In Vitro Evaluation of DSPE-PEG (5000) Amine SWCNT Toxicity and Efficacy as a Novel Nanovector Candidate in Photothermal Therapy by Response Surface Methodology (RSM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
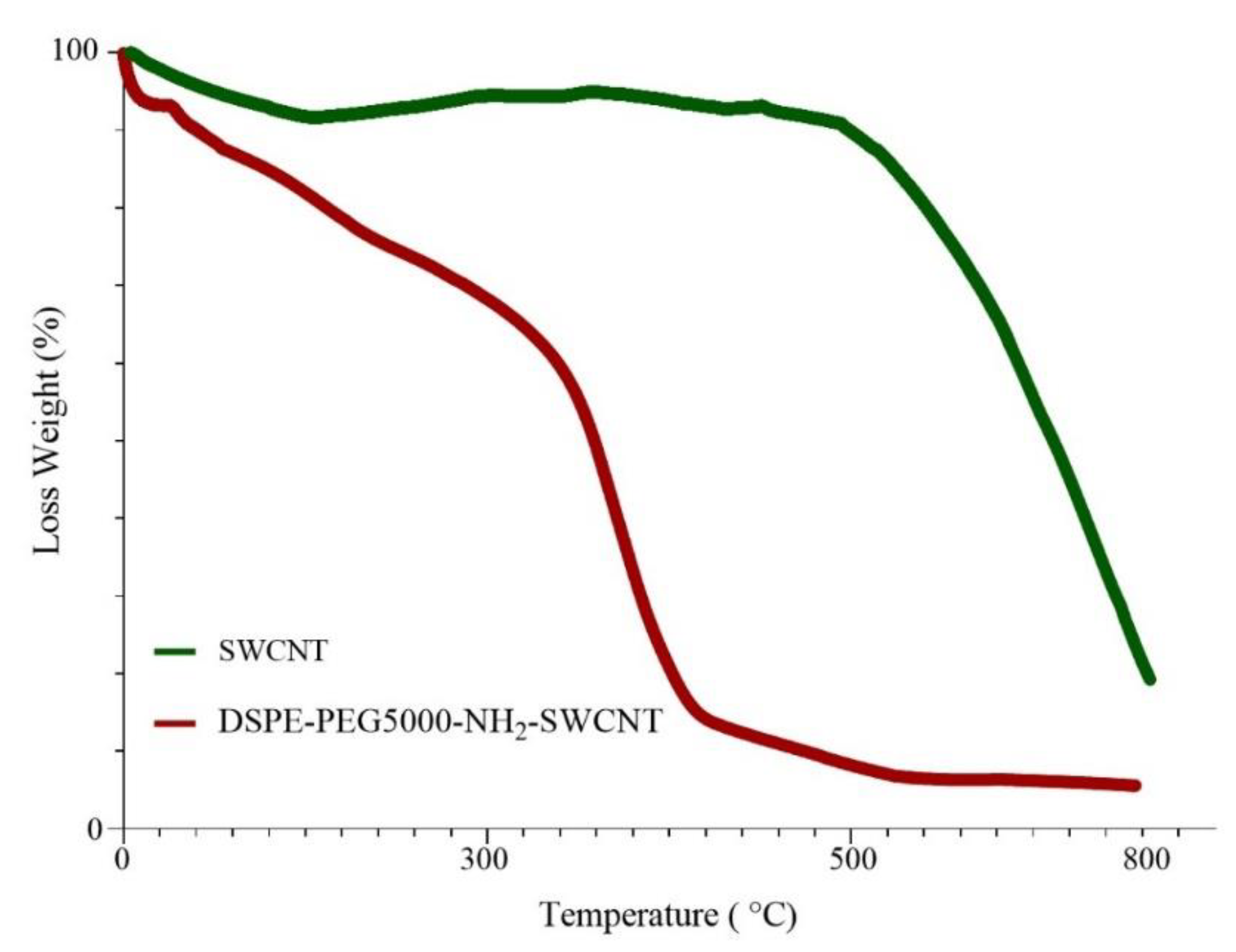
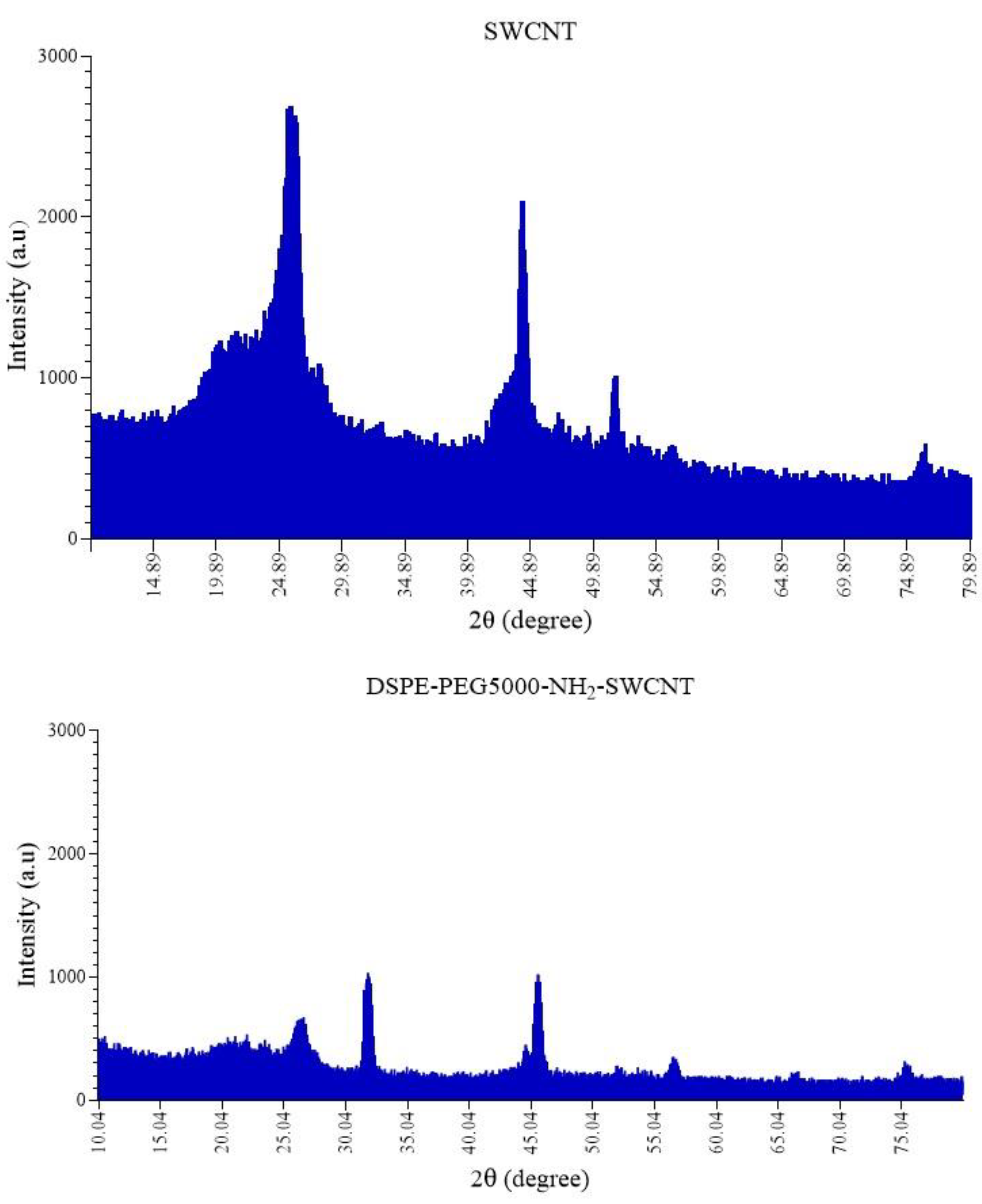
2.2.1. Preparation and Characterization of DSPE-PEG (5000) Amine SWCNTs
2.2.2. In Vitro Toxicity Studies
Trypan Blue Exclusion Test
2.2.3. Transmission Electron Microscopy (TEM)
2.2.4. In Vitro Photothermal Therapy Test in SKOV3 Cells
Experimental Design
NIR Exposure
Temperature Measurement
Cell Viability Assessment
Optimization
2.3. Statistical Analysis
3. Results
3.1. Characterization of DSPE-PEG (5000) Amine SWCNTs
3.2. In Vitro Toxicity Studies
Trypan Blue Exclusion Test
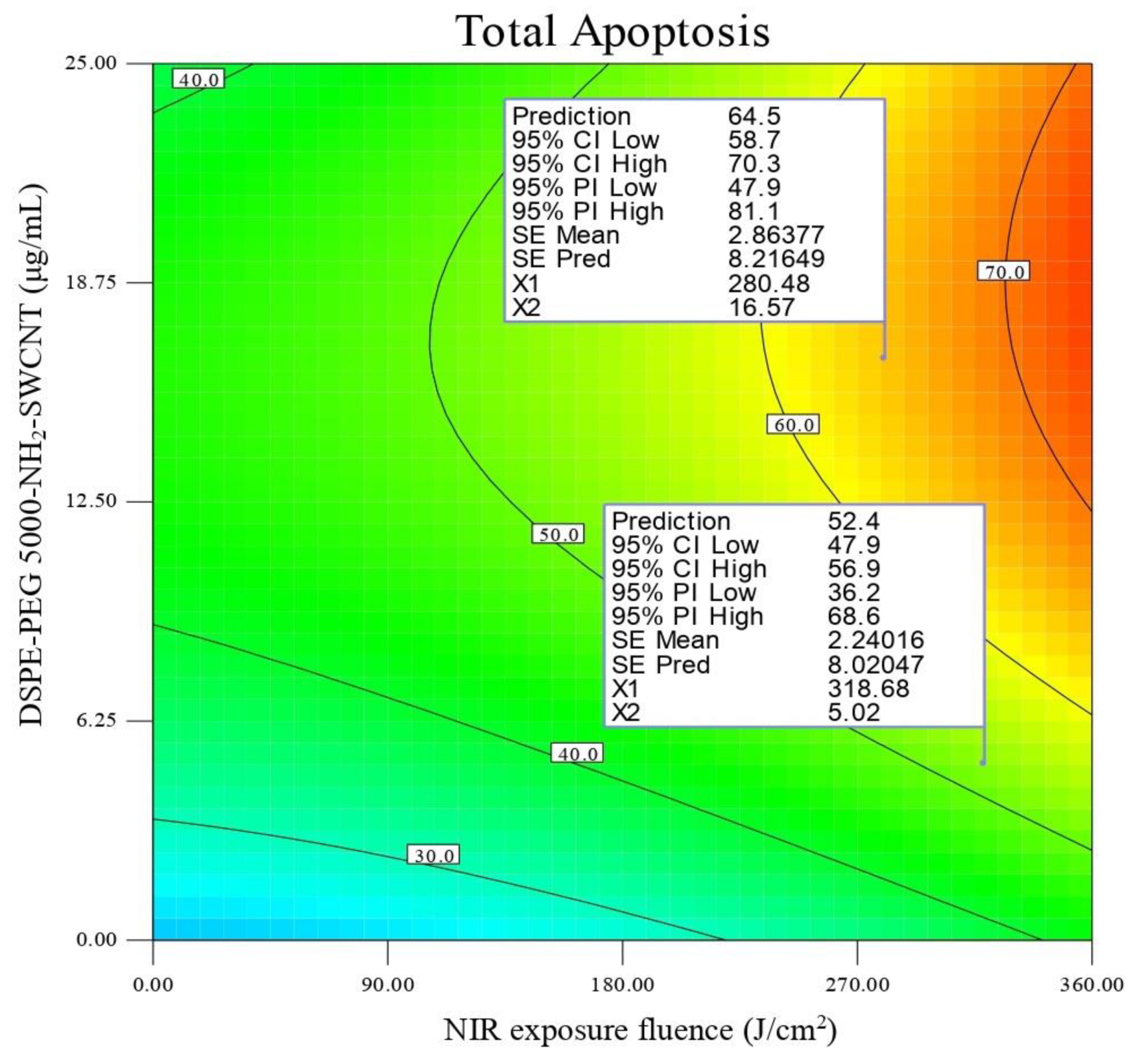
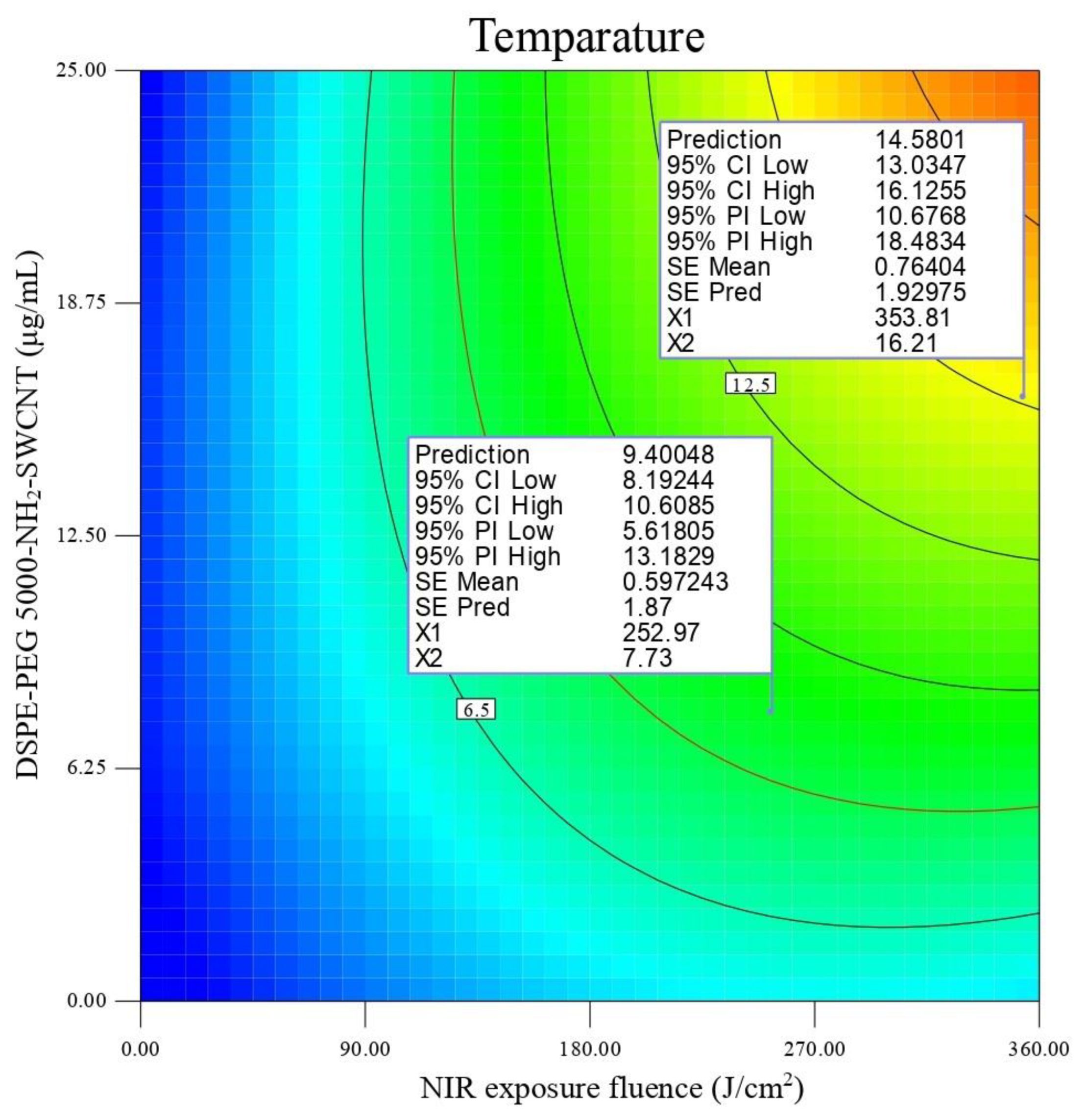
3.3. In Vitro Photothermal Therapy Test in SKOV3 Cells
Experimental Design
| Y1 (Total apoptosis) = 59 + 10.9 A + 8.71 B − 2.09 C − 0.28 − 0.74 B C + 1.75 A2 − 11.33 B2 CV (%) = CV (%) = 8.04 R2 = 0.90 adjusted R2 = 0.86 | (1) |
| Y2 (Temperature changes (∆T)) = 11.58 + 3 A + 3.78 B − 0.34 C + 1.82 A B − 0.2 A C + 0.55 B C − 1.10 A2 − 1.28 B2 CV (%) = 6.64 R2 = 0.92 adjusted R2 = 0.90 | (2) |
| Y3 (Post NIR exposure time) = 36 + 0 A + 0 B + 0 C CV (%) = 7.82 R2 = 0.81 adjusted R2 = 0.75 | (3) |
| Y4 (Necrosis) = 5.87 + 1.07 A + 0.8 B – 1.47 C + 0.42 A B + 0.029 A C – 0.91 B C + 0.4 A2 – 0.22 B2 CV (%) = 6.12 R2 = 0.89 adjusted R2 = 0.77 | (4) |
3.4. Optimization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoyanov, P.; Chromik, R.R. Scaling Effects on Materials Tribology: From Macro to Micro Scale. Materials 2017, 10, 550. [Google Scholar] [CrossRef]
- da Rocha, E.L.; Porto, L.M.; Rambo, C.R. Nanotechnology meets 3D in vitro models: Tissue engineered tumors and cancer therapies. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 270–279. [Google Scholar] [CrossRef]
- Yang, S.; You, Q.; Yang, L.; Li, P.; Lu, Q.; Wang, S.; Tan, F.; Ji, Y.; Li, N. Rodlike MSN@Au Nanohybrid-Modified Supermolecular Photosensitizer for NIRF/MSOT/CT/MR Quadmodal Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 6777–6788. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Yi, X.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Drug delivery micelles with efficient near-infrared photosensitizer for combined image-guided photodynamic therapy and chemotherapy of drug-resistant cancer. Biomaterials 2019, 218, 119330. [Google Scholar] [CrossRef]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L.; Zhao, C.; Li, Z. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Chen, Y.; Fang, Z. In-vitro photothermal therapy using plant extract polyphenols functionalized graphene sheets for treatment of lung cancer. J. Photochem. Photobiol. B 2020, 204, 111587. [Google Scholar] [CrossRef] [PubMed]
- Girma, W.M.; Dehvari, K.; Ling, Y.C.; Chang, J.Y. Albumin-functionalized CuFeS2/photosensitizer nanohybrid for single-laser-induced folate receptor-targeted photothermal and photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 179–189. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I.; Ziemkowska, W.; Brzózka, Z.; et al. 2D Ti2C (MXene) as a novel highly efficient and selective agent for photothermal therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 874–886. [Google Scholar] [CrossRef]
- Moon, H.K.; Lee, S.H.; Choi, H.C. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 2009, 3, 3707–3713. [Google Scholar] [CrossRef]
- Lei, W.; Sun, C.; Jiang, T.; Gao, Y.; Yang, Y.; Zhao, Q.; Wang, S. Polydopamine-coated mesoporous silica nanoparticles for multi-responsive drug delivery and combined chemo-photothermal therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110103. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Yan, Y.; Wang, D.; Zhang, Y.; Jiang, T.; Wang, S. The advantage of hollow mesoporous carbon as a near-infrared absorbing drug carrier in chemo-photothermal therapy compared with IR-820. Eur. J. Pharm. Sci. 2017, 99, 66–74. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.K.; Kim, Y.J.; Lee, T.J.; Jeong, G.J.; Kim, D.I.; Bhang, S.H. Enhancing therapeutic efficacy of photothermal therapy using poloxamer-reduced graphene oxide and mesenchymal stem cells. J. Ind. Eng. Chem. 2019, 80, 846–853. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of organic nanomaterials in photothermal cancer therapy. Cancer Res. 2016, 2, 67–84. [Google Scholar] [CrossRef]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Thanh, N.T. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and risk factor for cancer treatment. Achiev. Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Tian, X.; Ke, H.; Guo, M.; Zhu, A.; Yang, T.; Guo, Z.; Ge, Z.; Yang, X.; et al. Multipronged Design of Light-Triggered Nanoparticles To Overcome Cisplatin Resistance for Efficient Ablation of Resistant Tumor. ACS Nano 2015, 9, 9626–9637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Studer, A. Transition-Metal-Free Three-Component Radical 1,2-Amidoalkynylation of Unactivated Alkenes. Chemistry 2019, 25, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Roh, Y.H.; Shim, M.S.; Bong, K.W. Microfluidic fabrication of fatty alcohol-based microparticles for NIR light-triggered drug release. J. Ind. Eng. Chem. 2019, 80, 778–783. [Google Scholar] [CrossRef]
- Manikandan, M.; Hasan, N.; Wu, H.F. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials 2013, 34, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Zou, H.; Tang, D.; Wang, N.; Jia, S.; Sun, Z.; Yang, X.; Peng, J. Polyethylene glycol–modified molybdenum oxide as NIR photothermal agent and its ablation ability for HeLa cells. Colloid Polym. Sci. 2019, 297, 249–260. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Zou, R.; Liu, X.; Xu, K.; Li, W.; Hu, J. Cu7.2S4 nanocrystals: A novel photothermal agent with a 56.7% photothermal conversion efficiency for photothermal therapy of cancer cells. Nanoscale 2014, 6, 3274–3282. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, Y.; Shi, S.; Hao, S.; Wei, J.; Chen, X. Copper Sulfide Nanoparticles with Phospholipid-PEG Coating for In Vivo Near-Infrared Photothermal Cancer Therapy. Chem.–Asian J. 2015, 10, 370–376. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Aioub, M.; Panikkanvalappil, S.R.; El-Sayed, M.A. Platinum-Coated Gold Nanorods: Efficient Reactive Oxygen Scavengers That Prevent Oxidative Damage toward Healthy, Untreated Cells during Plasmonic Photothermal Therapy. ACS Nano 2017, 11, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Ghavami, M.; Shanehsazzadeh, S.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surfaces B Biointerfaces 2016, 143, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-W.; Huang, W.-Y.; Liaw, J.-W.; Rau, L.-R. Photothermal effects of laser-activated surface plasmonic gold nanoparticles on the apoptosis and osteogenesis of osteoblast-like cells. Int. J. Nanomed. 2016, 11, 3461–3473. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Zhang, X.; Ji, R.; Wang, Y.; Hu, H.; Peng, B.; Deng, Z. Preparation of colloidal polydopamine/Au hollow spheres for enhanced ultrasound contrast imaging and photothermal therapy. Mater. Sci. Eng. C 2020, 106, 110174. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Huang, N.; Tian, Y.; Wang, H.; Zhao, J.; Liu, H. Photothermal Effect of PEG-Functionalized Single-Walled Carbon Nanotubes. Nanotechnol. Nanomed. Nanobiotechnol. 2017, 4, 1–5. [Google Scholar] [CrossRef]
- Souslova, T.; Averill-Bates, D.A. Multidrug-resistant hela cells overexpressing MRP1 exhibit sensitivity to cell killing by hyperthermia: Interactions with etoposide. Int. J. Radiat. Oncol. 2004, 60, 1538–1551. [Google Scholar] [CrossRef]
- Robinson, J.T.; Welsher, K.; Tabakman, S.M.; Sherlock, S.P.; Wang, H.; Luong, R.; Dai, H. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010, 3, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.F.F.; Krais, J.J.; Van Rite, B.D.; Ramesh, R.; Resasco, D.E.; Harrison, R.G. Targeting single-walled carbon nanotubes for the treatment of breast cancer using photothermal therapy. Nanotechnology 2013, 24, 375104. [Google Scholar] [CrossRef]
- Eldridge, B.N.; Bernish, B.W.; Fahrenholtz, C.D.; Singh, R. Photothermal Therapy of Glioblastoma Multiforme Using Multiwalled Carbon Nanotubes Optimized for Diffusion in Extracellular Space. ACS Biomater. Sci. Eng. 2016, 2, 963–976. [Google Scholar] [CrossRef]
- Liang, X.; Shang, W.; Chi, C.; Zeng, C.; Wang, K.; Fang, C.; Chen, Q.; Liu, H.; Fan, Y.; Tian, J. Dye-conjugated single-walled carbon nanotubes induce photothermal therapy under the guidance of near-infrared imaging. Cancer Lett. 2016, 383, 243–249. [Google Scholar] [CrossRef]
- Sobhani, Z.; Behnam, M.A.; Emami, F.; Dehghanian, A.; Jamhiri, I. Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes. Int. J. Nanomed. 2017, 12, 4509–4517. [Google Scholar] [CrossRef] [PubMed]
- Virani, N.; Davis, C.; McKernan, P.; Hauser, P.; Hurst, R.E.; Slaton, J.; Silvy, R.P.; Resasco, D.E.; Harrison, R.G. Phosphatidylserine targeted single-walled carbon nanotubes for photothermal ablation of bladder cancer. Nanotechnology 2017, 29, 035101. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Hou, X.; Liu, Y.; Li, K.; Xu, S.; Wang, J. miR-185 acts as a tumor suppressor by targeting AKT1 in non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 11854–11862. [Google Scholar] [PubMed]
- Xiao, R.; Wang, R.; Zeng, Z.; Xu, L.; Wang, J. Application of poly(ethylene glycol)–distearoylphosphatidylethanolamine (PEG-DSPE) block copolymers and their derivatives as nanomaterials in drug delivery. Int. J. Nanomed. 2012, 7, 4185–4198. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Harris-Birtill, D.C.C.; Zhou, Y.; Gallina, M.E.; Cass, A.E.G.; Hanna, G.B.; Elson, D.S. Application of Gold Nanorods for Photothermal Therapy in Ex Vivo Human Oesophagogastric Adenocarcinoma. J. Biomed. Nanotechnol. 2016, 12, 481–490. [Google Scholar] [CrossRef]
- Hadidi, N.; Sharifnia, Z.; Eteghadi, A.; Shokrgozar, M.A.; Mosaffa, N. PEGylated single-walled carbon nanotubes as co-adjuvants enhance expression of maturation markers in monocyte-derived dendritic cells. Nanomedicine 2021, 16, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Aboofazeli, R.; Hadidi, N.; Kobarfard, F.; Nafissi-Varcheh, N. Optimization of single-walled carbon nanotube solubility by noncovalent PEGylation using experimental design methods. Int. J. Nanomed. 2011, 6, 737–746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van de Broek, B.; Devoogdt, N.; D’Hollander, A.; Gijs, H.-L.; Jans, K.; Lagae, L.; Muyldermans, S.; Maes, G.; Borghs, G. Specific Cell Targeting with Nanobody Conjugated Branched Gold Nanoparticles for Photothermal Therapy. ACS Nano 2011, 5, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, N.; Hosseini Shirazi, S.F.; Kobarfard, F.; Nafissi-Varchehd, N.; Aboofazeli, R. Evaluation of the Effect of PEGylated Sin-gle-Walled Carbon Nanotubes on Viability and Proliferation of Jurkat Cells. Iran J. Pharm. Res. 2012, 11, 27–37. [Google Scholar]
- Hadidi, N.; Ramezani, L.; Saffari, M. Evaluation of the cytotoxicity of original and functional carbon nanotubes in human lung cells. FEYZ 2015, 19, 326–333. [Google Scholar]
- Markovic, Z.M.; Harhaji-Trajkovic, L.; Marković, B.T.; Kepić, D.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramicanin, M.; Trajkovic, V. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Cojocaru, I.; Ilie, I.; Tabaran, F.A.; Zaharie, F.; Iancu, C.; Bartoş, D.; Mocan, L. Photothermal Treatment of Human Pancreatic Cancer Using PEGylated Multi-Walled Carbon Nanotubes Induces Apoptosis by Triggering Mitochondrial Membrane Depolarization Mechanism. J. Cancer 2014, 5, 679–688. [Google Scholar] [CrossRef]
- Bafkary, R.; Khoee, S. Carbon nanotube-based stimuli-responsive nanocarriers for drug delivery. RSC Adv. 2016, 6, 82553–82565. [Google Scholar] [CrossRef]
- Singh, S.; Vardharajula, S.; Tiwari, P.; Eroğlu, E.; Vig, K.; Dennis, V.; Ali, S.Z. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar] [CrossRef]
- Ghanbari, F.; Nasarzadeh, P.; Seydi, E.; Ghasemi, A.; Joghataei, M.T.; Ashtari, K.; Akbari, M. Mitochondrial oxidative stress and dysfunction induced by single- and multiwall carbon nanotubes: A comparative study. J. Biomed. Mater. Res. Part A 2017, 105, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Wu, F.; Yuan, P.; Chi, C.; Zhou, N. Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice. Carbon 2017, 123, 70–83. [Google Scholar] [CrossRef]
- Madani, S.Y.; Tan, A.; Naderi, N.; Seifalian, A. Application of OctaAmmonium-POSS functionalized single walled carbon nanotubes for thermal treatment of cancer. J. Nanosci. Nanotechnol. 2012, 12, 9018–9028. [Google Scholar] [CrossRef]
- Burke, A.R.; Singh, R.; Carroll, D.L.; Wood, J.C.; D’Agostino, R.B.; Ajayan, P.M.; Torti, F.M.; Torti, S.V. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012, 33, 2961–2970. [Google Scholar] [CrossRef]
- Pattani, V.P.; Shah, J.; Atalis, A.; Sharma, A.; Tunnell, J. Role of apoptosis and necrosis in cell death induced by nanoparticle-mediated photothermal therapy. J. Nanoparticle Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Jang, B.; Moorthy, M.S.; Manivasagan, P.; Xu, L.; Song, K.; Lee, K.D.; Kwak, M.; Oh, J.; Jin, J.-O. Fucoidan-coated CuS nanoparticles for chemo-and photothermal therapy against cancer. Oncotarget 2018, 9, 12649–12661. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, L.; Yang, X.; Ren, J.; Wang, Y.; Zhang, H.; Feng, Q.; Shi, Y.; Shan, X.; Yuan, Y. A novel redox-sensitive system based on single-walled carbon nanotubes for chemo-photothermal therapy and magnetic resonance imaging. Int. J. Nanomed. 2016, 11, 607–624. [Google Scholar] [CrossRef]
- Chuanzhi, L.I.; Ping, G.O.; Liang, Y.; Zuobin, W.A.; Li, W.A. The Application of Gold Nanorods for Photothermal Therapy of Ovarian Cancer. Mater. Sci. Medzg. 2020, 26, 243–248. [Google Scholar]
- Raphey, V.; Henna, T.; Nivitha, K.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Cho, E.-C. Carboxylated carbon nanomaterials in cell cycle and apoptotic cell death regulation. J. Biotechnol. 2019, 296, 14–21. [Google Scholar] [CrossRef]
- Halperin, E.C.; Wazner, D.E.; Perez, C.A.; Brady, L.W. Perez and Brady’s Principles and Practice of Radiation Oncology, 7th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 2260–2262. [Google Scholar]
- Oh, Y.; Je, J.-Y.; Moorthy, M.S.; Seo, H.; Cho, W.H. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int. J. Pharm. 2017, 531, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gong, J.; Cao, Y. Multi-Walled Carbon Nanotubes (MWCNTs) Activate Apoptotic Pathway through ER Stress: Does Surface Chemistry Matter? Int. J. Nanomed. 2019, 14, 9285–9294. [Google Scholar] [CrossRef] [PubMed]
- Przypis, L.; Krzywiecki, M.; Niidome, Y.; Aoki, H.; Shiraki, T.; Janas, D. Enhancing near-infrared photoluminescence from single-walled carbon nanotubes by defect-engineering using benzoyl peroxide. Sci. Rep. 2020, 10, 19877. [Google Scholar] [CrossRef]

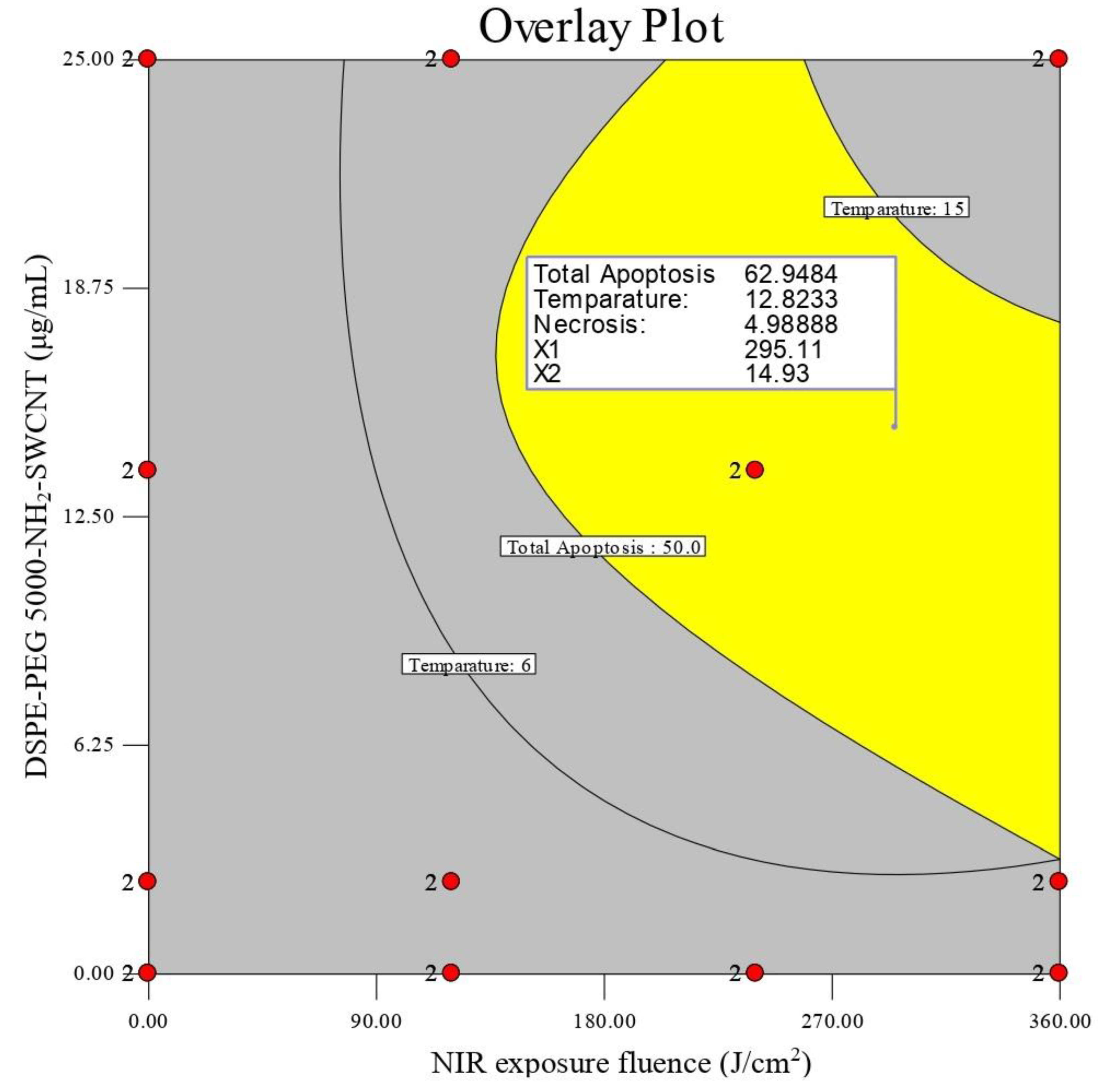
| Factor | Variables | Constraints | Optimization Criteria | Weight | Importance |
| A | NIR exposure fluence (J/cm2) | 120–360 | In range | 1 | 3 |
| B | DSPE-PEG 5000-NH2-SWCNT (μg/mL) | 2.5–25 | In range | 1 | 3 |
| C | Cell Treatment Time (hour) | 18, 24 | 24 | 1 | 3 |
| Response | |||||
| Y1 | Total Apoptosis (%) | 6.2–78.87 | Maximize (≥50%) | 1 | 3 |
| Y2 | Temperature change (∆T ≈ °C) | 0–19.75 | In range (10–15 °C) | 1 | 3 |
| Y3 | Post NIR exposure time (h) | 24, 48 | none | 1 | 3 |
| Y4 | Necrosis (%) | 1.52–12.53 | Minimize (≤8%) | 1 | 3 |
| Ra (pm) | Rq (pm) | Rt (nm) | |
|---|---|---|---|
| SWCNT | 1161 ± 7.82 | 2456 ± 1.29 | 48.99 ± 4.99 |
| DSPE-PEG 5000-NH2-SWCNT | 312.35 ± 6.65 | 455 ± 2.86 | 7.628 ± 2.23 |
| Cell Line | Samples | IC50 (µg/mL) after | |
|---|---|---|---|
| 24 h | 72 h | ||
| HEPG2 | Pure SWCNT DSPE-PEG 5000-NH2-SWNTs | 150 ± 6.35 300 ± 8.1 | 50 ± 7.50 250 ± 6.95 |
| A549 | Pure SWCNT DSPE-PEG 5000-NH2-SWNTs | 150 ± 7.6 370 ± 4.5 | 50 ± 4.95 240 ± 6.78 |
| SKOV3 | Pure SWCNT DSPE-PEG 5000-NH2-SWNTs | 150 ± 7.56 50 ± 6.77 | 50 ± 4.8 20 ± 2.6 |
| Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Run | A: DSPE-PEG5000-NH2-SWCNT Concentration (μg/mL) | B: NIR Exposure Fluence (J/cm2) | C: Cell Treatment Time (h) | Y1: Total Apoptosis (%) | Y2: ∆T (°C) | Y3: Post NIR Exposure (h) | Y4: Necrosis (%) |
| 1 | 2.5 | 120 | 18 | 36.65 ± 2.46 | 4 ± 2.86 | 24 | 5.93 ± 3.24 |
| 2 | 2.5 | 120 | 18 | 31.10 ± 2.41 | 4.4 ± 4.29 | 48 | 2.70 ± 1.12 |
| 3 | 2.5 | 120 | 24 | 18.13 ± 1.09 | 4 ± 1.73 | 24 | 7.11 ± 2.60 |
| 4 | 2.5 | 120 | 24 | 38.97 ± 1.31 | 0.65 ± 0.69 | 48 | 2.99 ± 1.06 |
| 5 | 0 | 120 | 18 | 24.92 ± 2.25 | 5.5 ± 5.05 | 24 | 5.08 ± 1.29 |
| 6 | 0 | 120 | 18 | 30.42 ± 2.16 | 3.6 ± 1.3 | 48 | 2.52 ± 1.21 |
| 7 | 0 | 120 | 24 | 20.47 ± 2.98 | 2.85 ± 1.05 | 24 | 6.80 ± 1.64 |
| 8 | 0 | 120 | 24 | 33.18 ± 1.68 | 2.26 ± 0.33 | 48 | 3.06 ± 1.67 |
| 9 | 25 | 120 | 18 | 51.93 ± 3.84 | 10.3 ± 0.49 | 24 | 11.58 ± 4.13 |
| 10 | 25 | 120 | 18 | 42.93 ± 2.14 | 6.73 ± 3.30 | 48 | 4.95 ± 0.17 |
| 11 | 25 | 120 | 24 | 28.44 ± 1.74 | 10.03 ± 0.46 | 24 | 1.52 ± 1.20 |
| 12 | 25 | 120 | 24 | 43.94 ± 1.85 | 8.9 ± 2.5 | 48 | 2.77 ± 1.72 |
| 13 | 2.5 | 0 | 18 | 37.03 ± 1.27 | 0 | 24 | 7.50 ± 0.92 |
| 14 | 2.5 | 0 | 18 | 30.88 ± 1.46 | 0 | 48 | 4.03 ± 1.75 |
| 15 | 2.5 | 0 | 24 | 32.63 ± 1.74 | 0 | 24 | 7.05 ± 3.45 |
| 16 | 2.5 | 0 | 24 | 36.59 ± 5.19 | 0 | 48 | 3.53 ± 1.86 |
| 17 | 25 | 0 | 18 | 44.04 ± 2.33 | 0 | 24 | 7.20 ± 1.97 |
| 18 | 25 | 0 | 18 | 35.02 ± 0.38 | 0 | 48 | 5.08 ± 1.75 |
| 19 | 25 | 0 | 24 | 36.63 ± 2.38 | 0 | 24 | 5.26 ± 1.61 |
| 20 | 25 | 0 | 24 | 46.12 ± 4.15 | 0 | 48 | 3.55 ± 1.16 |
| 21 | 13.75 | 0 | 18 | 50.43 ± 1.55 | 0 | 24 | 4.82 ± 3.58 |
| 22 | 13.75 | 0 | 18 | 38.98 ± 0.94 | 0 | 48 | 10.42 ± 1.38 |
| 23 | 13.75 | 0 | 24 | 37.69 ± 4.37 | 0 | 24 | 2.62 ± 1.89 |
| 24 | 13.75 | 0 | 24 | 46.64 ± 5.97 | 0 | 48 | 2.46 ± 1.02 |
| 25 | 13.75 | 240 | 18 | 61.54 ± 4.49 | 12.4 ± 1.49 | 24 | 7.43 ± 3.42 |
| 26 | 13.75 | 240 | 18 | 59.02 ± 3.27 | 14.13 ± 1.40 | 48 | 4.57 ± 1.79 |
| 27 | 13.75 | 240 | 24 | 46.45 ± 3.48 | 14.13 ± 2.46 | 24 | 6.74 ± 4.74 |
| 28 | 13.75 | 240 | 24 | 63.53 ± 5.36 | 9.68 ± 1.04 | 48 | 4.45 ± 2.41 |
| 29 | 0 | 240 | 18 | 42.30 ± 3.85 | 3.5 ± 1.30 | 24 | 6.43 ± 5.15 |
| 30 | 0 | 240 | 18 | 38.16 ± 2.84 | 6.43 ± 2.0 | 48 | 2.81 ± 0.42 |
| 31 | 0 | 240 | 24 | 22.84 ± 1.17 | 2.6 ± 0.7 | 24 | 8.00 ± 2.10 |
| 32 | 0 | 240 | 24 | 32.84 ± 2.72 | 3 ± 0.1 | 48 | 1.77 ± 0.95 |
| 33 | 2.5 | 360 | 18 | 43.23 ± 4.85 | 12.1 ± 4.95 | 24 | 6.56 ± 2.22 |
| 34 | 2.5 | 360 | 18 | 48.88 ± 3.24 | 7.42 ± 1.28 | 48 | 5.83 ± 3.41 |
| 35 | 2.5 | 360 | 24 | 45.68 ± 2.84 | 5.95 ± 1.39 | 24 | 6.59 ± 0.79 |
| 36 | 2.5 | 360 | 24 | 60.22 ± 3.33 | 5.74 ± 1.38 | 48 | 6.33 ± 1.31 |
| 37 | 0 | 360 | 18 | 37.85 ± 2.46 | 3.33 ± 1.32 | 24 | 5.03 ± 1.81 |
| 38 | 0 | 360 | 18 | 44.25 ± 2.83 | 6.76 ± 0.49 | 48 | 7.58 ± 1.53 |
| 39 | 0 | 360 | 24 | 31.97 ± 3.20 | 3.36 ± 0.23 | 24 | 4.81 ± 1.65 |
| 40 | 0 | 360 | 24 | 46.08 ± 5.17 | 3.76 ± 0.57 | 48 | 2.58 ± 0.80 |
| 41 | 25 | 360 | 18 | 78.87 ± 2.99 | 19.62 ± 1.8 | 24 | 11.46 ± 1.38 |
| 42 | 25 | 360 | 18 | 73.38 ± 2.25 | 12.87 ± 1.74 | 48 | 12.53 ± 3.91 |
| 43 | 25 | 360 | 24 | 61.43 ± 5.93 | 19.75 ± 1.24 | 24 | 3.22 ± 2.15 |
| 44 | 25 | 360 | 24 | 76.64 ± 2.60 | 16.07 ± 0.65 | 48 | 6.25 ± 1.22 |
| 45 | 0 | 0 | 18 | 18.95 ± 4.66 | 0 | 24 | 10.89 ± 4.75 |
| 46 | 0 | 0 | 18 | 8.52 ± 3.02 | 0 | 48 | 4.21 ± 2.13 |
| 47 | 0 | 0 | 24 | 6.20 ± 1.19 | 0 | 24 | 2.47 ± 2.16 |
| 48 | 0 | 0 | 24 | 18.98 ± 4.91 | 0 | 48 | 1.52 ± 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadidi, N.; Shahbahrami Moghadam, N.; Pazuki, G.; Parvin, P.; Shahi, F. In Vitro Evaluation of DSPE-PEG (5000) Amine SWCNT Toxicity and Efficacy as a Novel Nanovector Candidate in Photothermal Therapy by Response Surface Methodology (RSM). Cells 2021, 10, 2874. https://doi.org/10.3390/cells10112874
Hadidi N, Shahbahrami Moghadam N, Pazuki G, Parvin P, Shahi F. In Vitro Evaluation of DSPE-PEG (5000) Amine SWCNT Toxicity and Efficacy as a Novel Nanovector Candidate in Photothermal Therapy by Response Surface Methodology (RSM). Cells. 2021; 10(11):2874. https://doi.org/10.3390/cells10112874
Chicago/Turabian StyleHadidi, Naghmeh, Niloufar Shahbahrami Moghadam, Gholamreza Pazuki, Parviz Parvin, and Fatemeh Shahi. 2021. "In Vitro Evaluation of DSPE-PEG (5000) Amine SWCNT Toxicity and Efficacy as a Novel Nanovector Candidate in Photothermal Therapy by Response Surface Methodology (RSM)" Cells 10, no. 11: 2874. https://doi.org/10.3390/cells10112874
APA StyleHadidi, N., Shahbahrami Moghadam, N., Pazuki, G., Parvin, P., & Shahi, F. (2021). In Vitro Evaluation of DSPE-PEG (5000) Amine SWCNT Toxicity and Efficacy as a Novel Nanovector Candidate in Photothermal Therapy by Response Surface Methodology (RSM). Cells, 10(11), 2874. https://doi.org/10.3390/cells10112874








