Neurogenic Potential of the 18-kDa Mitochondrial Translocator Protein (TSPO) in Pluripotent P19 Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Western Blot Analysis
2.3. Cell Cycle
2.4. Cell Proliferation
2.5. Live/Dead Cell Assay
2.6. Mitochondrial Membrane Potential (∆Ψm)
2.7. Mitochondrial Calcium Level Measurement
2.8. Reactive Oxygen Species (ROS) Measurement
2.9. ATP Measurement
2.10. Statistical Analyses
3. Results
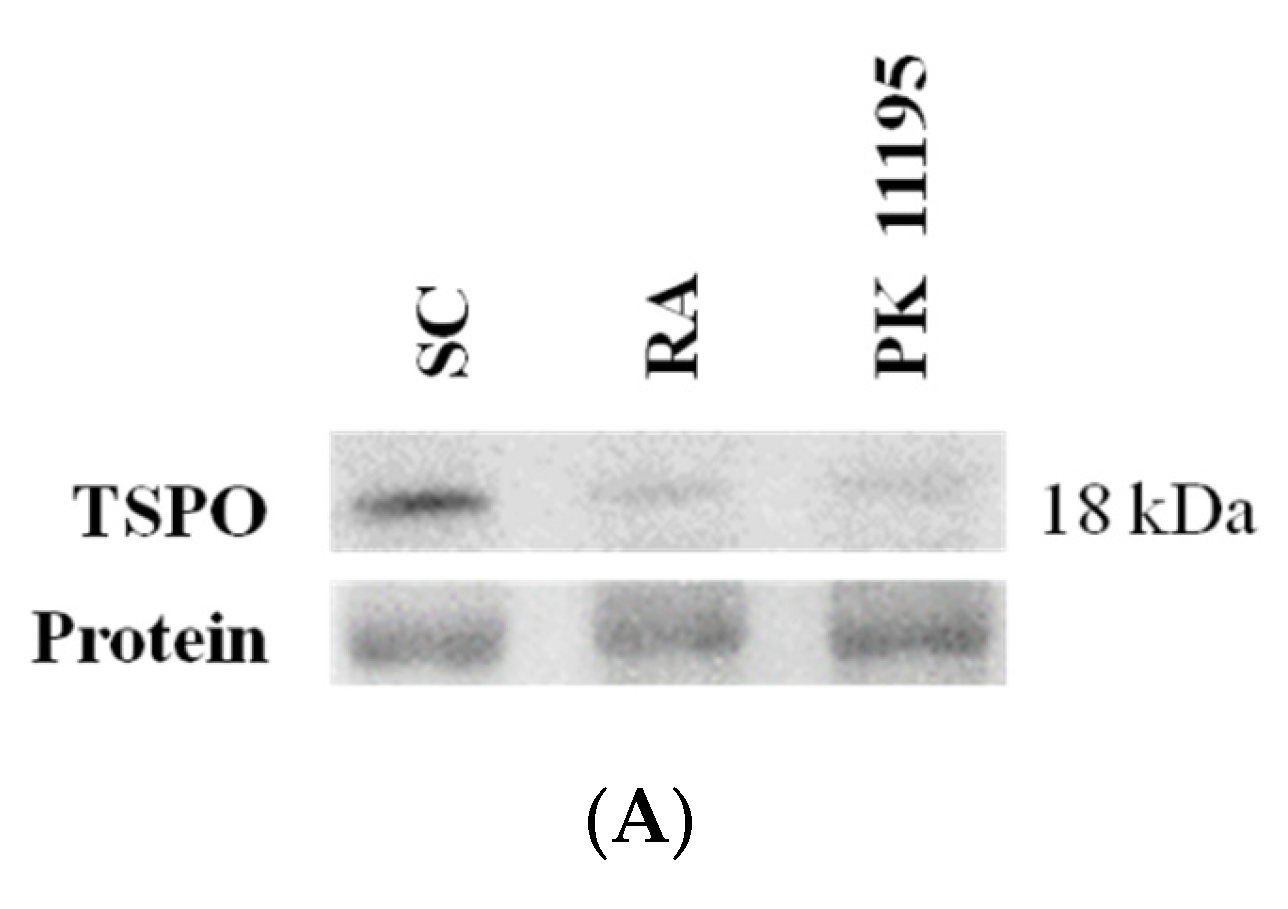
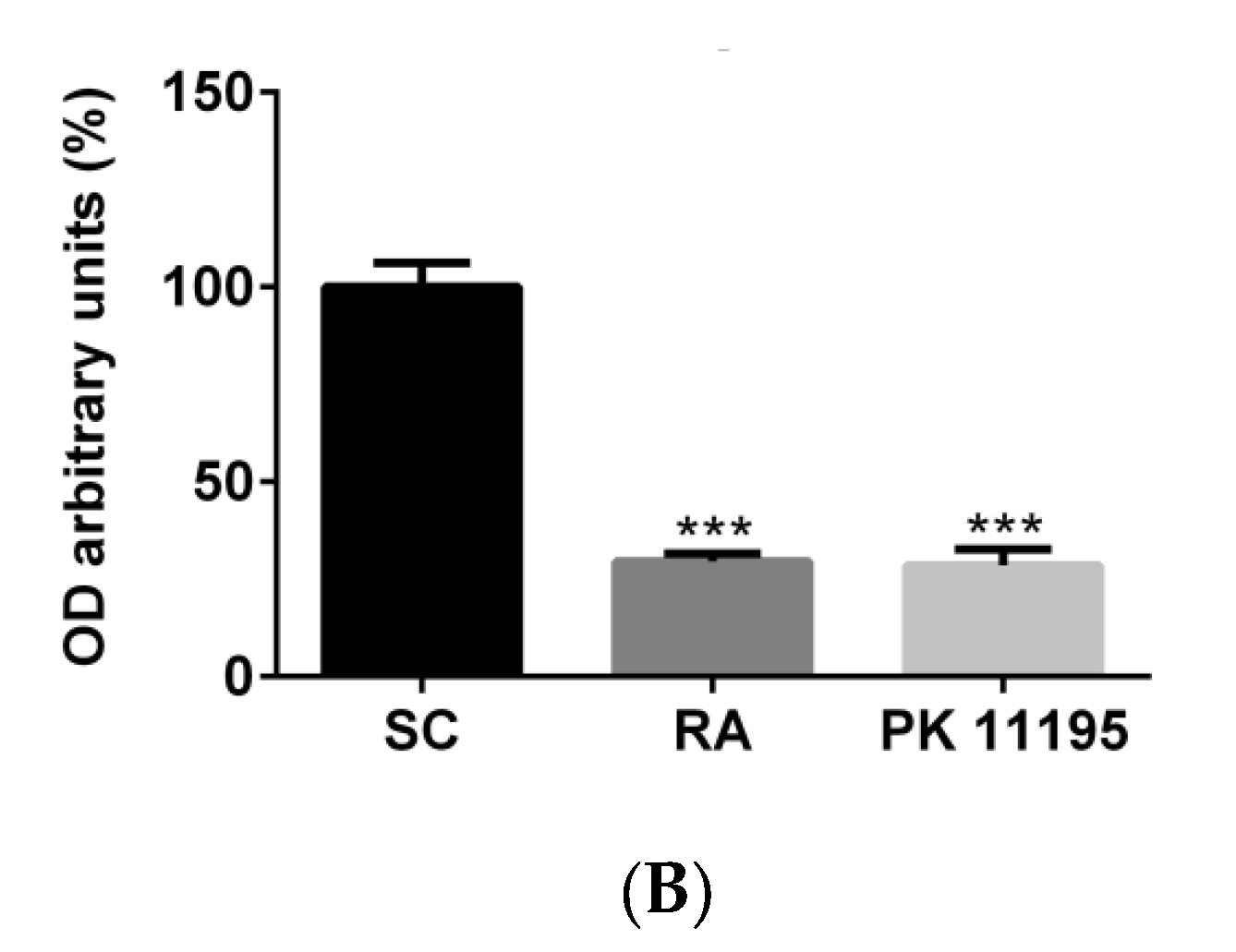
3.1. TSPO Levels in Pluripotent P19 Stem Cells
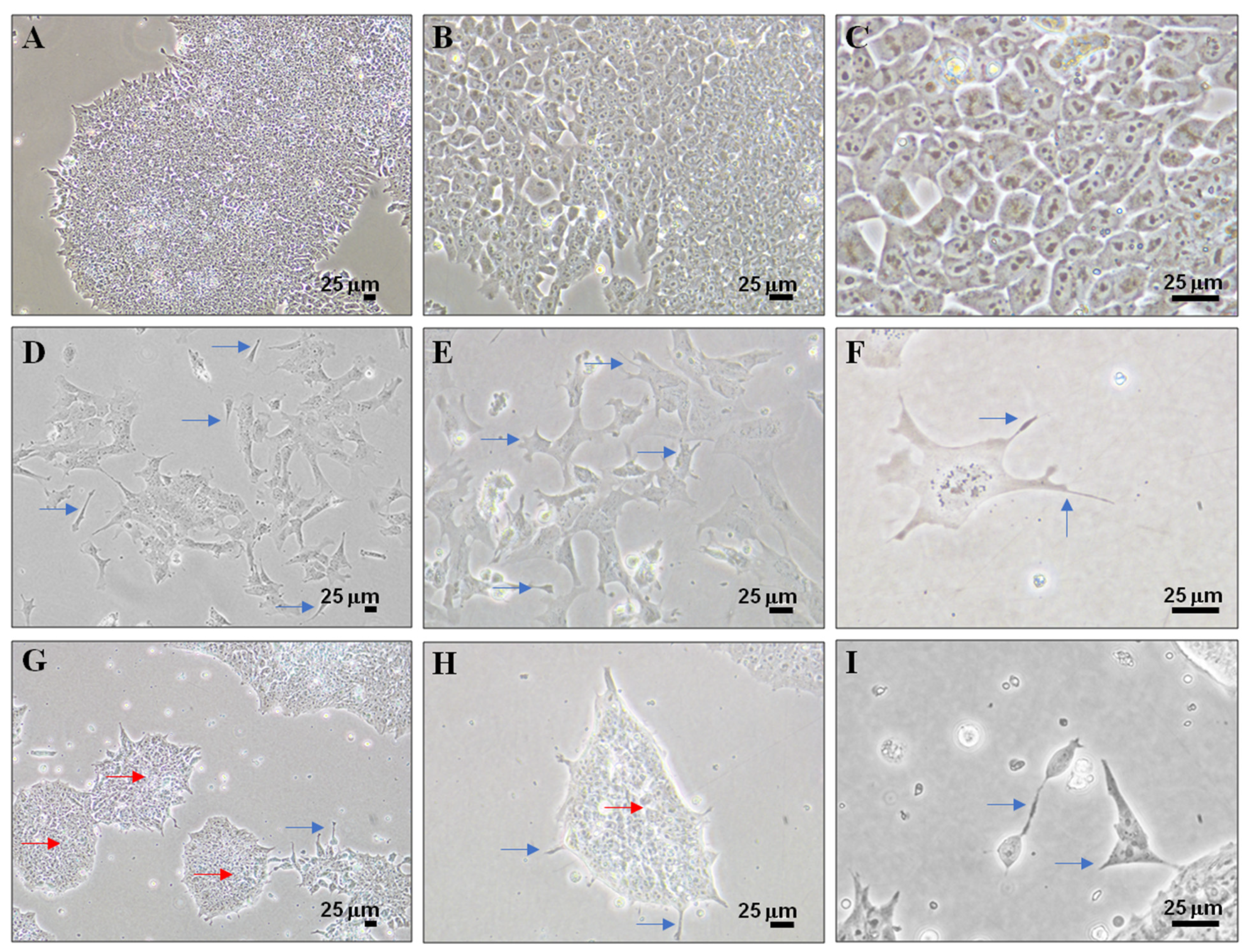
3.2. Cell Differentiation of P19 Stem Cells by RA or PK 11195
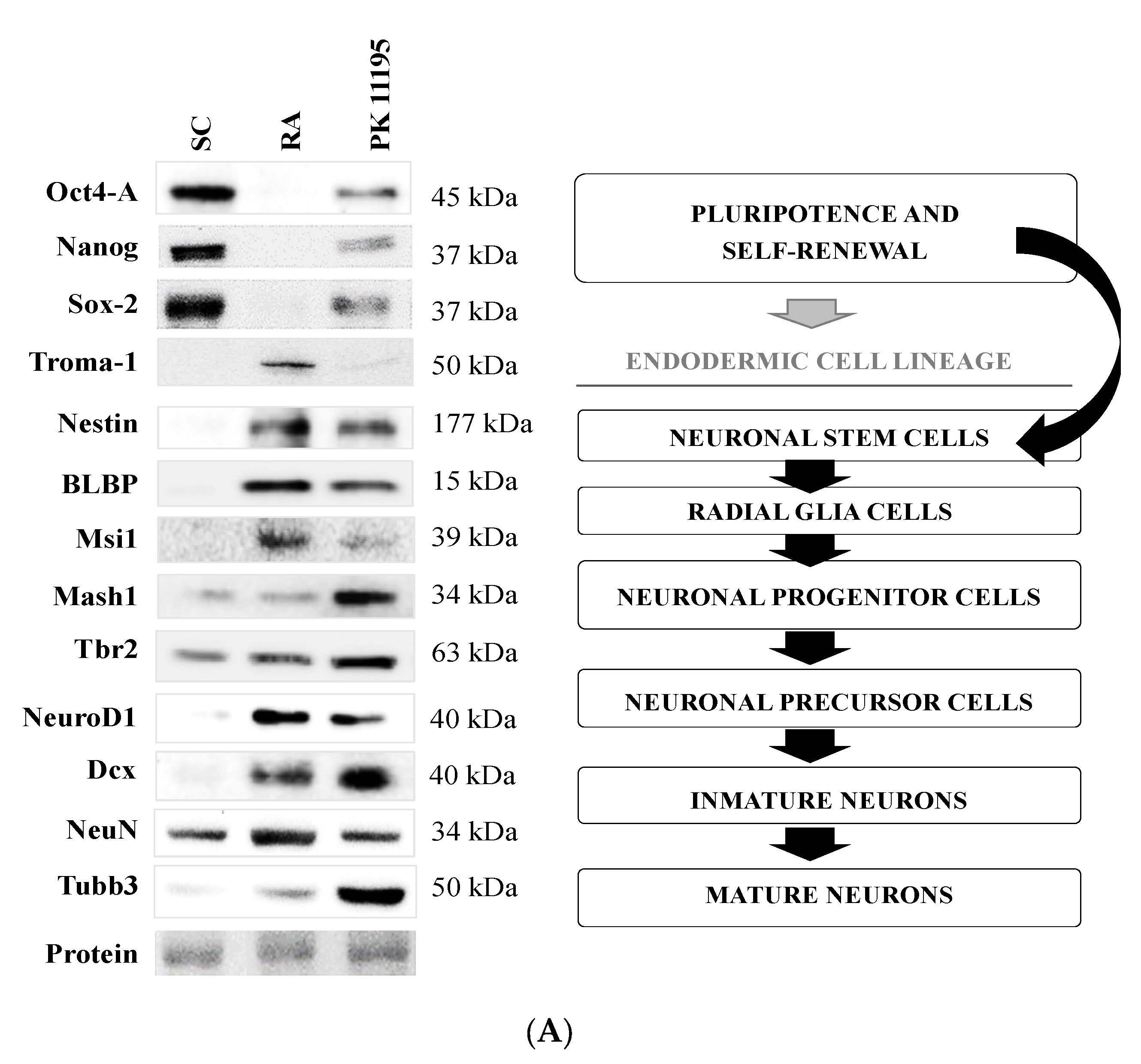
3.3. Pluripotency and Neuronal Markers during the Differentiation of P19 Stem Cells
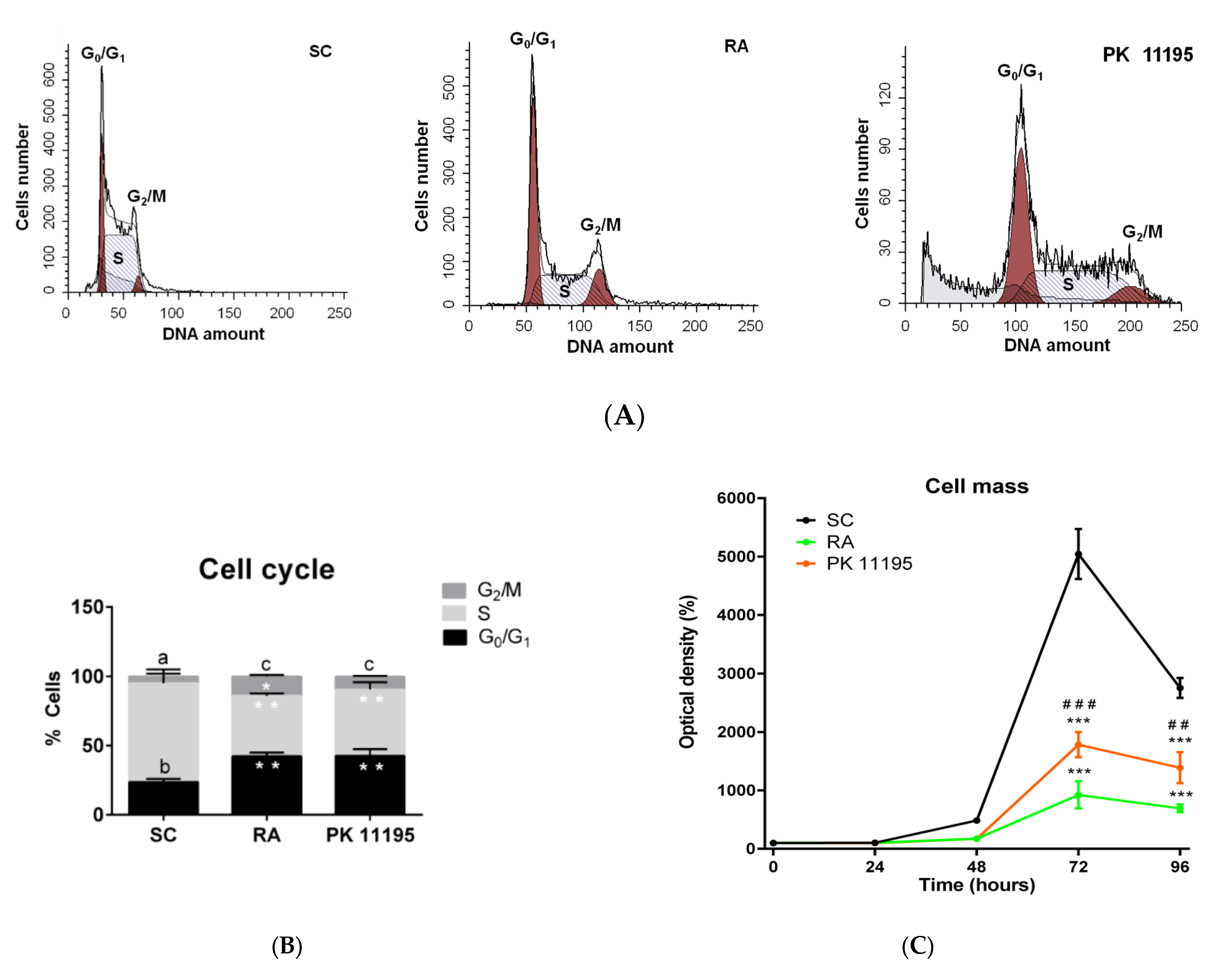
3.4. Cell Cycle during Cell Differentiation of P19 Stem Cells
3.5. Cell Death during Cell Differentiation of P19 Stem Cells
3.6. Mitochondrial Function during the Differentiation of P19 Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavish, M.; Bachman, I.; Shoukrun, R.; Katz, Y.; Veenman, L.; Weisinger, G.; Weizman, A. Enigma of the peripheral benzodiazepine receptor. Pharmacol. Rev 1999, 51, 629–650. [Google Scholar]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Gavish, M.; Veenman, L. Regulation of Mitochondrial, Cellular, and Organismal Functions by TSPO. Adv. Pharmacol. 2018, 82, 103–136. [Google Scholar] [CrossRef]
- Bode, J.; Veenman, L.; Caballero, B.; Lakomek, M.; Kugler, W.; Gavish, M. The 18 kDa translocator protein influences angiogenesis, as well as aggressiveness, adhesion, migration, and proliferation of glioblastoma cells. Pharmacogenet. Genom. 2012, 22, 538–550. [Google Scholar] [CrossRef]
- Caballero, B.; Veenman, L.; Gavish, M. Role of mitochondrial translocator protein (18 kDa) on mitochondrial- related cell death processes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2013, 7, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling. Int. J. Mol. Sci. 2017, 18, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, B.; Marko, K.; Hadinger, N.; Jelitai, M.; Demeter, K.; Tihanyi, K.; Vas, A.; Madarasz, E. Translocator protein (TSPO 18kDa) is expressed by neural stem and neuronal precursor cells. Neurosci. Lett. 2009, 462, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.F.; Weng, J.F.; Cen, M.; Dong, X.Q.; Yu, W.H.; Du, Q.; Yang, D.B.; Zheng, Y.K.; Hu, W.; Yu, L.; et al. Prognostic significance of serum translocator protein in patients with traumatic brain injury. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 488, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Palzur, E.; Sharon, A.; Shehadeh, M.; Soustiel, J.F. Investigation of the mechanisms of neuroprotection mediated by Ro5-4864 in brain injury. Neuroscience 2016, 329, 162–170. [Google Scholar] [CrossRef]
- Notter, T.; Schalbetter, S.M.; Clifton, N.E.; Mattei, D.; Richetto, J.; Thomas, K.; Meyer, U.; Hall, J. Neuronal activity increases translocator protein (TSPO) levels. Mol. Psychiatry 2020, 26, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Veenman, L.; Bode, J.; Leschiner, S.; Gavish, M. Concentration-Dependent Bimodal Effect of Specific 18 kDa Translocator Protein (TSPO) Ligands on Cell Death Processes Induced by Ammonium Chloride: Potential Implications for Neuropathological Effects Due to Hyperammonemia. Cns Neurol. Disord.-Drug Targets 2014, 13, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Veenman, L.; Shterenberg, A.; Singh, S.; Masarwa, A.; Dutta, B.; Island, B.; Tsoglin, E.; Levin, E.; Leschiner, S.; et al. Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease. Cell Death Discov. 2015, 1, 15027. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Vainshtein, A.; Gavish, M. TSPO as a target for treatments of diseases, including neuropathological disorders. Cell Death Dis 2015, 6, e1911. [Google Scholar] [CrossRef] [Green Version]
- Azrad, M.; Zeineh, N.; Weizman, A.; Veenman, L.; Gavish, M. The TSPO Ligands 2-Cl-MGV-1, MGV-1, and PK11195 Differentially Suppress the Inflammatory Response of BV-2 Microglial Cell to LPS. Int. J. Mol. Sci. 2019, 20, 594. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.K.; Choi, H.B.; McLarnon, J.G. Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol. Dis. 2005, 20, 550–561. [Google Scholar] [CrossRef]
- Chen, Y.; Veenman, L.; Singh, S.; Ouyang, F.; Liang, J.; Huang, W.; Marek, I.; Zeng, J.; Gavish, M. 2-Cl-MGV-1 Ameliorates Apoptosis in the Thalamus and Hippocampus and Cognitive Deficits After Cortical Infarct in Rats. Stroke 2017, 48, 3366–3374. [Google Scholar] [CrossRef]
- Sauerbeck, A.; Gao, J.; Readnower, R.; Liu, M.; Pauly, J.R.; Bing, G.; Sullivan, P.G. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol. 2011, 227, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Krestinina, O.V.; Myakisheva, S.N.; Baburina, Y.; Fadeev, R.S.; Azarashavili, T.S.; Akatov, V.S. The effects of isoquinoline carboxamide and melatonin on the differentation of N1E-115 mouse neuroblastoma cells (clone C-1300) and on the expression of the the TSPO translocation protein and 2’,3´-cyclonucleotide-3´-phosphodiesterase in these cells. Neurochem. J. 2017, 11, 31–37. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Traumatic Brain Injury and Stem Cells: An Overview of Clinical Trials, the Current Treatments and Future Therapeutic Approaches. Medicina 2020, 56, 137. [Google Scholar] [CrossRef] [Green Version]
- McBurney, M.W.; Jones-Villeneuve, E.M.; Edwards, M.K.; Anderson, P.J. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature 1982, 299, 165–167. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. BioMed Res. Int. 2015, 2015, 727542. [Google Scholar] [CrossRef] [Green Version]
- Pachernik, J.; Bryja, V.; Esner, M.; Kubala, L.; Dvorak, P.; Hampl, A. Neural differentiation of pluripotent mouse embryonal carcinoma cells by retinoic acid: Inhibitory effect of serum. Physiol. Res. 2005, 54, 115–122. [Google Scholar] [PubMed]
- Verma, I.; Seshagiri, P.B. Directed differentiation of mouse P19 embryonal carcinoma cells to neural cells in a serum- and retinoic acid-free culture medium. Vitr. Cell Dev. Biol. Anim. 2018, 54, 567–579. [Google Scholar] [CrossRef]
- Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. Int. J. Mol. Sci. 2018, 19, 2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanimoto, Y.; Yamasaki, T.; Nagoshi, N.; Nishiyama, Y.; Nori, S.; Nishimura, S.; Iida, T.; Ozaki, M.; Tsuji, O.; Ji, B.; et al. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Transl. Med. 2020, 9, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, R.; Magalhaes-Novais, S.; Mesquita, K.A.; Baldeiras, I.; Sousa, I.S.; Tavares, L.C.; Barbosa, I.A.; Oliveira, P.J.; Vega-Naredo, I. Melatonin antiproliferative effects require active mitochondrial function in embryonal carcinoma cells. Oncotarget 2015, 6, 17081–17096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryoo, H.D.; Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miho, Y.; Kouroku, Y.; Fujita, E.; Mukasa, T.; Urase, K.; Kasahara, T.; Isoai, A.; Momoi, M.Y.; Momoi, T. bFGF inhibits the activation of caspase-3 and apoptosis of P19 embryonal carcinoma cells during neuronal differentiation. Cell Death Differ. 1999, 6, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Ghani, M.; Dufort, D.; Stiles, R.; De Repentigny, Y.; Kothary, R.; Megeney, L.A. Wnt11 promotes cardiomyocyte development by caspase-mediated suppression of canonical Wnt signals. Mol. Cell. Biol. 2011, 31, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Vega-Naredo, I.; Loureiro, R.; Mesquita, K.A.; Barbosa, I.A.; Tavares, L.C.; Branco, A.F.; Erickson, J.R.; Holy, J.; Perkins, E.L.; Carvalho, R.A.; et al. Mitochondrial metabolism directs stemness and differentiation in P19 embryonal carcinoma stem cells. Cell Death Differ. 2014, 21, 1560–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, R.; Mesquita, K.A.; Magalhaes-Novais, S.; Oliveira, P.J.; Vega-Naredo, I. Mitochondrial biology in cancer stem cells. Semin. Cancer Biol. 2017, 47, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, P.; Kannan, P.; Mlody, B.; Prigione, A. Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep. 2018, 19, e45432. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes-Novais, S.; Bermejo-Millo, J.C.; Loureiro, R.; Mesquita, K.A.; Domingues, M.R.; Maciel, E.; Melo, T.; Baldeiras, I.; Erickson, J.R.; Holy, J.; et al. Cell quality control mechanisms maintain stemness and differentiation potential of P19 embryonic carcinoma cells. Autophagy 2020, 16, 313–333. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W. P19 embryonal carcinoma cells. Int. J. Dev. Biol. 1993, 37, 135–140. [Google Scholar]
- Breuss, M.W.; Leca, I.; Gstrein, T.; Hansen, A.H.; Keays, D.A. Tubulins and brain development-The origins of functional specification. Mol. Cell. Neurosci. 2017, 84, 58–67. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, M.; Zhang, N.; Yin, H.; Shu, B.; Duan, W. A pilot study on searching for peri-nuclear NeuN-positive cells. PeerJ 2020, 8, e8254. [Google Scholar] [CrossRef] [Green Version]
- Foudah, D.; Monfrini, M.; Donzelli, E.; Niada, S.; Brini, A.T.; Orciani, M.; Tredici, G.; Miloso, M. Expression of neural markers by undifferentiated mesenchymal-like stem cells from different sources. J. Immunol. Res. 2014, 2014, 987678. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Blanco, L.; Bermejo-Millo, J.C.; Oliveira, G.; Potes, Y.; Antuña, E.; Menéndez-Valle, I.; Vega-Naredo, I.; Coto-Montes, A.; Caballero, B. Neurogenic Potential of the 18-kDa Mitochondrial Translocator Protein (TSPO) in Pluripotent P19 Stem Cells. Cells 2021, 10, 2784. https://doi.org/10.3390/cells10102784
González-Blanco L, Bermejo-Millo JC, Oliveira G, Potes Y, Antuña E, Menéndez-Valle I, Vega-Naredo I, Coto-Montes A, Caballero B. Neurogenic Potential of the 18-kDa Mitochondrial Translocator Protein (TSPO) in Pluripotent P19 Stem Cells. Cells. 2021; 10(10):2784. https://doi.org/10.3390/cells10102784
Chicago/Turabian StyleGonzález-Blanco, Laura, Juan Carlos Bermejo-Millo, Gabriela Oliveira, Yaiza Potes, Eduardo Antuña, Iván Menéndez-Valle, Ignacio Vega-Naredo, Ana Coto-Montes, and Beatriz Caballero. 2021. "Neurogenic Potential of the 18-kDa Mitochondrial Translocator Protein (TSPO) in Pluripotent P19 Stem Cells" Cells 10, no. 10: 2784. https://doi.org/10.3390/cells10102784
APA StyleGonzález-Blanco, L., Bermejo-Millo, J. C., Oliveira, G., Potes, Y., Antuña, E., Menéndez-Valle, I., Vega-Naredo, I., Coto-Montes, A., & Caballero, B. (2021). Neurogenic Potential of the 18-kDa Mitochondrial Translocator Protein (TSPO) in Pluripotent P19 Stem Cells. Cells, 10(10), 2784. https://doi.org/10.3390/cells10102784







