Development of Functional Thyroid C Cell-like Cells from Human Pluripotent Cells in 2D and in 3D Scaffolds
Abstract
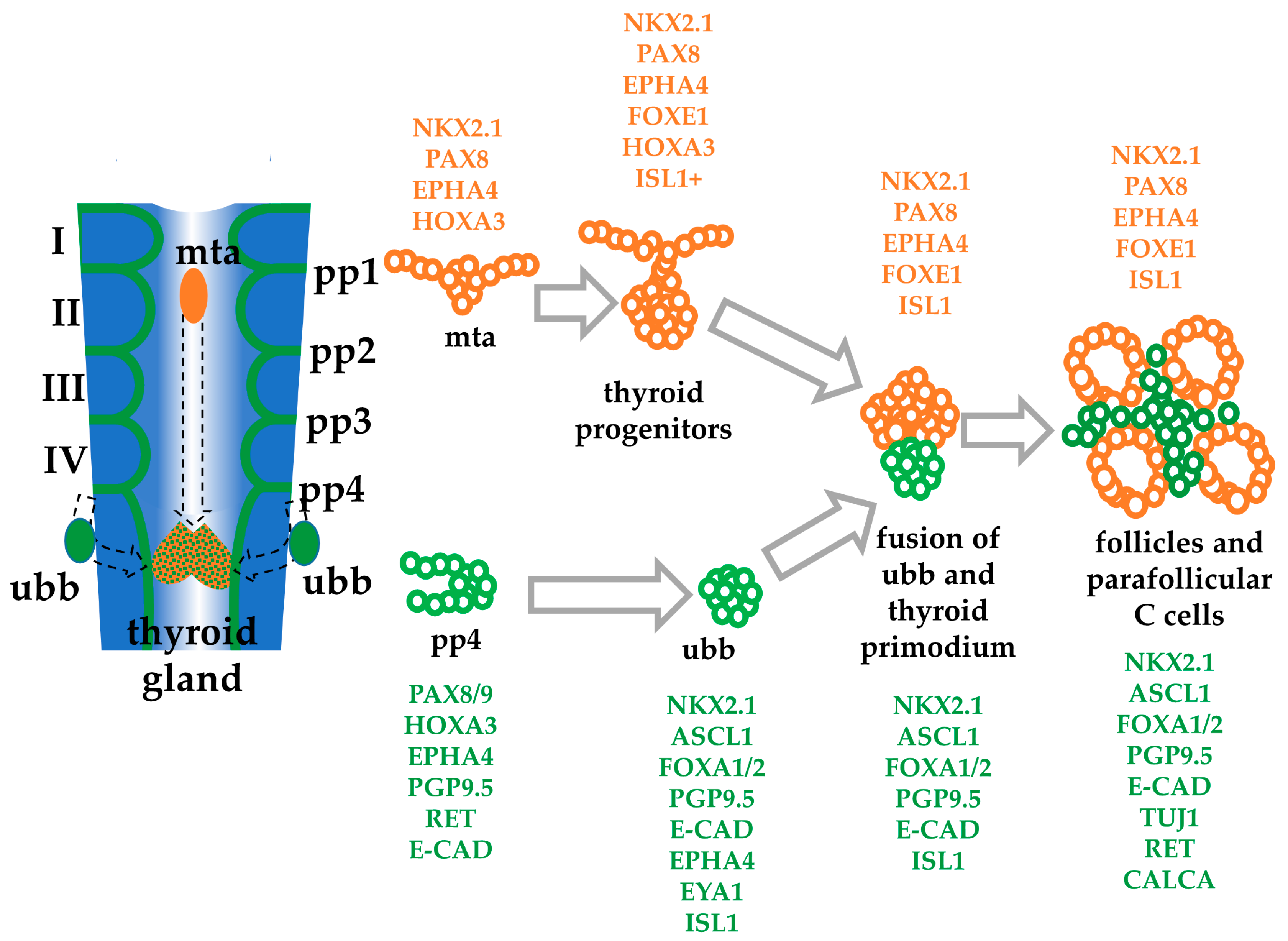
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Human Embryonic Stem Cells, hESC Culture, and Thyroid C Cell Differentiation in 2D Cultures
2.3. Thyroid C Cell Differentiation Using 3D Gel Matrigel Scaffold Cultures
2.4. Immunocytochemistry/Immunofluorescence
2.5. RNA Extraction and qPCR
2.6. FACs Sorting and Analysis
2.7. Calcitonin Production and Secretion
2.8. ELISA Assay
2.9. BCA Protein Assay (PIERCE)
2.10. Statistical Analyses
3. Results
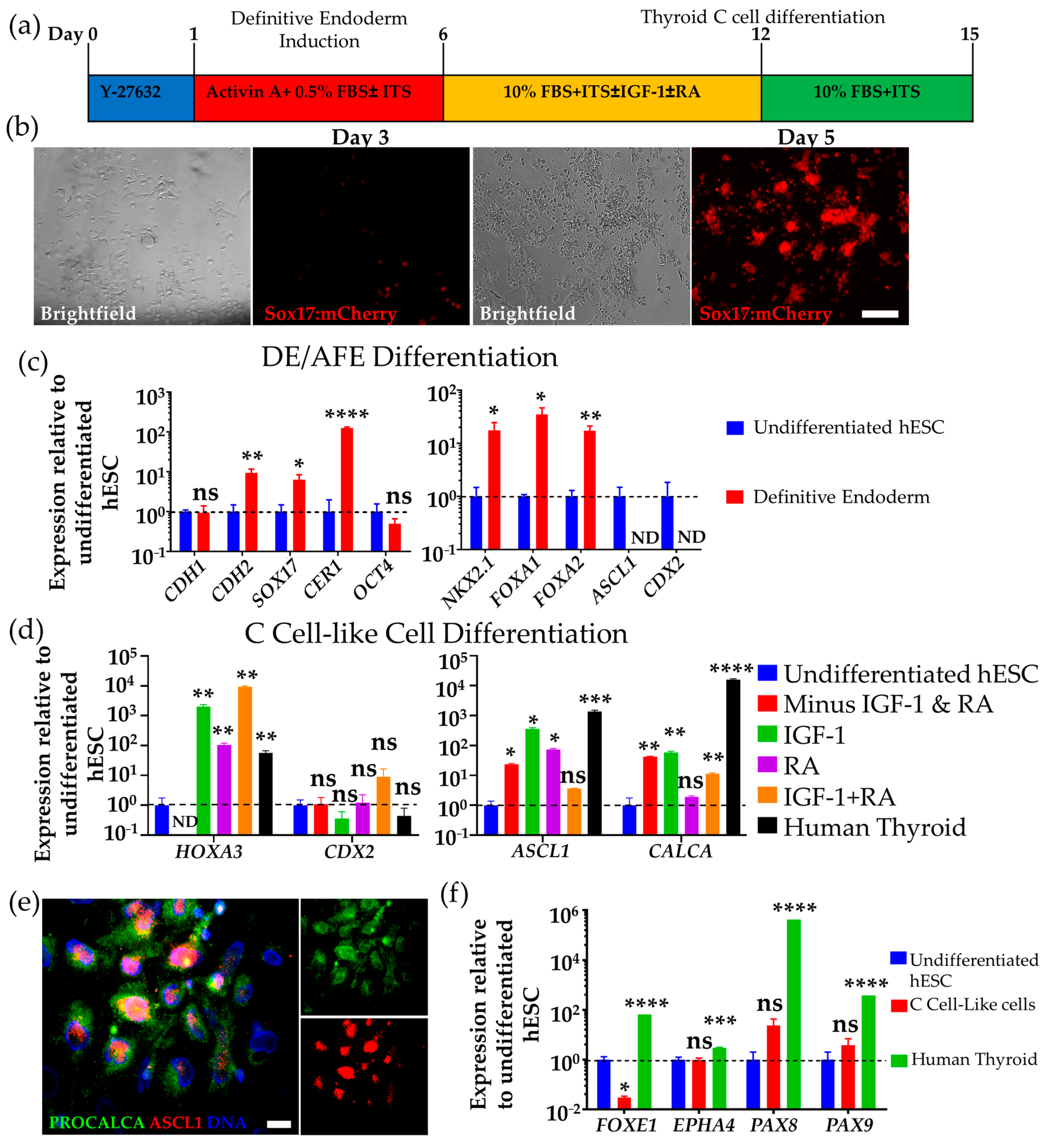
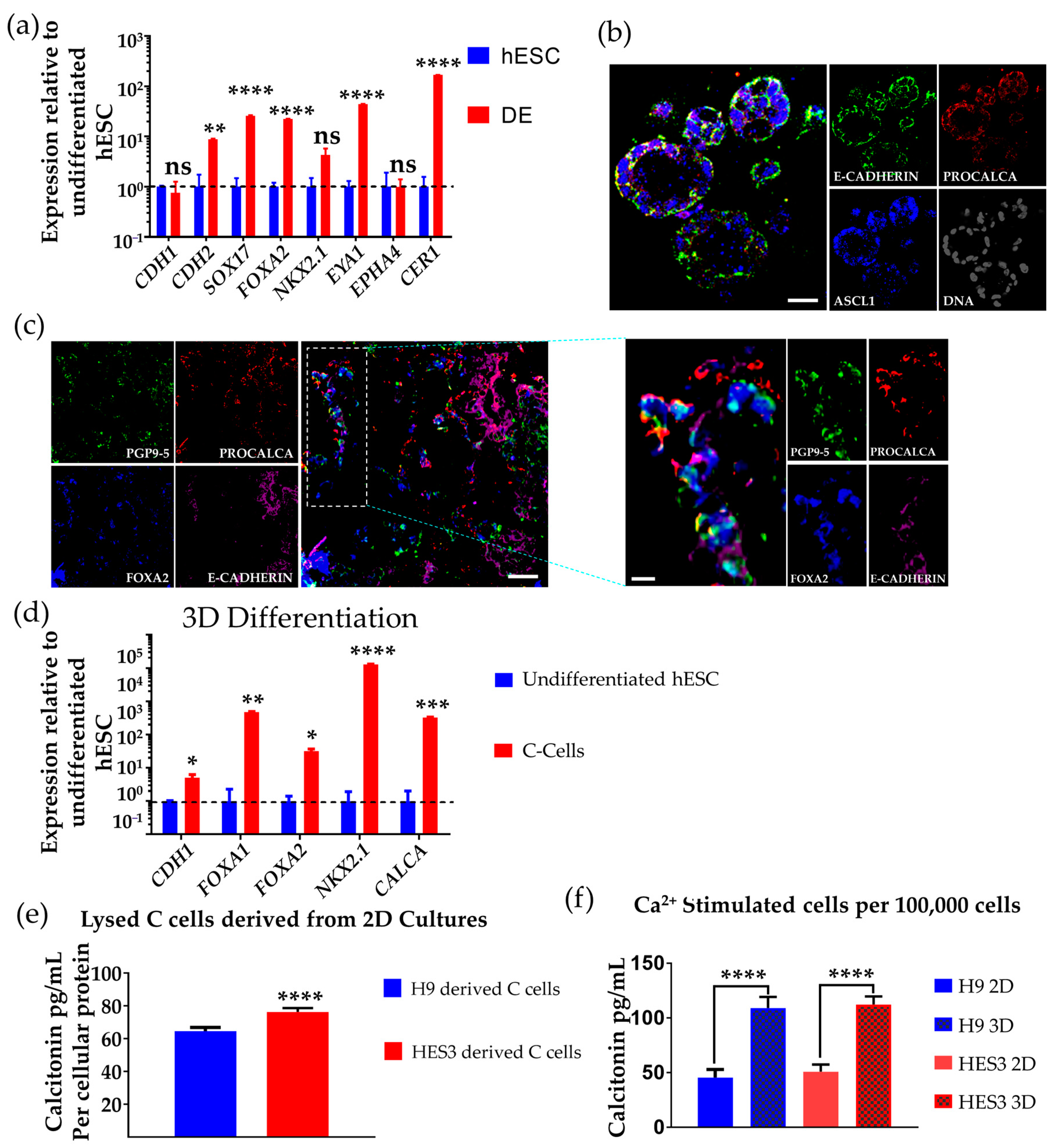
3.1. Differentiation of hESCs to Definitive Endoderm and Anterior Foregut Endoderm-like Cells
3.2. Differentiation of Human DE/AFE-like Cells to Thyroid C Cell-like Cells
3.3. Differentiation of hESCs to Neuroendocrine Thyroid C Cell-like Cells in 3D Matrigel
3.4. Determination of Calcitonin Production and Secretion on Ca2+ Challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21 (Suppl. 2), S37–S43. [Google Scholar] [CrossRef]
- Lin, R.Y. Thyroid cancer stem cells. Nat. Rev. Endocrinol. 2011, 7, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Hundahl, S.A.; Fleming, I.D.; Fremgen, A.M.; Menck, H.R. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 1998, 83, 2638–2648. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Vergamini, L.B.; Frazier, A.L.; Abrantes, F.L.; Ribeiro, K.B.; Rodriguez-Galindo, C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: A population-based study. J. Pediatr. 2014, 164, 1481–1485. [Google Scholar] [CrossRef]
- Chen, A.Y.; Jemal, A.; Ward, E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009, 115, 3801–3807. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Pacini, F.; Robinson, B.G.; Santoro, M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: An update. J. Clin. Endocrinol. Metab. 2013, 98, 3149–3164. [Google Scholar] [CrossRef]
- Vaclavikova, E.; Kavalcova, L.; Skaba, R.; Dvorakova, S.; Macokova, P.; Rouskova, B.; Bendlova, B. Hirschsprung’s disease and medullary thyroid carcinoma: 15-year experience with molecular genetic screening of the RET proto-oncogene. Pediatric Surg. Int. 2012, 28, 123–128. [Google Scholar] [CrossRef]
- Waguespack, S.G.; Rich, T.A.; Perrier, N.D.; Jimenez, C.; Cote, G.J. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat. Rev. Endocrinol. 2011, 7, 596–607. [Google Scholar] [CrossRef]
- Roy, M.; Chen, H.; Sippel, R.S. Current understanding and management of medullary thyroid cancer. Oncologist 2013, 18, 1093–1100. [Google Scholar] [CrossRef]
- Frendo, J.L.; Delage-Mourroux, R.; Cohen, R.; Pichaud, F.; Pidoux, E.; Guliana, J.M.; Jullienne, A. Calcitonin receptor mRNA expression in TT cells: Effect of dexamethasone. Mol. Cell. Endocrinol. 1998, 139, 37–43. [Google Scholar] [CrossRef]
- Hazard, J.B. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. Am. J. Pathol. 1977, 88, 213–250. [Google Scholar]
- Dionigi, G.; Bianchi, V.; Rovera, F.; Boni, L.; Piantanida, E.; Tanda, M.L.; Dionigi, R.; Bartalena, L. Medullary thyroid carcinoma: Surgical treatment advances. Expert Rev. Anticancer Ther. 2007, 7, 877–885. [Google Scholar] [CrossRef]
- Dionigi, G.; Tanda, M.L.; Piantanida, E. Medullary thyroid carcinoma: Surgical treatment advances. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 158–162. [Google Scholar] [CrossRef]
- Perez, C.A.; Arango, B.A.; Velez, M.; Raez, L.E.; Santos, E.S. Emerging role of multikinase inhibitors for refractory thyroid cancer. Biologics 2012, 6, 257–265. [Google Scholar] [CrossRef][Green Version]
- Mologni, L.; Gambacorti-Passerini, C.; Goekjian, P.; Scapozza, L. RET kinase inhibitors: A review of recent patents (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Colman, A.; Dreesen, O. Pluripotent stem cells and disease modeling. Cell Stem Cell 2009, 5, 244–247. [Google Scholar] [CrossRef]
- Gingold, J.; Zhou, R.; Lemischka, I.R.; Lee, D.F. Modeling Cancer with Pluripotent Stem Cells. Trends Cancer 2016, 2, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.F.; Su, J.; Kim, H.S.; Chang, B.; Papatsenko, D.; Zhao, R.; Yuan, Y.; Gingold, J.; Xia, W.; Darr, H.; et al. Modeling familial cancer with induced pluripotent stem cells. Cell 2015, 161, 240–254. [Google Scholar] [CrossRef]
- Passier, R.; Orlova, V.; Mummery, C. Complex Tissue and Disease Modeling using hiPSCs. Cell Stem Cell 2016, 18, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.V.; Russ, H.A.; Khan, I.S.; LaFlam, T.N.; Metzger, T.C.; Anderson, M.S.; Hebrok, M. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell 2013, 13, 219–229. [Google Scholar] [CrossRef]
- Dame, K.; Cincotta, S.; Lang, A.H.; Sanghrajka, R.M.; Zhang, L.; Choi, J.; Kwok, L.; Wilson, T.; Kandula, M.M.; Monti, S.; et al. Thyroid Progenitors Are Robustly Derived from Embryonic Stem Cells through Transient, Developmental Stage-Specific Overexpression of Nkx2-1. Stem Cell Rep. 2017, 8, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Longmire, T.A.; Ikonomou, L.; Hawkins, F.; Christodoulou, C.; Cao, Y.; Jean, J.C.; Kwok, L.W.; Mou, H.; Rajagopal, J.; Shen, S.S.; et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 2012, 10, 398–411. [Google Scholar] [CrossRef]
- Fagman, H.; Nilsson, M. Morphogenesis of the thyroid gland. Mol. Cell. Endocrinol. 2010, 323, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, T.; Hoshi, N.; Kimura, S. Origin of the ultimobranchial body cyst: T/ebp/Nkx2.1 expression is required for development and fusion of the ultimobranchial body to the thyroid. Dev. Dyn. 2006, 235, 1300–1309. [Google Scholar] [CrossRef]
- Kameda, Y.; Nishimaki, T.; Chisaka, O.; Iseki, S.; Sucov, H.M. Expression of the epithelial marker E-cadherin by thyroid C cells and their precursors during murine development. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 1075–1088. [Google Scholar] [CrossRef]
- Cote, G.J.; Grubbs, E.G.; Hofmann, M.C. Thyroid C-Cell Biology and Oncogenic Transformation. Recent Results Cancer Res. 2015, 204, 1–39. [Google Scholar] [CrossRef]
- Kameda, Y. Morphological and molecular evolution of the ultimobranchial gland of nonmammalian vertebrates, with special reference to the chicken C cells. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2017, 246, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.; Fontaine, J.; Le Lievre, C. New studies on the neural crest origin of the avian ultimobranchial glandular cells--interspecific combinations and cytochemical characterization of C cells based on the uptake of biogenic amine precursors. Histochemistry 1974, 38, 297–305. [Google Scholar] [CrossRef]
- Zabel, M. Parafollicular cells of the rat thyroid gland after treatment with vitamin D. Acta Anat. (Basel) 1984, 118, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Andersson, L.; Ornros, J.; Carlsson, T.; Ingeson-Carlsson, C.; Liang, S.; Dahlberg, J.; Jansson, S.; Parrillo, L.; Zoppoli, P.; et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development 2015, 142, 3519–3528. [Google Scholar] [CrossRef]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Li, E.; Weiner, L.M. 3D Culture Systems for Exploring Cancer Immunology. Cancers 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Krokker, L.; Szabo, B.; Nemeth, K.; Tohati, R.; Sarkadi, B.; Meszaros, K.; Patocs, A.; Butz, H. Three Dimensional Cell Culturing for Modeling Adrenal and Pituitary Tumors. Pathol. Oncol. Res. 2021, 27, 640676. [Google Scholar] [CrossRef]
- Fagman, H.; Amendola, E.; Parrillo, L.; Zoppoli, P.; Marotta, P.; Scarfo, M.; De Luca, P.; de Carvalho, D.P.; Ceccarelli, M.; De Felice, M.; et al. Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev. Biol. 2011, 359, 163–175. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef]
- Kumar, N.; Brafman, D.; Willert, K. Endoderm Differentiation from Human Pluripotent Stem Cells. In Working with Stem Cells; Springer International Publishing: Cham, Switzerland, 2016; pp. 237–255. [Google Scholar] [CrossRef]
- Loh, K.M.; Ang, L.T.; Zhang, J.; Kumar, V.; Ang, J.; Auyeong, J.Q.; Lee, K.L.; Choo, S.H.; Lim, C.Y.; Nichane, M.; et al. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell 2014, 14, 237–252. [Google Scholar] [CrossRef]
- Ang, S.L.; Wierda, A.; Wong, D.; Stevens, K.A.; Cascio, S.; Rossant, J.; Zaret, K.S. The formation and maintenance of the definitive endoderm lineage in the mouse: Involvement of HNF3/forkhead proteins. Development 1993, 119, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Kanai-Azuma, M.; Kanai, Y.; Gad, J.M.; Tajima, Y.; Taya, C.; Kurohmaru, M.; Sanai, Y.; Yonekawa, H.; Yazaki, K.; Tam, P.P.; et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 2002, 129, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Nishimaki, T.; Miura, M.; Jiang, S.X.; Guillemot, F. Mash1 regulates the development of C cells in mouse thyroid glands. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2007, 236, 262–270. [Google Scholar] [CrossRef]
- Kadzik, R.S.; Morrisey, E.E. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell 2012, 10, 355–361. [Google Scholar] [CrossRef]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef]
- Goss, A.M.; Tian, Y.; Tsukiyama, T.; Cohen, E.D.; Zhou, D.; Lu, M.M.; Yamaguchi, T.P.; Morrisey, E.E. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 2009, 17, 290–298. [Google Scholar] [CrossRef]
- Gouon-Evans, V.; Boussemart, L.; Gadue, P.; Nierhoff, D.; Koehler, C.I.; Kubo, A.; Shafritz, D.A.; Keller, G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 2006, 24, 1402–1411. [Google Scholar] [CrossRef]
- Xu, X.; Browning, V.L.; Odorico, J.S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 2011, 128, 412–427. [Google Scholar] [CrossRef]
- Nilsson, M.; Fagman, H. Mechanisms of thyroid development and dysgenesis: An analysis based on developmental stages and concurrent embryonic anatomy. Curr. Top. Dev. Biol. 2013, 106, 123–170. [Google Scholar] [CrossRef]
- Westerlund, J. Misguided Migration of C Cell Precursors to Extra-Thyroidal Locations Related to Defective Pharyngeal Pouch Development in Shh Deficient Mice. Cell Dev. Biol. 2013, 2, 129. [Google Scholar] [CrossRef]
- Brooker, G.J.; Kalloniatis, M.; Russo, V.C.; Murphy, M.; Werther, G.A.; Bartlett, P.F. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J. Neurosci. Res. 2000, 59, 332–341. [Google Scholar] [CrossRef]
- Nieto-Estevez, V.; Defterali, C.; Vicario-Abejon, C. IGF-I: A.A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Laurino, L.; Wang, X.X.; de la Houssaye, B.A.; Sosa, L.; Dupraz, S.; Caceres, A.; Pfenninger, K.H.; Quiroga, S. PI3K activation by IGF-1 is essential for the regulation of membrane expansion at the nerve growth cone. J. Cell Sci. 2005, 118, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Villegas, S.N.; Rothova, M.; Barrios-Llerena, M.E.; Pulina, M.; Hadjantonakis, A.K.; Le Bihan, T.; Astrof, S.; Brickman, J.M. PI3K/Akt1 signalling specifies foregut precursors by generating regionalized extra-cellular matrix. Elife 2013, 2, e00806. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, J.L.; Smith, E.R.; Capo-Chichi, C.D.; Frolov, A.; Yang, D.H.; Godwin, A.K.; Xu, X.X. Ras/MAPK pathway confers basement membrane dependence upon endoderm differentiation of embryonic carcinoma cells. J. Biol. Chem. 2002, 277, 40911–40918. [Google Scholar] [CrossRef]
- Gajovic, S.; St-Onge, L.; Yokota, Y.; Gruss, P. Retinoic acid mediates Pax6 expression during in vitro differentiation of embryonic stem cells. Differentiation 1997, 62, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wendling, O.; Dennefeld, C.; Chambon, P.; Mark, M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development 2000, 127, 1553–1562. [Google Scholar] [CrossRef]
- Miura, M.; Kameda, Y. Neuronal properties in cultured ultimobranchial C cells of chick embryos: Process outgrowth and expression of TuJ1 and enkephalin. Brain Res. 2001, 905, 1–11. [Google Scholar] [CrossRef]
- Kameda, Y. Cellular and molecular events on the development of mammalian thyroid C cells. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2016, 245, 323–341. [Google Scholar] [CrossRef]
- Westerlund, J.; Andersson, L.; Carlsson, T.; Zoppoli, P.; Fagman, H.; Nilsson, M. Expression of Islet1 in thyroid development related to budding, migration, and fusion of primordia. Dev. Dyn. 2008, 237, 3820–3829. [Google Scholar] [CrossRef]
- Kimura, S. Thyroid-specific transcription factors and their roles in thyroid cancer. J. Thyroid. Res. 2011, 2011, 710213. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Morshed, S.A.; Latif, R.; Davies, T.F. Thyroid cell differentiation from murine induced pluripotent stem cells. Front. Endocrinol. (Lausanne) 2015, 6, 56. [Google Scholar] [CrossRef]
- Andersson, L.; Westerlund, J.; Liang, S.; Carlsson, T.; Amendola, E.; Fagman, H.; Nilsson, M. Role of EphA4 Receptor Signaling in Thyroid Development: Regulation of Folliculogenesis and Propagation of the C-Cell Lineage. Endocrinology 2011, 152, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Katagiri, N.; Ueda, M.; Tanaka, S. Functional analysis of Nkx2.1 and Pax9 for calcitonin gene transcription. Gen. Comp. Endocrinol. 2007, 152, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.; Neubuser, A.; Kratochwil, K.; Balling, R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998, 12, 2735–2747. [Google Scholar] [CrossRef]
- Philp, D.; Chen, S.S.; Fitzgerald, W.; Orenstein, J.; Margolis, L.; Kleinman, H.K. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells 2005, 23, 288–296. [Google Scholar] [CrossRef]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016, 11, 1681–1692. [Google Scholar] [CrossRef]
- Watson, C.L.; Mahe, M.M.; Munera, J.; Howell, J.C.; Sundaram, N.; Poling, H.M.; Schweitzer, J.I.; Vallance, J.E.; Mayhew, C.N.; Sun, Y.; et al. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014, 20, 1310–1314. [Google Scholar] [CrossRef]
- Munera, J.O.; Sundaram, N.; Rankin, S.A.; Hill, D.; Watson, C.; Mahe, M.; Vallance, J.E.; Shroyer, N.F.; Sinagoga, K.L.; Zarzoso-Lacoste, A.; et al. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell 2017, 21, 51–64. [Google Scholar] [CrossRef]
- Wang, W.; Jin, S.; Ye, K. Development of Islet Organoids from H9 Human Embryonic Stem Cells in Biomimetic 3D Scaffolds. Stem Cells Dev. 2017, 26, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Kurmann, A.A.; Serra, M.; Hawkins, F.; Rankin, S.A.; Mori, M.; Astapova, I.; Ullas, S.; Lin, S.; Bilodeau, M.; Rossant, J.; et al. Regeneration of Thyroid Function by Transplantation of Differentiated Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Mishra, S.; Kaul, J.M. Development of Parafollicular Cells and their Relationship with Developing Thyroid Follicles in Human Foetuses. J. Clin. Diagn. Res. 2017, 11, AC01–AC04. [Google Scholar] [CrossRef] [PubMed]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018, 13, 27. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Bonsrah, K.D.; Newgreen, D.F.; Dottori, M. Development of Functional Thyroid C Cell-like Cells from Human Pluripotent Cells in 2D and in 3D Scaffolds. Cells 2021, 10, 2897. https://doi.org/10.3390/cells10112897
Abu-Bonsrah KD, Newgreen DF, Dottori M. Development of Functional Thyroid C Cell-like Cells from Human Pluripotent Cells in 2D and in 3D Scaffolds. Cells. 2021; 10(11):2897. https://doi.org/10.3390/cells10112897
Chicago/Turabian StyleAbu-Bonsrah, Kwaku Dad, Donald F. Newgreen, and Mirella Dottori. 2021. "Development of Functional Thyroid C Cell-like Cells from Human Pluripotent Cells in 2D and in 3D Scaffolds" Cells 10, no. 11: 2897. https://doi.org/10.3390/cells10112897
APA StyleAbu-Bonsrah, K. D., Newgreen, D. F., & Dottori, M. (2021). Development of Functional Thyroid C Cell-like Cells from Human Pluripotent Cells in 2D and in 3D Scaffolds. Cells, 10(11), 2897. https://doi.org/10.3390/cells10112897





