A Microfluidic Flip-Chip Combining Hydrodynamic Trapping and Gravitational Sedimentation for Cell Pairing and Fusion
Abstract
:1. Introduction
2. Materials and Methods
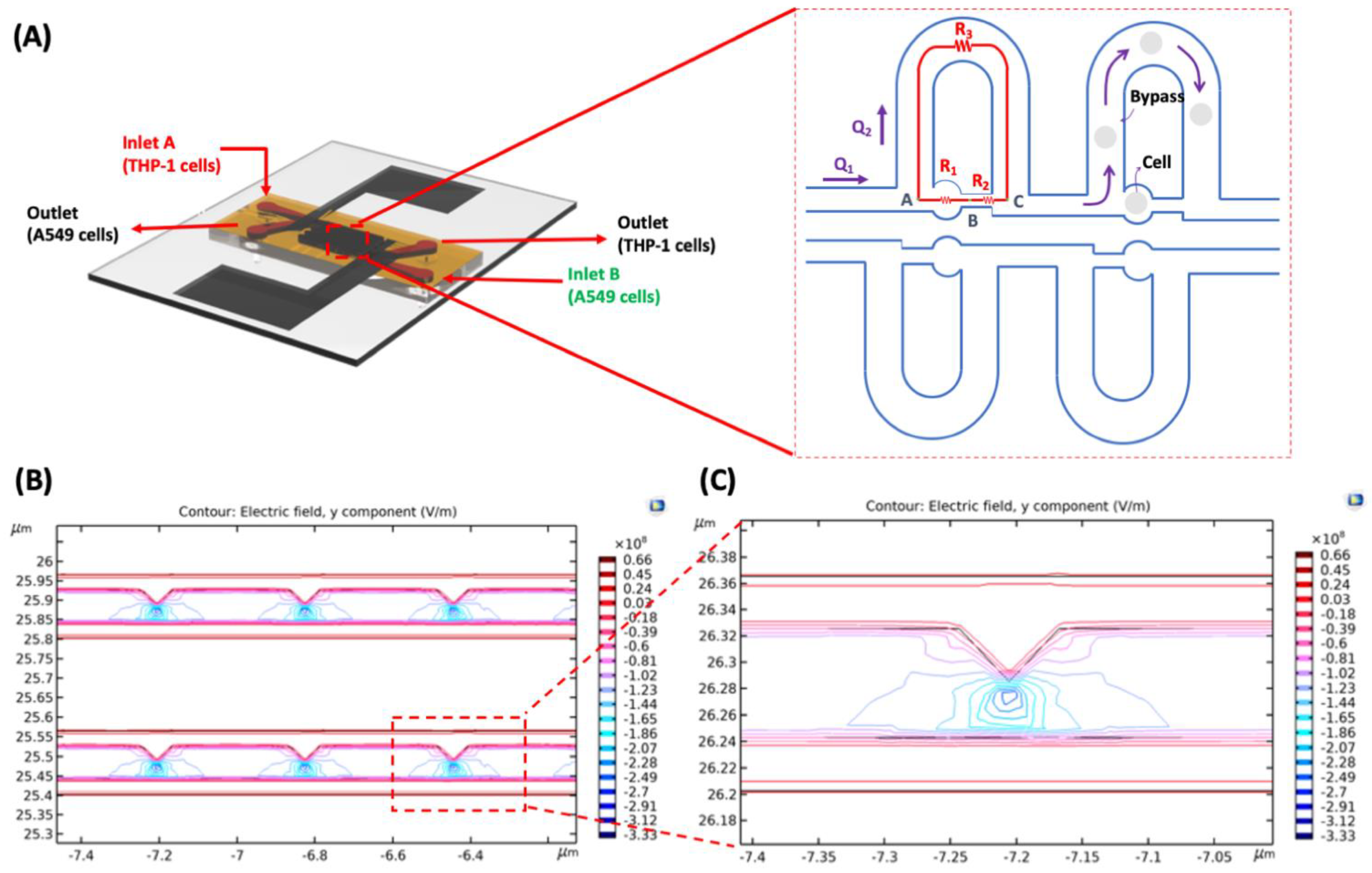
2.1. Device Design
2.2. Device Fabrication
2.3. PDMS Membrane Optimization
2.4. Modeling and Simulation
2.5. Cell Preparation
2.6. Experimental Setup
2.7. Device Operation
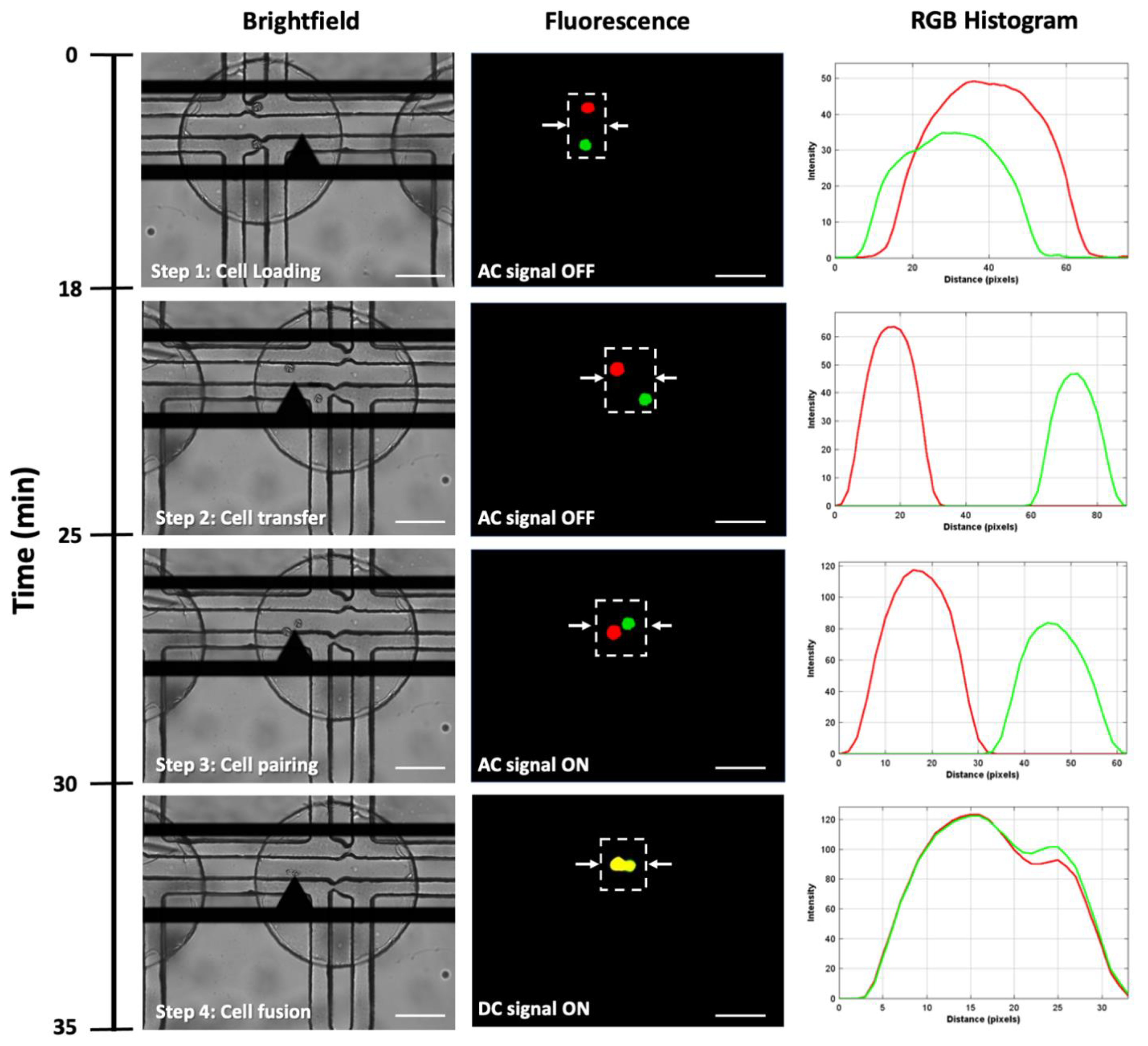
2.7.1. Cell Trapping
2.7.2. Cell Pairing and Fusion
2.8. Image Acquisition and Analysis
2.9. Statistical Analysis
3. Results
3.1. Cell Pairing
3.2. Cell Electrofusion
Effect of Electric Field on Fusion Efficiency
3.3. Effect of Membrane Thickness
3.4. Effect of Washing Flow Rate
3.5. Characterization of Fused Cells
3.6. Cell Viability in 96-Well Plate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Chen, D.; Kashiwaba, M.; Kufe, D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat. Med. 1997, 3, 558–561. [Google Scholar] [CrossRef]
- Koido, S.; Hara, E.; Homma, S.; Torii, A.; Toyama, Y.; Kawahara, H.; Watanabe, M.; Yanaga, K.; Fujise, K.; Tajiri, H.; et al. Dendritic Cells Fused with Allogeneic Colorectal Cancer Cell Line Present Multiple Colorectal Cancer–Specific Antigens and Induce Antitumor Immunity against Autologous Tumor Cells. Clin. Cancer Res. 2005, 11, 7891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-L.; Zou, M.-Z.; Liu, T.; Zeng, J.-Y.; Li, X.; Yu, W.-Y.; Li, C.-X.; Ye, J.-J.; Song, W.; Feng, J.; et al. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019, 10, 3199. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Kufe, D.; Avigan, D. Dendritic cell fusion vaccines for cancer immunotherapy. Expert Opin. Biol. Ther. 2005, 5, 703–715. [Google Scholar] [CrossRef]
- Kemna, E.W.M.; Wolbers, F.; Vermes, I.; van den Berg, A. On chip electrofusion of single human B cells and mouse myeloma cells for efficient hybridoma generation. Electrophoresis 2011, 32, 3138–3146. [Google Scholar] [CrossRef]
- Tomita, M.; Tsumoto, K. Hybridoma technologies for antibody production. Immunotherapy 2011, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.A.; Atienza, J.; Melton, D.A.; Eggan, K. Nuclear Reprogramming of Somatic Cells After Fusion with Human Embryonic Stem Cells. Science 2005, 309, 1369. [Google Scholar] [CrossRef] [Green Version]
- Oback, B.; Wiersema, A.T.; Gaynor, P.; Laible, G.; Tucker, F.C.; Oliver, J.E.; Miller, A.L.; Troskie, H.E.; Wilson, K.L.; Forsyth, J.T.; et al. Cloned Cattle Derived from a Novel Zona-Free Embryo Reconstruction System. Cloning Stem Cells 2003, 5, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oback, B.; Wells, D.N. Cloning Cattle. In Somatic Cell Nuclear Transfer; Sutovsky, P., Ed.; Springer: New York, NY, USA, 2007; pp. 30–57. [Google Scholar]
- White, J.; Matlin, K.; Helenius, A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 1981, 89, 674–679. [Google Scholar] [CrossRef]
- Zakai, N.; Kulka, R.G.; Loyter, A. Fusion of human erythrocyte ghosts promoted by the combined action of calcium and phosphate ions. Nature 1976, 263, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.T.-S.; Sun, T.-P.; Huang, W.-T.; Huang, S.-H.; Hsiao, C.-S.; Chang, K.-M. A circuit design of a low-cost, portable and programmable electroporation device for biomedical applications. Sens. Actuators Chem. 2012, 166–167, 292–300. [Google Scholar] [CrossRef]
- KÖHler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Dessain, S.K.; Adekar, S.P.; Stevens, J.B.; Carpenter, K.A.; Skorski, M.L.; Barnoski, B.L.; Goldsby, R.A.; Weinberg, R.A. High efficiency creation of human monoclonal antibody-producing hybridomas. J. Immunol. Methods 2004, 291, 109–122. [Google Scholar] [CrossRef]
- Clow, A.L.; Gaynor, P.T.; Oback, B.J. A novel micropit device integrates automated cell positioning by dielectrophoresis and nuclear transfer by electrofusion. Biomed. Microdev. 2010, 12, 777–786. [Google Scholar] [CrossRef]
- Tresset, G.; Takeuchi, S. A Microfluidic Device for Electrofusion of Biological Vesicles. Biomed Microdev. 2004, 6, 213–218. [Google Scholar] [CrossRef]
- Gel, M.; Kimura, Y.; Kurosawa, O.; Oana, H.; Kotera, H.; Washizu, M. Dielectrophoretic cell trapping and parallel one-to-one fusion based on field constriction created by a micro-orifice array. Biomicrofluidics 2010, 4, 022808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Gel, M.; Techaumnat, B.; Oana, H.; Kotera, H.; Washizu, M. Dielectrophoresis-assisted massively parallel cell pairing and fusion based on field constriction created by a micro-orifice array sheet. Electrophoresis 2011, 32, 2496–2501. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, J.; Qian, S.; Joo, S.W.; Zheng, X. A cell electrofusion microfluidic device integrated with 3D thin-film microelectrode arrays. Biomicrofluidics 2011, 5, 034121. [Google Scholar] [CrossRef] [Green Version]
- Skelley, A.M.; Kirak, O.; Suh, H.; Jaenisch, R.; Voldman, J. Microfluidic control of cell pairing and fusion. Nat. Methods 2009, 6, 147–152. [Google Scholar] [CrossRef]
- Zhang, K.; Chou, C.-K.; Xia, X.; Hung, M.-C.; Qin, L. Block-Cell-Printing for live single-cell printing. Proc. Natl. Acad. Sci. USA 2014, 111, 2948. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Liu, Y.; Liu, Y.; Zhang, M.; Zhang, X. Microfluidic Control of Tumor and Stromal Cell Spheroids Pairing and Merging for Three-Dimensional Metastasis Study. Anal. Chem. 2020, 92, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, J.; Joo, S.W.; Banerjee, A.N.; Qian, S. Cell electrofusion in microfluidic devices: A review. Sens. Actuators B Chem. 2013, 178, 63–85. [Google Scholar] [CrossRef]
- Hu, N.; Yang, J.; Yin, Z.-Q.; Ai, Y.; Qian, S.; Svir, I.B.; Xia, B.; Yan, J.-W.; Hou, W.-S.; Zheng, X.-L. A high-throughput dielectrophoresis-based cell electrofusion microfluidic device. Electrophoresis 2011, 32, 2488–2495. [Google Scholar] [CrossRef]
- Ju, J.; Ko, J.-M.; Cha, H.-C.; Park, J.Y.; Im, C.-H.; Lee, S.-H. An electrofusion chip with a cell delivery system driven by surface tension. J. Micromech. Microeng. 2008, 19, 015004. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, J.; Yin, Z.Q.; Luo, H.Y.; Yang, M.; Hu, N.; Yang, J.; Huo, D.Q.; Hou, C.J.; Jiang, Z.Z.; et al. Study of high-throughput cell electrofusion in a microelectrode-array chip. Microfluid. Nanofluid. 2008, 5, 669–675. [Google Scholar] [CrossRef]
- Hu, N.; Yang, J.; Qian, S.; Zhang, X.; Joo, S.W.; Zheng, X. A cell electrofusion microfluidic chip using discrete coplanar vertical sidewall microelectrodes. Electrophoresis 2012, 33, 1980–1986. [Google Scholar] [CrossRef]
- Qu, Y.; Hu, N.; Xu, H.; Yang, J.; Xia, B.; Zheng, X.; Yin, Z.Q. Somatic and stem cell pairing and fusion using a microfluidic array device. Microfluid. Nanofluid. 2011, 11, 633–641. [Google Scholar] [CrossRef]
- Masuda, S.; Washizu, M.; Nanba, T. Novel method of cell fusion in field constriction area in fluid integration circuit. IEEE Trans. Ind. Appl. 1989, 25, 732–737. [Google Scholar] [CrossRef]
- Techaumnat, B.; Tsuda, K.; Kurosawa, O.; Murat, G.; Oana, H.; Washizu, M. High-yield electrofusion of biological cells based on field tailoring by microfabricated structures. IET Nanobiotechnol. 2008, 2, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gel, M.; Suzuki, S.; Kimura, Y.; Kurosawa, O.; Techaumnat, B.; Oana, H.; Washizu, M. Microorifice-Based High-Yield Cell Fusion on Microfluidic Chip: Electrofusion of Selected Pairs and Fusant Viability. IEEE Trans. NanoBiosci. 2009, 8, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, R.M.; van den Beld, W.T.E.; Kemna, E.W.M.; Wolbers, F.; Eijkel, J.C.T.; van den Berg, A. Electrofusion of single cells in picoliter droplets. Sci. Rep. 2018, 8, 3714. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-C.; Wang, C.-H.; Lee, W.-B.; Lee, G.-B. Automatic cell fusion via optically-induced dielectrophoresis and optically-induced locally-enhanced electric field on a microfluidic chip. Biomicrofluidics 2018, 12, 034108. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-F.; Wang, C.-H.; Lee, G.-B. Optically-Induced Cell Fusion on Cell Pairing Microstructures. Sci. Rep. 2016, 6, 22036. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Y.; Huang, W.; Wu, H. Cell pairing and polyethylene glycol (PEG)-mediated cell fusion using two-step centrifugation-assisted single-cell trapping (CAScT). Lab. A Chip. 2018, 18, 1113–1120. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.-H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendharkar, G.; Lu, Y.-T.; Chang, C.-M.; Lu, M.-P.; Lu, C.-H.; Chen, C.-C.; Liu, C.-H. A Microfluidic Flip-Chip Combining Hydrodynamic Trapping and Gravitational Sedimentation for Cell Pairing and Fusion. Cells 2021, 10, 2855. https://doi.org/10.3390/cells10112855
Pendharkar G, Lu Y-T, Chang C-M, Lu M-P, Lu C-H, Chen C-C, Liu C-H. A Microfluidic Flip-Chip Combining Hydrodynamic Trapping and Gravitational Sedimentation for Cell Pairing and Fusion. Cells. 2021; 10(11):2855. https://doi.org/10.3390/cells10112855
Chicago/Turabian StylePendharkar, Gaurav, Yen-Ta Lu, Chia-Ming Chang, Meng-Ping Lu, Chung-Huan Lu, Chih-Chen Chen, and Cheng-Hsien Liu. 2021. "A Microfluidic Flip-Chip Combining Hydrodynamic Trapping and Gravitational Sedimentation for Cell Pairing and Fusion" Cells 10, no. 11: 2855. https://doi.org/10.3390/cells10112855
APA StylePendharkar, G., Lu, Y.-T., Chang, C.-M., Lu, M.-P., Lu, C.-H., Chen, C.-C., & Liu, C.-H. (2021). A Microfluidic Flip-Chip Combining Hydrodynamic Trapping and Gravitational Sedimentation for Cell Pairing and Fusion. Cells, 10(11), 2855. https://doi.org/10.3390/cells10112855






