsdottir, G.; Thorgeirsson, T.E.; Ellingsen, L.M.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Stefansson, K.; Ulfarsson, M.O. Brain age prediction using deep learning uncovers associated sequence variants. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.S.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell 2018, 172, 1122–1131.e9. [Google Scholar] [CrossRef]
- Nguyen, B.P.; Pham, H.N.; Tran, H.; Nghiem, N.; Nguyen, Q.H.; Do, T.T.; Tran, C.T.; Simpson, C.R. Predicting the onset of type 2 diabetes using wide and deep learning with electronic health records. Comput. Methods Programs Biomed. 2019, 182, 105055. [Google Scholar] [CrossRef]
- Suresh, H.; Hunt, N.; Johnson, A.; Celi, L.A.; Szolovits, P.; Ghassemi, M. Clinical Intervention Prediction and Understanding with Deep Neural Networks. In Proceedings of the Machine Learning for Healthcare Conference, Boston, MA, USA, 18–19 August 2017; pp. 322–337. [Google Scholar]
- Quang, D.; Chen, Y.; Xie, X. DANN: A deep learning approach for annotating the pathogenicity of genetic variants. Bioinformatics 2015, 31, 761–763. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, D.; Seki, T.; Sawada, H.; Kunitomi, A.; Katsuki, T.; Kimura, M.; Ito, S.; Komuro, J.; Hashimoto, H.; Fukuda, K.; et al. Anti-senescent drug screening by deep learning-based morphology senescence scoring. Nat. Commun. 2021, 12, 257. [Google Scholar]
- Ahmad, J.; Farman, H.; Jan, Z. Deep Learning Methods and Applications. In Briefs in Computer Science; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Dongare, A.D.; Kharde, R.R.; Kachare, A.D. Introduction to Artificial Neural Network. Certif. Int. J. Eng. Innov. Technol. 2008, 9001, 2277–3754. [Google Scholar]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Mo, T.; Feng, Q.; Zhong, P.; Lai, M.; Chang, E.I.C. Deep learning of feature representation with multiple instance learning for medical image analysis. In Proceedings of the 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy, 4–9 May 2014; pp. 1626–1630. [Google Scholar]
- Liu, W.; Wang, Z.; Liu, X.; Zeng, N.; Liu, Y.; Alsaadi, F.E. A survey of deep neural network architectures and their applications. Neurocomputing 2017, 234, 11–26. [Google Scholar] [CrossRef]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Mohamed, N.A.E.; Arshad, H. State-of-the-art in artificial neural network applications: A survey. Heliyon 2018, 4, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Fang, S.; Zhao, Y.; Wang, P.; Zhang, J. Implementation of Training Convolutional Neural Networks. arXiv 2015, arXiv:1506.01195. [Google Scholar]
- Larochelle, H.; Bengio, Y.; Louradour, J.; Ca, L.U. Exploring Strategies for Training Deep Neural Networks Pascal Lamblin. J. Mach. Learn. Res. 2009, 1, 1–40. [Google Scholar]
- Alom, M.Z.; Taha, T.M.; Yakopcic, C.; Westberg, S.; Sidike, P.; Nasrin, M.S.; Hasan, M.; Van Essen, B.C.; Awwal, A.A.S.; Asari, V.K. A State-of-the-Art Survey on Deep Learning Theory and Architectures. Electronics 2019, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Ca, H.L.; Mandel, M.; Pascanu, R.; Bengio, Y.; Ca, B.U. Learning Algorithms for the Classification Restricted Boltzmann Machine Hugo Larochelle. J. Mach. Learn. Res. 2012, 13, 643–669. [Google Scholar]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative Adversarial Networks: An Overview. IEEE Signal Process. Mag. 2018, 35, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Salakhutdinov, R.; Hinton, G. Deep Boltzmann Machines. In Proceedings of the Twelth International Conference on Artificial Intelligence and Statistics, Clearwater Beach, FL, USA, 16–18 April 2009; pp. 448–455. [Google Scholar]
- Eddy, S.R. What is a hidden Markov model? Nat. Biotechnol. 2004, 22, 1315–1316. [Google Scholar] [CrossRef] [Green Version]
- Hagan, M.T.; Demuth, H.B.; Beale, M.H. Neural Network Design; PWS Publishing Co.: Boston, MA, USA, 1996; Volume 20. [Google Scholar]
- LeCun, Y.; Denker, J.S.; Solla, S.A. Optimal Brain Damage (Pruning). Adv. Neural Inf. Process. Syst. 1990, 598–605. [Google Scholar]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 1–74. [Google Scholar] [CrossRef]
- Agarap, A.F. Deep Learning using Rectified Linear Units (ReLU). arXiv 2018, arXiv:1803.08375. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Sherstinsky, A. Fundamentals of Recurrent Neural Network (RNN) and Long Short-Term Memory (LSTM) network. Phys. D Nonlinear Phenom. 2020, 404, 132306. [Google Scholar] [CrossRef] [Green Version]
- Bengio, Y.; Lamblin, P.; Popovici, D.; Larochelle, H. Greedy Layer-Wise Training of Deep Networks. In Proceedings of the 19th International Conference on Neural Information Processing Systems, Cambridge, MA, USA, 4–7 December 2006; pp. 153–160. [Google Scholar]
- Filonenko, A.; Kurnianggoro, L.; Jo, K.H. Comparative study of modern convolutional neural networks for smoke detection on image data. In Proceedings of the 2017 10th International Conference on Human System Interactions (HSI), Ulsan, Korea, 17–19 July 2017; pp. 64–68. [Google Scholar]
- Roddick, J.F.; Fule, P.; Graco, W.J. Exploratory medical knowledge discovery: Experiences and issues. ACM SIGKDD Explor. Newsl. 2003, 5, 94–99. [Google Scholar] [CrossRef]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M.; et al. TensorFlow: A System for Large-Scale Machine Learning TensorFlow: A system for large-scale machine learning. In Proceedings of the 12th USENIX Symposium on Operating Systems Design and Implementation (OSDI’16), Savannah, GA, USA, 2–4 November 2016; pp. 265–284. [Google Scholar]
- Asgari, E.; Garakani, K.; Mofrad, M.R.K. A New Approach for Scalable Analysis of Microbial Communities. arXiv 2015, arXiv:1512.00397. [Google Scholar]
- Shen, D.; Wu, G.; Suk, H.-I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef] [Green Version]
- Fabris, F.; Palmer, D.; Salama, K.M.; De Magalhães, J.P.; Freitas, A.A. Using deep learning to associate human genes with age-related diseases. Bioinformatics 2020, 36, 2202–2208. [Google Scholar] [CrossRef]
- Guberman, J.M.; Ai, J.; Arnaiz, O.; Baran, J.; Blake, A.; Baldock, R.; Chelala, C.; Croft, D.; Cros, A.; Cutts, R.J.; et al. BioMart Central Portal: An open database network for the biological community. Database J. Biol. Databases Curation 2011, 2011, bar041. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.; Wan, C.; Tacutu, R.; Barardo, D.; Rajput, A.; Wang, J.; Thoppil, H.; Thornton, D.; Yang, C.; Freitas, A.; et al. Systematic analysis of the gerontome reveals links between aging and age-related diseases. Hum. Mol. Genet. 2016, 25, 4804–4818. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Sodhro, A.H.; Luo, Z.; Zahid, N.; Nawaz, M.W.; Pirbhulal, S.; Muzammal, M. A Joint Deep Learning and Internet of Medical Things Driven Framework for Elderly Patients. IEEE Access 2020, 8, 75822–75832. [Google Scholar] [CrossRef]
- Wu, M.; Luo, J. Wearable Technology Applications in Health Care. Online J. Nurs. Inform. 2019, 23. [Google Scholar]
- Chase, J.A. Methodological challenges in physical activity research with older adults. West. J. Nurs. Res. 2013, 35, 76–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aylward.org. Open-Access Medical Image Repositories. Available online: https://www.aylward.org/notes/open-access-medical-image-repositories (accessed on 17 October 2021).
- Blobel, B. Interoperable EHR Systems—Challenges, Standards and Solution. Eur. J. Biomed. Inform. 2018, 14, 10–19. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017, 2, 230. [Google Scholar] [CrossRef]
- Yue, L.; Tian, D.; Chen, W.; Han, X.; Yin, M. Deep learning for heterogeneous medical data analysis. World Wide Web 2020, 23, 2715–2737. [Google Scholar] [CrossRef]
- Torres-Soto, J.; Ashley, E.A. Multi-task deep learning for cardiac rhythm detection in wearable devices. npj Digit. Med. 2020, 3. [Google Scholar] [CrossRef]
- Sundararajan Kalaivani, L.W. Deep Learning for Biometrics. ACM Comput. Surv. 2018, 51, 1–34. [Google Scholar] [CrossRef]
- Chassagnon, G.; Vakalopoulou, M.; Régent, A.; Zacharaki, E.I.; Aviram, G.; Martin, C.; Marini, R.; Bus, N.; Jerjir, N.; Mekinian, A.; et al. Deep Learning–based Approach for Automated Assessment of Interstitial Lung Disease in Systemic Sclerosis on CT Images. Radiol. Artif. Intell. 2020, 2, e190006. [Google Scholar] [CrossRef]
- Summers, M.J.; Madl, T.; Vercelli, A.E.; Aumayr, G.; Bleier, D.M.; Ciferri, L. DL for detection of preclinical neurodegenerative diseases of aging. DigitCult-Sci. J. Digit. Cul. 2017, 2, 9–24. [Google Scholar]
- Kaymak, S.; Esmaili, P.; Serener, A. Deep Learning for Two-Step Classification of Malignant Pigmented Skin Lesions. In Proceedings of the 2018 14th Symposium on Neural Networks and Applications (NEUREL), Belgrade, Serbia, 20–21 November 2018; pp. 1–6. [Google Scholar]
- Page, M.J.; Moher, D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Shetty, A.; Mehta, D.; Rane, P.; Dodani, S.N. Detection and Prediction of Alzheimer’s disease using Deep learning: A review. In Proceedings of the 2021 4th Biennial International Conference on Nascent Technologies in Engineering (ICNTE), NaviMumbai, India, 15–16 January 2021; pp. 1–7. [Google Scholar]
- Su, C.; Xu, Z.; Pathak, J.; Wang, F. Deep learning in mental health outcome research: A scoping review. Transl. Psychiatry 2020, 10, 1–26. [Google Scholar] [CrossRef]
- Saxena, V. Old Age Diseases. Available online: https://gomedii.com/blogs/english/health-a2z/top-7-common-diseases-in-old-age (accessed on 17 October 2021).
- Yu-Chuan, E.; Yeung, L.; Lee, Y.-L.; Wu, C.-H.; Peng, S.-Y.; Chen, Y.-P.; Gao, Q.-Z.; Lin, C.; Kuo, C.-F.; Lai, C.-C. A Multimodal Imaging–Based Deep Learning Model for Detecting Treatment-Requiring Retinal Vascular Diseases: Model Development and Validation Study. JMIR Med Inf. 2021, 9, e28868. [Google Scholar]
- Peng, Y.; Dharssi, S.; Chen, Q.; Keenan, T.D.; Agrón, E.; Wong, W.T.; Chew, E.Y.; Lu, Z. DeepSeeNet: A Deep Learning Model for Automated Classification of Patient-based Age-related Macular Degeneration Severity from Color Fundus Photographs. Ophthalmology 2019, 126, 565–575. [Google Scholar] [CrossRef]
- Burlina, P.M.; Joshi, N.; Pacheco, K.D.; Freund, D.E.; Kong, J.; Bressler, N.M. Use of Deep Learning for Detailed Severity Characterization and Estimation of 5-Year Risk Among Patients With Age-Related Macular Degeneration. JAMA Ophthalmol. 2018, 136, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- ZHANG, P.; XU, F. Effect of AI deep learning techniques on possible complications and clinical nursing quality of patients with coronary heart disease. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Goallec, A.L.; Prost, J.-B.; Collin, S.; Diai, S.; Vincent, T.; Patel, C.J. Dissecting heart age using cardiac magnetic resonance videos, electrocardiograms, biobanks, and deep learning. medRxiv 2021. [Google Scholar] [CrossRef]
- Schroeder, J.D.; Lanfredi, R.B.; Li, T.; Chan, J.; Vachet, C.; III, R.P.; Srikumar, V.; Tasdizen, T. Prediction of Obstructive Lung Disease from Chest Radiographs via Deep Learning Trained on Pulmonary Function Data. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 3455. [Google Scholar] [CrossRef]
- Gang Nam, J.; Kim, M.; Park, J.; Jin Hwang, E.; Hyuk Lee, J.; Hee Hong, J.; Mo Goo, J.; Min Park, C. Development and validation of a deep learning algorithm detecting 10 common abnormalities on chest radiographs. Eur. Respir. J. 2021, 57, 2003061. [Google Scholar]
- Yang, H.-C.; Wang, Y.-H.; Bai, K.-J.; Wang, H.-H.; Li, Y.-C. Artificial Intelligence–Based Prediction of Lung Cancer Risk Using Nonimaging Electronic Medical Records: Deep Learning Approach. J. Med. Internet Res. 2021, 23, e26256. [Google Scholar]
- Kalweit, M.; Walker, U.A.; Finckh, A.; Müller, R.; Kalweit, G.; Scherer, A.; Boedecker, J.; Hügle, T. Personalized prediction of disease activity in patients with rheumatoid arthritis using an adaptive deep neural network. PLoS ONE 2021, 16, e0252289. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Zhang, B.; Tan, J.; Shen, Y.; Geras, K.J.; Babb, J.S.; Cho, K.; Chang, G.; Deniz, C.M. Prediction of Total Knee Replacement and Diagnosis of Osteoarthritis by Using Deep Learning on Knee Radiographs: Data from the Osteoarthritis Initiative. Radiology 2020, 296, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Norgeot, B.; Glicksberg, B.S.; Trupin, L.; Lituiev, D.; Gianfrancesco, M.; Oskotsky, B.; Schmajuk, G.; Yazdany, J.; Butte, A.J. Assessment of a Deep Learning Model Based on Electronic Health Record Data to Forecast Clinical Outcomes in Patients With Rheumatoid Arthritis. JAMA Netw. Open 2019, 2, e190606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, T.; Nishide, M.; Nonaka, N.; Seita, J.; Ebina, K.; Sakurada, K.; Kumanogoh, A. Development and validation of a deep-learning model for scoring of radiographic finger joint destruction in rheumatoid arthritis. Rheumatol. Adv. Pract. 2019, 3, rkz047. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Liu, J.; Adalı, T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 2009, 45, S163–S172. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Lv, Y.; Cao, C.; Wang, Z.; Li, A. Multivariate Deep Learning Classification of Alzheimer’s Disease Based on Hierarchical Partner Matching Independent Component Analysis. Front. Aging Neurosci. 2018, 10, 417. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.N.I.; Ryu, S.; Song, J.; Lee, K.H.; Lee, B. Evaluation of Functional Decline in Alzheimer’s Dementia Using 3D Deep Learning and Group ICA for rs-fMRI Measurements. Front. Aging Neurosci. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Jin, K.H. Predicting cognitive decline with deep learning of brain metabolism and amyloid imaging. Behav. Brain Res. 2018, 344, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Aparici, C.M.; et al. A deep learning model to predict a diagnosis of Alzheimer disease by using 18 F-FDG PET of the brain. Radiology 2019, 290, 456–464. [Google Scholar] [CrossRef]
- Lu, B.; Li, H.-X.; Chang, Z.-K.; Li, L.; Chen, N.-X.; Zhu, Z.-C.; Zhou, H.-X.; Li, X.-Y.; Wang, Y.-W.; Cui, S.-X.; et al. A Practical Alzheimer Disease Classifier via Brain Imaging-Based Deep Learning on 85,721 Samples. bioRxiv 2021. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, J.; Zhou, Y.; Tong, T.; Zhuang, X. Diagnosis of Alzheimer’s Disease via Multi-Modality 3D Convolutional Neural Network. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Prince, J.; De Vos, M. A Deep Learning Framework for the Remote Detection of Parkinson’S Disease Using Smart-Phone Sensor Data. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3144–3147. [Google Scholar]
- Mishkhal, I.; Kareem, S.A.A.; Saleh, H.H.; Alqayyar, A. Deep Learning with network of Wearable sensors for preventing the Risk of Falls for Older People. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 032050. [Google Scholar] [CrossRef]
- Nath, R.K.; Thapliyal, H.; Caban-Holt, A. Machine Learning Based Stress Monitoring in Older Adults Using Wearable Sensors and Cortisol as Stress Biomarker. J. Signal Process. Syst. 2021, 1–13. [Google Scholar]
- Titano, J.J.; Badgeley, M.; Schefflein, J.; Pain, M.; Su, A.; Cai, M.; Swinburne, N.; Zech, J.; Kim, J.; Bederson, J.; et al. Automated deep-neural-network surveillance of cranial images for acute neurologic events. Nat. Med. 2018, 24, 1337–1341. [Google Scholar] [CrossRef]
- Betancur, J.; Commandeur, F.; Motlagh, M.; Sharir, T.; Einstein, A.J.; Bokhari, S.; Fish, M.B.; Ruddy, T.D.; Kaufmann, P.; Sinusas, A.J.; et al. Deep Learning for Prediction of Obstructive Disease From Fast Myocardial Perfusion SPECT: A Multicenter Study. JACC Cardiovasc. Imaging 2018, 11, 1654–1663. [Google Scholar] [CrossRef]
- Shi, W.; Peng, X.; Liu, T.; Cheng, Z.; Lu, H.; Yang, S.; Zhang, J.; Wang, M.; Gao, Y.; Shi, Y.; et al. A deep learning-based quantitative computed tomography model for predicting the severity of COVID-19: A retrospective study of 196 patients. Ann. Transl. Med. 2021, 9, 216. [Google Scholar] [CrossRef]
- Varadarajan, A.V.; Poplin, R.; Blumer, K.; Angermueller, C.; Ledsam, J.; Chopra, R.; Keane, P.A.; Corrado, G.S.; Peng, L.; Webster, D.R. Deep Learning for Predicting Refractive Error From Retinal Fundus Images. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2861–2868. [Google Scholar] [CrossRef] [Green Version]
- Basheer, S.; Bhatia, S.; Sakri, S.B. Computational Modeling of Dementia Prediction Using Deep Neural Network: Analysis on OASIS Dataset. IEEE Access 2021, 9, 42449–42462. [Google Scholar] [CrossRef]
- Cheon, S.; Kim, J.; Lim, J. The Use of Deep Learning to Predict Stroke Patient Mortality. Int. J. Environ. Res. Public Health 2019, 16, 1876. [Google Scholar] [CrossRef] [Green Version]
- Papagiannaki, A.; Zacharaki, E.I.; Kalouris, G.; Kalogiannis, S.; Deltouzos, K.; Ellul, J.; Megalooikonomou, V. Recognizing Physical Activity of Older People from Wearable Sensors and Inconsistent Data. Sensors 2019, 19, 880. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Ling, C.X. Using AUC and accuracy in evaluating learning algorithms. IEEE Trans. Knowl. Data Eng. 2005, 17, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Willmott, C.J.; Matsuura, K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Clim. Res. 2005, 30, 79–82. [Google Scholar] [CrossRef]
- Kwon, S.B.; Han, H.S.; Lee, M.C.; Kim, H.C.; Ku, Y.; Ro, D.H. Machine Learning-Based Automatic Classification of Knee Osteoarthritis Severity Using Gait Data and Radiographic Images. IEEE Access 2020, 8, 120597–120603. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Paschali, M.; Naeem, M.F.; Simson, W.; Steiger, K.; Mollenhauer, M.; Navab, N. Deep Learning Under the Microscope: Improving the Interpretability of Medical Imaging Neural Networks. arXiv 2019, arXiv:1904.03127. [Google Scholar]
- Richards, B.A.; Lillicrap, T.P.; Therien, D.; Kording, K.P.; Beaudoin, P.; Bengio, Y.; Bogacz, R.; Christensen, A. FOCUS | PersPective A deep learning framework for neuroscience. Nat. Neurosci. 2019, 22, 1761–1770. [Google Scholar] [CrossRef]
- Geerts, H.; Dacks, P.A.; Devanarayan, V.; Haas, M.; Khachaturian, Z.S.; Gordon, M.F.; Maudsley, S.; Romero, K.; Stephenson, D. Big data to smart data in Alzheimer’s disease: The brain health modeling initiative to foster actionable knowledge. Alzheimer’s Dement. 2016, 12, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Bonnett, L.J.; Snell, K.I.E.; Collins, G.S.; Riley, R.D. Guide to presenting clinical prediction models for use in clinical settings. BMJ 2019, 365, l737. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Wolpert, D.H.; Macready, W.G. No Free Lunch Theorems for Optimization. IEEE Trans. Evol. Comput. 1997, 1, 67. [Google Scholar] [CrossRef] [Green Version]
- EfficientNet Explained | Papers with Code. Available online: https://paperswithcode.com/method/efficientnet (accessed on 27 August 2021).
| ANN [28] | Artificial Neural Network |
| CNN [29] | Convolutional neural network |
| DNN [30] | Deep neural network |
| RNN [31] | Recurrent neural network |
| RBM [32] | Restricted Boltzmann machine |
| GAN [33] | Generative adversarial networks |
| DBM [34] | Deep Boltzmann machine |
| DBN [31] | Deep Belief Network |
| AE [31] | Auto Encoder |
| SAE [5] | Stacked auto-encoder |
| HMM [35] | Hidden Markov Model |
| Scoping Review Title | Role of DL in Predicting Age-Related Diseases: A Scoping Review |
|---|---|
| Review objective | To investigate the current state of DL methods in detecting age-related diseases and provide better patient care (such as early detection of age-related diseases). |
| Review question | How DL can help in predicting age-related diseases to understand the fundamental mechanism? |
| Population | Elderly human population is considered, excluding the children and youth. |
| Concept | The current review studies the trend in the last five years (2017–2021) of using DL, to classify predictive stages of common age-related diseases (such as AMD, Arthritis, Cardiovascular and Respiratory diseases, etc.) |
| Context | Use of medical imaging data, genomic data, Electronic Health Record data and data collected from wearable sensors to predict the human diseases. |
| Types of evidence source | Analysis of heterogenous and unstructured data to derive relevant diagnostic information aiding patients in old age. |
| Participants | Mostly participants with a mean age of 60–75 (nearly). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassan, J.T.; Zheng, H.; Wang, H. Role of Deep Learning in Predicting Aging-Related Diseases: A Scoping Review. Cells 2021, 10, 2924. https://doi.org/10.3390/cells10112924
Wassan JT, Zheng H, Wang H. Role of Deep Learning in Predicting Aging-Related Diseases: A Scoping Review. Cells. 2021; 10(11):2924. https://doi.org/10.3390/cells10112924
Chicago/Turabian StyleWassan, Jyotsna Talreja, Huiru Zheng, and Haiying Wang. 2021. "Role of Deep Learning in Predicting Aging-Related Diseases: A Scoping Review" Cells 10, no. 11: 2924. https://doi.org/10.3390/cells10112924
APA StyleWassan, J. T., Zheng, H., & Wang, H. (2021). Role of Deep Learning in Predicting Aging-Related Diseases: A Scoping Review. Cells, 10(11), 2924. https://doi.org/10.3390/cells10112924







