The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications
Abstract
1. Introduction
1.1. Dendritic Cells’ Role and Regulation in Anti-Tumor Immunity
1.2. Myeloid-Derived Suppressor Cells’ Role and Regulation in Anti-Tumor Immunity
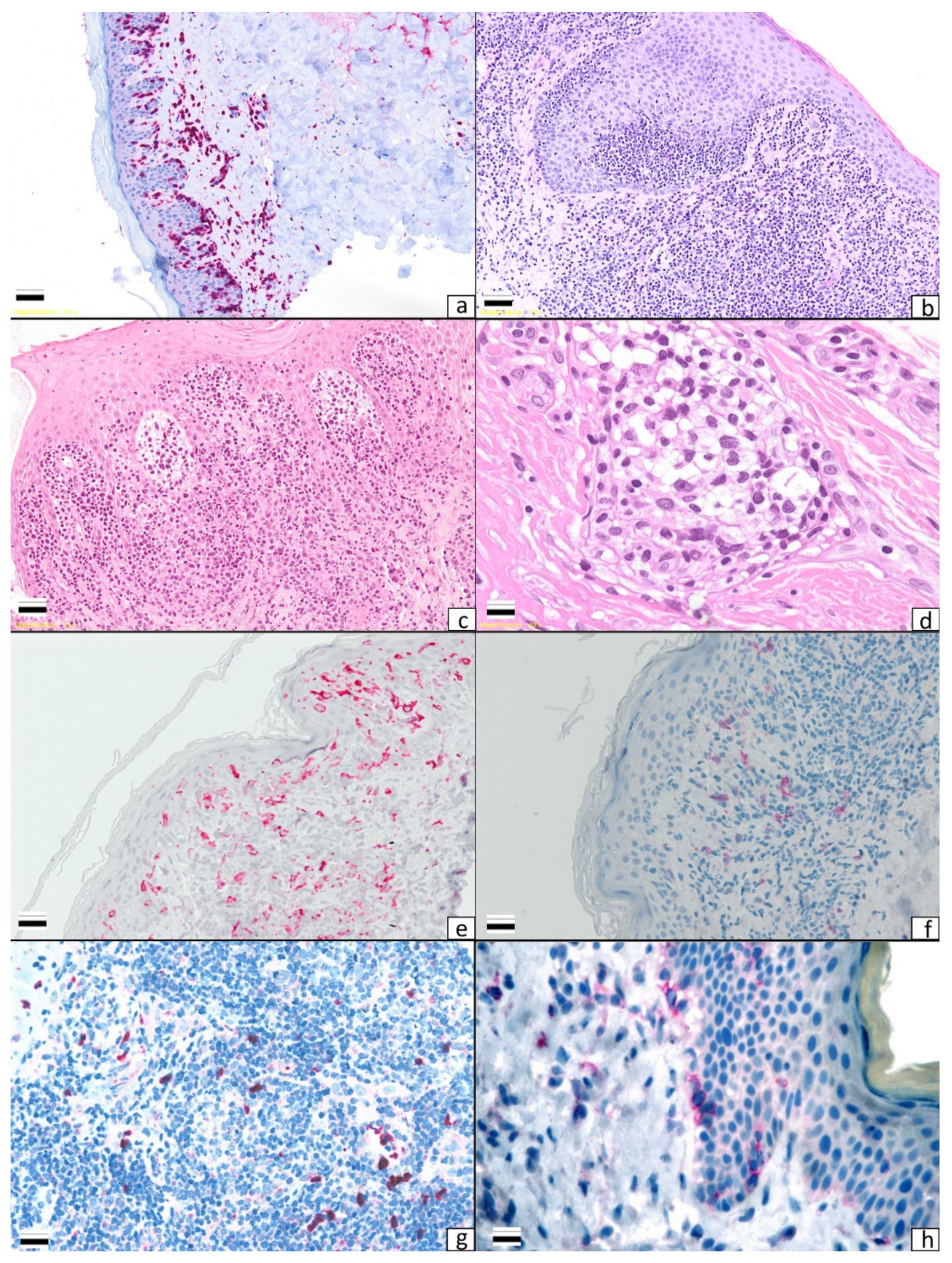
1.3. LCs, DDCs, and MDSCs in MF and SS
1.4. Regulatory B-Cells (Breg Cells)
1.5. Brigs in MF
1.6. Regulatory T-Cells (Treg Cells)
1.7. Tregs in MF and SS
1.8. Macrophages
1.9. Tumor-Associated Macrophages (TAMs) in MF and SS
1.10. Keratinocytes
1.11. Keratinocytes in MF and SS
1.12. Endothelial Cells
1.13. Endothelial Cells in MF and SS
1.14. Tumor-Infiltrating Lymphocytes (TILs)
1.15. Tumor-Infiltrating Lymphocytes in MF and SS
1.16. NK Cells
1.17. NK Cells in MF and SS
1.18. Eosinophils
1.19. Eosinophils’ Role in MF and SS
1.20. Fibroblasts
1.21. Fibroblasts’ Role in MF and SS
1.22. Cytokines’ Influence on the Tumor Microenvironment’s Composition in MF/SS
2. Materials and Methods
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bradford, P.T.; Devesa, S.S.; Anderson, W.F.; Toro, J.R. Cutaneous Lymphoma Incidence Patterns in the United States: A Population-Based Study of 3884 Cases. Blood 2009, 113, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Lessin, S.R.; Duvic, M.; Guitart, J.; Pandya, A.G.; Strober, B.E.; Olsen, E.A.; Hull, C.M.; Knobler, E.H.; Rook, A.H.; Kim, E.J.; et al. Topical Chemotherapy in Cutaneous T-Cell Lymphoma: Positive Results of a Randomized, Controlled, Multi-Center Trial Testing the Efficacy and Safety of a Novel 0.02% Mechlorethamine Gel in Mycosis Fungoides. JAMA Dermatol. 2013, 149, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Maule, M.; Prince, H.M.; Porcu, P.; Horwitz, S.; Duvic, M.; Talpur, R.; Vermeer, M.; Bagot, M.; Guitart, J.; et al. Global Patterns of Care in Advanced Stage Mycosis Fungoides/Sezary Syndrome: A Multicenter Retrospective Follow-up Study from the Cutaneous Lymphoma International Consortium. Ann. Oncol. 2017, 28, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. Brentuximab Vedotin or Physician’s Choice in CD30-Positive Cutaneous T-Cell Lymphoma (ALCANZA): An International, Open-Label, Randomised, Phase 3, Multicentre Trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus Vorinostat in Previously Treated Cutaneous T-Cell Lymphoma (MAVORIC): An International, Open-Label, Randomised, Controlled Phase 3 Trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef]
- Quaglino, P.; Fava, P.; Pileri, A.; Grandi, V.; Sanlorenzo, M.; Panasiti, V.; Guglielmo, A.; Alberti-Violetti, S.; Novelli, M.; Astrua, C.; et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Dermatol. 2021, 141, 484–495. [Google Scholar] [CrossRef]
- Burnet Cancer: A Biological Approach. III. Viruses Associated with Neoplastic Conditions. IV. Practical Applications. Abstract —Europe PMC. Available online: http://europepmc.org/article/PMC/1973618 (accessed on 12 June 2021).
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Ni, X. Dendritic Cells and Cutaneous T-Cell Lymphomas. Giorn. Ital. Dermatol. Venereol. 2011, 146, 103–113. [Google Scholar]
- Schlapbach, C.; Ochsenbein, A.; Kaelin, U.; Hassan, A.S.; Hunger, R.E.; Yawalkar, N. High Numbers of DC-SIGN+ Dendritic Cells in Lesional Skin of Cutaneous T-Cell Lymphoma. J. Am. Acad. Dermatol. 2010, 62, 995–1004. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological Decision-Making: How Does the Immune System Decide to Mount a Helper T-Cell Response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef]
- Prokopi, A.; Tripp, C.; Tummers, B.; Hornsteiner, F.; Spoeck, S.; Crawford, J.C.; Clements, D.; Efremova, M.; Hutter, K.; Bellmann, L.; et al. Skin Dendritic Cells in Melanoma Are Key for Successful Checkpoint. Blockade Therapy. J. Immunother. Cancer 2021, 9, e000832. [Google Scholar] [CrossRef]
- Valladeau, J.; Saeland, S. Cutaneous Dendritic Cells. Semin. Immunol. 2005, 17, 273–283. [Google Scholar] [CrossRef]
- Siegal, F.P. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Jordan, K.R.; Amaria, R.N.; Ramirez, O.; Callihan, E.B.; Gao, D.; Borakove, M.; Manthey, E.; Borges, V.F.; McCarter, M.D. Myeloid-Derived Suppressor Cells Are Associated with Disease Progression and Decreased Overall Survival in Advanced-Stage Melanoma Patients. Cancer Immunol. Immunother. 2013, 62, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, K.; Liu, Y.; Lin, Y.; Zhang, X.; Zhou, J.; Zhang, H.; Pan, T.; Fu, Y. Finasteride Enhances the Generation of Human Myeloid-Derived Suppressor Cells by Up-Regulating the COX2/PGE2 Pathway. PLoS ONE 2016, 11, e0156549. [Google Scholar] [CrossRef] [PubMed]
- Lüftl, M.; Feng, A.; Licha, E.; Schuler, G. Dendritic Cells and Apoptosis in Mycosis Fungoides. Br. J. Dermatol. 2002, 147, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Der-Petrossian, M.; Valencak, J.; Jonak, C.; Klosner, G.; Dani, T.; Müllauer, L.; Pehamberger, H.; Knobler, R.; Trautinger, F. Dermal Infiltrates of Cutaneous T-Cell Lymphomas with Epidermotropism but Not Other Cutaneous Lymphomas Are Abundant with Langerin+ Dendritic Cells. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 922–927. [Google Scholar] [CrossRef]
- Schwingshackl, P.; Obermoser, G.; Nguyen, A.; Fritsch, P.; Sepp, N.; Romani, N. Distribution and Maturation of Skin Dendritic Cell Subsets in Two Forms of Cutaneous T-Cell Lymphoma: Mycosis Fungoides and Sézary Syndrome. Acta Derm.-Venereol. 2012, 92, 269–275. [Google Scholar] [CrossRef]
- Pileri, A.; Agostinelli, C.; Sessa, M.; Quaglino, P.; Santucci, M.; Tomasini, C.; Grandi, V.; Fava, P.; Astrua, C.; Righi, S.; et al. Langerhans, Plasmacytoid Dendritic and Myeloid-Derived Suppressor Cell Levels in Mycosis Fungoides Vary According to the Stage of the Disease. Virchows Arch. 2017, 470, 575–582. [Google Scholar] [CrossRef]
- Goos, M.; Kaiserling, E.; Lennert, K. Mycosis Fungoides: Model for T-Lymphocyte Homing to the Skin? Br. J. Dermatol. 1976, 94, 221–222. [Google Scholar] [CrossRef]
- Pimpinelli, N.; Santucci, M.; Romagnoli, P.; Giannotti, B. Dendritic Cells in T- and B-Cell Proliferation in the Skin. Dermatol. Clin. 1994, 12, 255–270. [Google Scholar] [CrossRef]
- Nestle, F.O.; Turka, L.A.; Nickoloff, B.J. Characterization of Dermal Dendritic Cells in Psoriasis. Autostimulation of T Lymphocytes and Induction of Th1 Type Cytokines. J. Clin. Investig. 1994, 94, 202–209. [Google Scholar] [CrossRef]
- Geskin, L.J.; Akilov, O.E.; Kwon, S.; Schowalter, M.; Watkins, S.; Whiteside, T.L.; Butterfield, L.H.; Falo, L.D. Therapeutic Reduction of Cell-Mediated Immunosuppression in Mycosis Fungoides and Sézary Syndrome. Cancer Immunol. Immunother. 2018, 67, 423–434. [Google Scholar] [CrossRef]
- Argyropoulos, K.V.; Pulitzer, M.; Perez, S.; Korkolopoulou, P.; Angelopoulou, M.; Baxevanis, C.; Palomba, M.L.; Siakantaris, M. Tumor-Infiltrating and Circulating Granulocytic Myeloid-Derived Suppressor Cells Correlate with Disease Activity and Adverse Clinical Outcomes in Mycosis Fungoides. Clin. Transl. Oncol. 2020, 22, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B Cell Regulation in Cancer and Anti-Tumor Immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Yanaba, K.; Tedder, T.F. B Cells Are Required for Optimal CD4+ and CD8+ T Cell Tumor Immunity: Therapeutic B Cell Depletion Enhances B16 Melanoma Growth in Mice. J. Immunol. 2010, 184, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Chavez-Rueda, K.A.; Evans, J.G.; Shlomchik, M.J.; Eddaoudi, A.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. Selective Targeting of B Cells with Agonistic Anti-CD40 Is an Efficacious Strategy for the Generation of Induced Regulatory T2-like B Cells and for the Suppression of Lupus in MRL/Lpr Mice. J. Immunol. 2009, 182, 3492–3502. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Noreña, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(Hi)CD38(Hi) B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef]
- Khan, A.R.; Hams, E.; Floudas, A.; Sparwasser, T.; Weaver, C.T.; Fallon, P.G. PD-L1hi B Cells Are Critical Regulators of Humoral Immunity. Nat. Commun. 2015, 6, 5997. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.-D.; Haas, K.M.; Poe, J.C.; Fujimoto, M.; Tedder, T.F. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity 2008, 28, 639–650. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.-D.; Matsushita, T.; Tsubata, T.; Tedder, T.F. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 Cells) Requires. Antigen Receptor Diversity and TLR Signals. J. Immunol. 2009, 182, 7459–7472. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Miyagaki, T.; DiLillo, D.J.; Matsushita, T.; Horikawa, M.; Kountikov, E.I.; Spolski, R.; Poe, J.C.; Leonard, W.J.; Tedder, T.F. Regulatory B Cells Control T-Cell Autoimmunity through IL-21-Dependent Cognate Interactions. Nature 2012, 491, 264–268. [Google Scholar] [CrossRef]
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive Plasma Cells Impede T-Cell-Dependent Immunogenic Chemotherapy. Nature 2015, 521, 94–98. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ Tumor-Infiltrating Lymphocytes Have an Atypical CD27- Memory Phenotype and Together with CD8+ T Cells Promote Favorable Prognosis in Ovarian Cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Elstrand, M.B.; Holth, A.; Silins, I.; Berner, A.; Trope, C.G.; Davidson, B.; Risberg, B. NK- and B-Cell Infiltration Correlates with Worse Outcome in Metastatic Ovarian Carcinoma. Am. J. Clin. Pathol. 2006, 125, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic Impact of Tumor-Associated B Cells and Plasma Cells in Epithelial Ovarian Cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, T.; Miyagaki, T.; Nakajima, R.; Kamijo, H.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; et al. Decreased IL-10-Producing Regulatory B Cells in Patients with Advanced Mycosis Fungoides. Eur. J. Dermatol. 2018, 28, 314–319. [Google Scholar] [CrossRef]
- Nikolaou, V.; Iliakis, T.; Marinos, L.; Voudouri, D.; Sidiropoulou, P.; Rigopoulos, D.; Stratigos, A.J. Another Window into Tumor Microenvironment: A Case of Β-Cell Rich Folliculotropic Mycosis Fungoides Responding to Rituximab. Australas. J. Dermatol. 2020, 61, e226–e228. [Google Scholar] [CrossRef]
- Tschetter, A.J.; Zafar, F.; Moye, M.S.; Ghahramani, G.K.; Swick, B.L.; Link, B.K.; Liu, V. CD20+ Cutaneous T-Cell Lymphoma with Phenotypic Shift after Treatment with Rituximab: Case Report and Review of the Literature. JAAD Case Rep. 2020, 6, 308–310. [Google Scholar] [CrossRef]
- Zou, W. Regulatory T Cells, Tumor Immunity and Immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef]
- Park, Y.-H.; Koo, S.-K.; Kim, Y.; Kim, H.-M.; Joe, I.-Y.; Park, C.-S.; Kim, S.-C.; Han, D.-J.; Lim, D.-G. Effect of in Vitroexpanded CD4(+)CD25(+)Foxp3(+) Regulatory T Cell Therapy Combined with Lymphodepletion in Murine Skin Allotransplantation. Clin. Immunol. 2010, 135, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.L.; Tigelaar, R.; Cohen, J.; Mariwalla, K.; Trinh, J.; Wang, N.; Edelson, R.L. Cutaneous T-Cell Lymphoma: Malignant Proliferation of T-Regulatory Cells. Blood 2005, 105, 1640–1647. [Google Scholar] [CrossRef]
- Hallermann, C.; Niermann, C.; Schulze, H.-J. Regulatory T-Cell Phenotype in Association with Large Cell Transformation of Mycosis Fungoides. Eur. J. Haematol. 2007, 78, 260–263. [Google Scholar] [CrossRef]
- Klemke, C.-D.; Fritzsching, B.; Franz, B.; Kleinmann, E.V.; Oberle, N.; Poenitz, N.; Sykora, J.; Banham, A.H.; Roncador, G.; Kuhn, A.; et al. Paucity of FOXP3+ Cells in Skin and Peripheral Blood Distinguishes Sézary Syndrome from Other Cutaneous T-Cell Lymphomas. Leukemia 2006, 20, 1123–1129. [Google Scholar] [CrossRef]
- Tiemessen, M.M.; Mitchell, T.J.; Hendry, L.; Whittaker, S.J.; Taams, L.S.; John, S. Lack of Suppressive CD4+CD25+FOXP3+ T Cells in Advanced Stages of Primary Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 2006, 126, 2217–2223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gjerdrum, L.M.; Woetmann, A.; Odum, N.; Burton, C.M.; Rossen, K.; Skovgaard, G.L.; Ryder, L.P.; Ralfkiaer, E. FOXP3+ Regulatory T Cells in Cutaneous T-Cell Lymphomas: Association with Disease Stage and Survival. Leukemia 2007, 21, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, E.; Vonderheid, E.C.; Thoburn, C.J.; Wasik, M.A.; Bahler, D.W.; Hess, A.D. Expression of T-Plastin, FoxP3 and Other Tumor-Associated Markers by Leukemic T-Cells of Cutaneous T-Cell Lymphoma. Leuk. Lymphoma 2008, 49, 1190–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solomon, G.J.; Magro, C.M. Foxp3 Expression in Cutaneous T-Cell Lymphocytic Infiltrates. J. Cutan. Pathol. 2008, 35, 1032–1039. [Google Scholar] [CrossRef]
- Wada, D.A.; Wilcox, R.A.; Weenig, R.H.; Gibson, L.E. Paucity of Intraepidermal FoxP3-Positive T Cells in Cutaneous T-Cell Lymphoma in Contrast with Spongiotic and Lichenoid Dermatitis. J. Cutan. Pathol. 2010, 37, 535–541. [Google Scholar] [CrossRef]
- Alcántara-Hernández, M.; Torres-Zárate, C.; Pérez-Montesinos, G.; Jurado-Santacruz, F.; Domínguez-Gómez, M.A.; Peniche-Castellanos, A.; Ferat-Osorio, E.; Neri, N.; Nambo, M.J.; Alvarado-Cabrero, I.; et al. Overexpression of Hypoxia-Inducible Factor 1 Alpha Impacts FoxP3 Levels in Mycosis Fungoides—Cutaneous T-Cell Lymphoma: Clinical Implications. Int. J. Cancer 2014, 134, 2136–2145. [Google Scholar] [CrossRef]
- Fried, I.; Cerroni, L. FOXP3 in Sequential Biopsies of Progressive Mycosis Fungoides. Am. J. Dermatol. 2012, 34, 263–265. [Google Scholar] [CrossRef]
- Shareef, M.M.; Elgarhy, L.H.; Wasfy, R.E.-S. Expression of Granulysin and FOXP3 in Cutaneous T Cell Lymphoma and Sézary Syndrome. Asian Pac. J. Cancer Prev. 2015, 16, 5359–5364. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Vonderheid, E.C.; Hess, A.D.; Eischen, C.M.; McGirt, L.Y. Genetic Markers Associated with Progression in Early Mycosis Fungoides. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-A.; Chen, Z.-Q.; Chen, M.-H.; Xu, Z.-D. The Number of Regular T Cells and Immature Dendritic Cells Involved in Mycosis Fungoides Is Linked to the Tumor Stage. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 553–558. [Google Scholar]
- Querfeld, C.; Rosen, S.T.; Guitart, J.; Duvic, M.; Kim, Y.H.; Dusza, S.W.; Kuzel, T.M. Results of an Open-Label Multicenter Phase 2 Trial of Lenalidomide Monotherapy in Refractory Mycosis Fungoides and Sézary Syndrome. Blood 2014, 123, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Jorgensen, J.L.; Goswami, M.; Challagundla, P.; Decker, W.K.; Kim, Y.H.; Duvic, M.A. Reduction of Regulatory T Cells by Mogamulizumab, a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients with Aggressive/Refractory Mycosis Fungoides and Sézary Syndrome. Clin. Cancer Res. 2015, 21, 274–285. [Google Scholar] [CrossRef]
- Shiue, L.H.; Couturier, J.; Lewis, D.E.; Wei, C.; Ni, X.; Duvic, M. The Effect of Extracorporeal Photopheresis Alone or in Combination Therapy on Circulating CD4(+) Foxp3(+) CD25(-) T Cells in Patients with Leukemic Cutaneous T-Cell Lymphoma. Photodermatol. Photoimmunol. Photomed. 2015, 31, 184–194. [Google Scholar] [CrossRef]
- Mantovani, A.; Bottazzi, B.; Colotta, F.; Sozzani, S.; Ruco, L. The Origin and Function of Tumor-Associated Macrophages. Immunol. Today 1992, 13, 265–270. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Hao, N.-B.; Lü, M.-H.; Fan, Y.-H.; Cao, Y.-L.; Zhang, Z.-R.; Yang, S.-M. Macrophages in Tumor Microenvironments and the Progression of Tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef]
- Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Suga, H.; Kai, H.; Kamata, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Association of the Numbers of CD163+ Cells in Lesional Skin and Serum Levels of Soluble CD163 with Disease Progression of Cutaneous T Cell Lymphoma. J. Dermatol. Sci. 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Wu, X.; Schulte, B.C.; Zhou, Y.; Haribhai, D.; Mackinnon, A.C.; Plaza, J.A.; Williams, C.B.; Hwang, S.T. Depletion of M2-Like Tumor-Associated Macrophages Delays Cutaneous T-Cell Lymphoma Development In Vivo. J. Investig. Dermatol. 2014, 134, 2814–2822. [Google Scholar] [CrossRef]
- Tada, K.; Hamada, T.; Asagoe, K.; Umemura, H.; Mizuno-Ikeda, K.; Aoyama, Y.; Otsuka, M.; Yamasaki, O.; Iwatsuki, K. Increase of DC-LAMP+ Mature Dendritic Cell Subsets in Dermatopathic Lymphadenitis of Mycosis Fungoides. Eur. J. Dermatol. 2014, 24, 670–675. [Google Scholar] [CrossRef]
- Günther, C.; Zimmermann, N.; Berndt, N.; Großer, M.; Stein, A.; Koch, A.; Meurer, M. Up-Regulation of the Chemokine CCL18 by Macrophages Is a Potential Immunomodulatory Pathway in Cutaneous T-Cell Lymphoma. Am. J. Pathol 2011, 179, 1434–1442. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Suga, H.; Ohmatsu, H.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. Increased CCL18 Expression in Patients with Cutaneous T-Cell Lymphoma: Association with Disease Severity and Prognosis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e60–e67. [Google Scholar] [CrossRef]
- Furudate, S.; Fujimura, T.; Kakizaki, A.; Kambayashi, Y.; Asano, M.; Watabe, A.; Aiba, S. The Possible Interaction between Periostin Expressed by Cancer Stroma and Tumor-Associated Macrophages in Developing Mycosis Fungoides. Exp. Dermatol. 2016, 25, 107–112. [Google Scholar] [CrossRef]
- Ando, T.; Xiao, W.; Gao, P.; Namiranian, S.; Matsumoto, K.; Tomimori, Y.; Hong, H.; Yamashita, H.; Kimura, M.; Kashiwakura, J.; et al. Critical Role for Mast Cell Stat5 Activity in Skin Inflammation. Cell Rep. 2014, 6, 366–376. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin Secreted by Glioblastoma Stem Cells Recruits M2 Tumor-Associated Macrophages and Promotes Malignant Growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin Immune Sentinels in Health and Disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- Takahashi, N.; Sugaya, M.; Suga, H.; Oka, T.; Kawaguchi, M.; Miyagaki, T.; Fujita, H.; Sato, S. Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-Cell Lymphoma. Cancer Res. 2016, 76, 6241–6252. [Google Scholar] [CrossRef]
- Tuzova, M.; Richmond, J.; Wolpowitz, D.; Curiel-Lewandrowski, C.; Chaney, K.; Kupper, T.; Cruikshank, W. CCR4+T Cell Recruitment to the Skin in Mycosis Fungoides: Potential Contributions by Thymic Stromal Lymphopoietin and Interleukin-16. Leuk. Lymphoma 2015, 56, 440–449. [Google Scholar] [CrossRef]
- Litvinov, I.V.; Cordeiro, B.; Fredholm, S.; Ødum, N.; Zargham, H.; Huang, Y.; Zhou, Y.; Pehr, K.; Kupper, T.S.; Woetmann, A.; et al. Analysis of STAT4 Expression in Cutaneous T-Cell Lymphoma (CTCL) Patients and Patient-Derived Cell Lines. Cell Cycle 2014, 13, 2975–2982. [Google Scholar] [CrossRef]
- Fredholm, S.; Willerslev-Olsen, A.; Met, Ö.; Kubat, L.; Gluud, M.; Mathiasen, S.L.; Friese, C.; Blümel, E.; Petersen, D.L.; Hu, T.; et al. SATB1 in Malignant T Cells. J. Investig. Dermatol. 2018, 138, 1805–1815. [Google Scholar] [CrossRef]
- Herrera, M.; Mezheyeuski, A.; Villabona, L.; Corvigno, S.; Strell, C.; Klein, C.; Hölzlwimmer, G.; Glimelius, B.; Masucci, G.; Sjöblom, T.; et al. Prognostic Interactions between FAP+ Fibroblasts and CD8a+ T Cells in Colon Cancer. Cancers 2020, 12, 3238. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Zhuang, S.; Liu, N. Recent Advances on Uric Acid Transporters. Oncotarget 2017, 8, 100852–100862. [Google Scholar] [CrossRef]
- Nakajima, R.; Miyagaki, T.; Hirakawa, M.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Fujita, H.; Asano, Y.; Sugaya, M.; et al. Interleukin-25 Is Involved in Cutaneous T-Cell Lymphoma Progression by Establishing a T Helper 2-Dominant Microenvironment. Br. J. Dermatol. 2018, 178, 1373–1382. [Google Scholar] [CrossRef]
- Geskin, L.J.; Viragova, S.; Stolz, D.B.; Fuschiotti, P. Interleukin-13 Is Overexpressed in Cutaneous T-Cell Lymphoma Cells and Regulates Their Proliferation. Blood 2015, 125, 2798–2805. [Google Scholar] [CrossRef]
- Vacca, A.; Moretti, S.; Ribatti, D.; Pellegrino, A.; Pimpinelli, N.; Bianchi, B.; Bonifazi, E.; Ria, R.; Serio, G.; Dammacco, F. Progression of Mycosis Fungoides Is Associated with Changes in Angiogenesis and Expression of the Matrix Metalloproteinases 2 and 9. Eur. J. Cancer 1997, 33, 1685–1692. [Google Scholar] [CrossRef]
- Rasheed, H.; Tolba Fawzi, M.M.; Abdel-Halim, M.R.E.; Eissa, A.M.; Mohammed Salem, N.; Mahfouz, S. Immunohistochemical Study of the Expression of Matrix Metalloproteinase-9 in Skin Lesions of Mycosis Fungoides. Am. J. Dermatol. 2010, 32, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mazur, G.; Woźniak, Z.; Wróbel, T.; Maj, J.; Kuliczkowski, K. Increased Angiogenesis in Cutaneous T-Cell Lymphomas. Pathol. Oncol. Res. 2004, 10, 34–36. [Google Scholar] [CrossRef]
- Pileri, A.; Agostinelli, C.; Righi, S.; Fuligni, F.; Bacci, F.; Sabattini, E.; Patrizi, A.; Pileri, S.A.; Piccaluga, P.P. Vascular Endothelial Growth Factor A (VEGFA) Expression in Mycosis Fungoides. Histopathology 2015, 66, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Karpova, M.B.; Fujii, K.; Jenni, D.; Dummer, R.; Urosevic-Maiwald, M. Evaluation of Lymphangiogenic Markers in Sézary Syndrome. Leuk. Lymphoma 2011, 52, 491–501. [Google Scholar] [CrossRef]
- Jankowska-Konsur, A.; Kobierzycki, C.; Grzegrzolka, J.; Piotrowska, A.; Gomulkiewicz, A.; Glatzel-Plucinska, N.; Olbromski, M.; Podhorska-Okolow, M.; Szepietowski, J.C.; Dziegiel, P. Expression of CD31 in Mycosis Fungoides. Anticancer Res. 2016, 36, 4575–4582. [Google Scholar] [CrossRef]
- Jankowska-Konsur, A.; Kobierzycki, C.; Reich, A.; Piotrowska, A.; Gomulkiewicz, A.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P.; Szepietowski, J.C. Expression of SOX18 in Mycosis Fungoides. Acta Derm.-Venereol. 2017, 97, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Roufail, S.; Inder, R.; Caesar, C.; Karnezis, T.; Shayan, R.; Farnsworth, R.H.; Sato, T.; Achen, M.G.; Mann, G.B.; et al. Signaling for Lymph angiogenesis via VEGFR-3 Is Required for the Early Events of Metastasis. Clin. Exp. Metastasis 2013, 30, 819–832. [Google Scholar] [CrossRef]
- El-Ashmawy, A.A.; Shamloula, M.M.; Elfar, N.N. Podoplanin as a Predictive Marker for Identification of High-Risk Mycosis Fungoides Patients: An Immunohistochemical Study. Indian J. Dermatol. 2020, 65, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Lauenborg, B.; Christensen, L.; Ralfkiaer, U.; Kopp, K.L.; Jønson, L.; Dabelsteen, S.; Bonefeld, C.M.; Geisler, C.; Gjerdrum, L.M.R.; Zhang, Q.; et al. Malignant T Cells Express Lymphotoxin α and Drive Endothelial Activation in Cutaneous T Cell Lymphoma. Oncotarget 2015, 6, 15235–15249. [Google Scholar] [CrossRef]
- Murray, D.; McMurray, J.L.; Eldershaw, S.; Pearce, H.; Davies, N.; Scarisbrick, J.J.; Moss, P. Progression of Mycosis Fungoides Occurs through Divergence of Tumor Immunophenotype by Differential Expression of HLA-DR. Blood Adv. 2019, 3, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Vowels, B.R.; Lessin, S.R.; Cassin, M.; Jaworsky, C.; Benoit, B.; Wolfe, J.T.; Rook, A.H. Th2 Cytokine MRNA Expression in Skin in Cutaneous T-Cell Lymphoma. J. Investig. Dermatol. 1994, 103, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, R.T.; Medeiros, L.J.; Warnke, R.A.; Wood, G.S. CD8-Positive Tumor-Infiltrating Lymphocytes Influence the Long-Term Survival of Patients with Mycosis Fungoides. J. Am. Acad. Dermatol. 1995, 32, 448–453. [Google Scholar] [CrossRef]
- Vermeer, M.H.; van Doorn, R.; Dukers, D.; Bekkenk, M.W.; Meijer, C.J.; Willemze, R. CD8+ T Cells in Cutaneous T-Cell Lymphoma: Expression of Cytotoxic Proteins, Fas Ligand, and Killing Inhibitory Receptors and Their Relationship with Clinical Behavior. J. Clin. Oncol. 2001, 19, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Hahtola, S.; Tuomela, S.; Elo, L.; Häkkinen, T.; Karenko, L.; Nedoszytko, B.; Heikkilä, H.; Saarialho-Kere, U.; Roszkiewicz, J.; Aittokallio, T.; et al. Th1 Response and Cytotoxicity Genes Are Down-Regulated in Cutaneous T-Cell Lymphoma. Clin. Cancer Res. 2006, 12, 4812–4821. [Google Scholar] [CrossRef]
- Hsi, A.C.; Lee, S.J.; Rosman, I.S.; Carson, K.R.; Kelley, A.; Viele, V.; Pang, X.; Musiek, A.; Schaffer, A. Expression of Helper T Cell Master Regulators in Inflammatory Dermatoses and Primary Cutaneous T-Cell Lymphomas: Diagnostic Implications. J. Am. Acad. Dermatol. 2015, 72, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, D.; Vivarelli, A.G.; Gonçalves, F.; Cury-Martins, J.; Sanches, J.A. Progression of Mycosis Fungoides after Treatment with Dupilumab: A Case Report. Dermatol. Ther. 2020, 33, e13880. [Google Scholar] [CrossRef]
- Russomanno, K.; Carver DeKlotz, C.M. Acceleration of Cutaneous T-Cell Lymphoma Following Dupilumab Administration. JAAD Case Rep. 2021, 8, 83–85. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Zeng, Z.; Wei, F.; Ren, X. Exhausted T Cells and Epigenetic Status. Cancer Biol. Med. 2020, 17, 923–936. [Google Scholar] [CrossRef]
- Scott, A.C.; Dündar, F.; Zumbo, P.; Chandran, S.S.; Klebanoff, C.A.; Shakiba, M.; Trivedi, P.; Menocal, L.; Appleby, H.; Camara, S.; et al. TOX Is a Critical Regulator of Tumor-Specific T Cell Differentiation. Nature 2019, 571, 270–274. [Google Scholar] [CrossRef]
- Khan, O.; Giles, J.R.; McDonald, S.; Manne, S.; Ngiow, S.F.; Patel, K.P.; Werner, M.T.; Huang, A.C.; Alexander, K.A.; Wu, J.E.; et al. TOX Transcriptionally and Epigenetically Programs CD8+ T Cell Exhaustion. Nature 2019, 571, 211–218. [Google Scholar] [CrossRef]
- Choi, J.; Goh, G.; Walradt, T.; Hong, B.S.; Bunick, C.G.; Chen, K.; Bjornson, R.D.; Maman, Y.; Wang, T.; Tordoff, J.; et al. Genomic Landscape of Cutaneous T Cell Lymphoma. Nat. Genet. 2015, 47, 1011–1019. [Google Scholar] [CrossRef]
- Da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The Mutational Landscape of Cutaneous T Cell Lymphoma and Sézary Syndrome. Nat. Genet. 2015, 47, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- McGirt, L.Y.; Degesys, C.A.; Johnson, V.E.; Zic, J.A.; Zwerner, J.P.; Eischen, C.M. TOX Expression and Role in CTCL. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Ungewickell, A.; Bhaduri, A.; Rios, E.; Reuter, J.; Lee, C.S.; Mah, A.; Zehnder, A.; Ohgami, R.; Kulkarni, S.; Armstrong, R.; et al. Genomic Analysis of Mycosis Fungoides and Sézary Syndrome Identifies Recurrent Alterations in TNFR2. Nat. Genet. 2015, 47, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ni, X.; Covington, K.R.; Yang, B.Y.; Shiu, J.; Zhang, X.; Xi, L.; Meng, Q.; Langridge, T.; Drummond, J.; et al. Genomic Profiling of Sézary Syndrome Identifies Alterations of Key T Cell Signaling and Differentiation Genes. Nat. Genet. 2015, 47, 1426–1434. [Google Scholar] [CrossRef]
- Woollard, W.J.; Pullabhatla, V.; Lorenc, A.; Patel, V.M.; Butler, R.M.; Bayega, A.; Begum, N.; Bakr, F.; Dedhia, K.; Fisher, J.; et al. Candidate Driver Genes Involved in Genome Maintenance and DNA Repair in Sézary Syndrome. Blood 2016, 127, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Wong, G.K.-S.; Gniadecki, R. Branched Evolution and Genomic Intratumor Heterogeneity in the Pathogenesis of Cutaneous T-Cell Lymphoma. Blood Adv. 2020, 4, 2489–2500. [Google Scholar] [CrossRef]
- Dulmage, B.O.; Akilov, O.; Vu, J.R.; Falo, L.D.; Geskin, L.J. Dysregulation of the TOX-RUNX3 Pathway in Cutaneous T-Cell Lymphoma. Oncotarget 2019, 10, 3104–3113. [Google Scholar] [CrossRef]
- Moerman-Herzog, A.M.; Acheampong, D.A.; Brooks, A.G.; Blair, S.M.; Hsu, P.-C.; Wong, H.K. Transcriptome Analysis of Sézary Syndrome and Lymphocytic-Variant Hypereosinophilic Syndrome T Cells Reveals Common and Divergent Genes. Oncotarget 2019, 10, 5052–5069. [Google Scholar] [CrossRef] [PubMed]
- Lefrançois, P.; Xie, P.; Wang, L.; Tetzlaff, M.T.; Moreau, L.; Watters, A.K.; Netchiporouk, E.; Provost, N.; Gilbert, M.; Ni, X.; et al. Gene Expression Profiling and Immune Cell-Type Deconvolution Highlight Robust Disease Progression and Survival Markers in Multiple Cohorts of CTCL Patients. Oncoimmunology 2018, 7, e1467856. [Google Scholar] [CrossRef]
- Schrader, A.M.R.; Jansen, P.M.; Willemze, R. TOX Expression in Cutaneous T-Cell Lymphomas: An Adjunctive Diagnostic Marker That Is Not Tumor Specific and Not Restricted to the CD4(+) CD8(-) Phenotype. Br. J. Dermatol. 2016, 175, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Fehlings, M.; Kløverpris, H.N.; McGovern, N.; Koo, S.-L.; Loh, C.Y.; Lim, S.; Kurioka, A.; Fergusson, J.R.; Tang, C.-L.; et al. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity 2018, 48, 1060. [Google Scholar] [CrossRef]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a Subset of Human Natural Killer Cells Expressing High Levels of Programmed Death 1: A Phenotypic and Functional Characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346.e3. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 Mediates Functional Exhaustion of Activated NK Cells in Patients with Kaposi Sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef]
- Vari, F.; Arpon, D.; Keane, C.; Hertzberg, M.S.; Talaulikar, D.; Jain, S.; Cui, Q.; Han, E.; Tobin, J.; Bird, R.; et al. Immune Evasion via PD-1/PD-L1 on NK Cells and Monocyte/Macrophages Is More Prominent in Hodgkin Lymphoma than DLBCL. Blood 2018, 131, 1809–1819. [Google Scholar] [CrossRef]
- Tumino, N.; Martini, S.; Munari, E.; Scordamaglia, F.; Besi, F.; Mariotti, F.R.; Bogina, G.; Mingari, M.C.; Vacca, P.; Moretta, L. Presence of Innate Lymphoid Cells in Pleural Effusions of Primary and Metastatic Tumors: Functional Analysis and Expression of PD-1 Receptor. Int. J. Cancer 2019, 145, 1660–1668. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA Class-I Molecules in Human Natural Killer Cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef]
- Godal, R.; Bachanova, V.; Gleason, M.; McCullar, V.; Yun, G.H.; Cooley, S.; Verneris, M.R.; McGlave, P.B.; Miller, J.S. Natural Killer Cell Killing of Acute Myelogenous Leukemia and Acute Lymphoblastic Leukemia Blasts by Killer Cell Immunoglobulin-like Receptor-Negative Natural Killer Cells after NKG2A and LIR-1 Blockade. Biol. Blood Marrow Transpl. 2010, 16, 612–621. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Söderström, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E Binds to Natural Killer Cell Receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- De Kruijf, E.M.; Sajet, A.; van Nes, J.G.H.; Natanov, R.; Putter, H.; Smit, V.T.H.B.M.; Liefers, G.J.; van den Elsen, P.J.; van de Velde, C.J.H.; Kuppen, P.J.K. HLA-E and HLA-G Expression in Classical HLA Class I-Negative Tumors Is of Prognostic Value for Clinical Outcome of Early Breast Cancer Patients. J. Immunol. 2010, 185, 7452–7459. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A Expression Contributes to NK Cell Exhaustion and Predicts a Poor Prognosis of Patients with Liver Cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef]
- Seliger, B.; Jasinski-Bergner, S.; Quandt, D.; Stoehr, C.; Bukur, J.; Wach, S.; Legal, W.; Taubert, H.; Wullich, B.; Hartmann, A. HLA-E Expression and Its Clinical Relevance in Human Renal Cell Carcinoma. Oncotarget 2016, 7, 67360–67372. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E Expression by Gynecological Cancers Restrains Tumor-Infiltrating CD8+ T Lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.M.; Bianchini, M.; Von Euw, E.M.; Barrio, M.M.; Bravo, A.I.; Furman, D.; Domenichini, E.; Macagno, C.; Pinsky, V.; Zucchini, C.; et al. Human Leukocyte Antigen-E Protein Is Overexpressed in Primary Human Colorectal Cancer. Int. J. Oncol. 2008, 32, 633–641. [Google Scholar] [CrossRef]
- Spaans, V.M.; Peters, A.A.W.; Fleuren, G.J.; Jordanova, E.S. HLA-E Expression in Cervical Adenocarcinomas: Association with Improved Long-Term Survival. J. Transl. Med. 2012, 10, 184. [Google Scholar] [CrossRef]
- Sako, N.; Schiavon, V.; Bounfour, T.; Dessirier, V.; Ortonne, N.; Olive, D.; Ram-Wolff, C.; Michel, L.; Sicard, H.; Marie-Cardine, A.; et al. Membrane Expression of NK Receptors CD160 and CD158k Contributes to Delineate a Unique CD4+ T-Lymphocyte Subset in Normal and Mycosis Fungoides Skin. Cytom. A 2014, 85, 869–882. [Google Scholar] [CrossRef]
- Mattei, F.; Andreone, S.; Marone, G.; Gambardella, A.R.; Loffredo, S.; Varricchi, G.; Schiavoni, G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 1–28. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils Orchestrate Cancer Rejection by Normalizing Tumor Vessels and Enhancing Infiltration of CD8(+) T Cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Woschnagg, C.; Rubin, J.; Venge, P. Eosinophil Cationic Protein (ECP) Is Processed during Secretion. J. Immunol. 2009, 183, 3949–3954. [Google Scholar] [CrossRef]
- Varricchi, G.; Bagnasco, D.; Borriello, F.; Heffler, E.; Canonica, G.W. Interleukin-5 Pathway Inhibition in the Treatment of Eosinophilic Respiratory Disorders: Evidence and Unmet Needs. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 186–200. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.-B.; Shang, W.-Q.; Chang, K.-K.; Meng, Y.-H.; Mei, J.; Yu, J.-J.; Li, D.-J.; Li, M.-Q. The Infiltration and Functional Regulation of Eosinophils Induced by TSLP Promote the Proliferation of Cervical Cancer Cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Onesti, C.E.; Josse, C.; Boulet, D.; Thiry, J.; Beaumecker, B.; Bours, V.; Jerusalem, G. Blood Eosinophilic Relative Count Is Prognostic for Breast Cancer and Associated with the Presence of Tumor at Diagnosis and at Time of Relapse. Oncoimmunology 2020, 9, 1761176. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, L.; Pollheimer, M.J.; Kornprat, P.; Lindtner, R.A.; Bokemeyer, C.; Langner, C. Peritumoral Eosinophils Predict Recurrence in Colorectal Cancer. Mod. Pathol. 2015, 28, 403–413. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E. HMGB1, IL-1α, IL-33 and S100 Proteins: Dual-Function Alarmins. Cell Mol. Immunol. 2017, 14, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Granata, F.; Frattini, A.; Loffredo, S.; Staiano, R.I.; Petraroli, A.; Ribatti, D.; Oslund, R.; Gelb, M.H.; Lambeau, G.; Marone, G.; et al. Production of Vascular Endothelial Growth Factors from Human Lung Macrophages Induced by Group IIA and Group X Secreted Phospholipases A2. J. Immunol. 2010, 184, 5232–5241. [Google Scholar] [CrossRef]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular Endothelial Growth Factors Synthesized by Human Lung Mast Cells Exert Angiogenic Effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149.e5. [Google Scholar] [CrossRef]
- Schratl, P.; Royer, J.F.; Kostenis, E.; Ulven, T.; Sturm, E.M.; Waldhoer, M.; Hoefler, G.; Schuligoi, R.; Lippe, I.T.; Peskar, B.A.; et al. The Role of the Prostaglandin D2 Receptor, DP, in Eosinophil Trafficking. J. Immunol. 2007, 179, 4792–4799. [Google Scholar] [CrossRef]
- Capelo, R.; Lehmann, C.; Ahmad, K.; Snodgrass, R.; Diehl, O.; Ringleb, J.; Flamand, N.; Weigert, A.; Stark, H.; Steinhilber, D.; et al. Cellular Analysis of the Histamine H4 Receptor in Human Myeloid Cells. Biochem. Pharm. 2016, 103, 74–84. [Google Scholar] [CrossRef]
- Simon, S.C.S.; Utikal, J.; Umansky, V. Opposing Roles of Eosinophils in Cancer. Cancer Immunol. Immunother. 2019, 68, 823–833. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The Unsung Heroes in Cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef]
- Iliadis, A.; Koletsa, T.; Patsatsi, A.; Georgiou, E.; Sotiriadis, D.; Kostopoulos, I. The Cellular Microenvironment and Neoplastic Population in Mycosis Fungoides Skin Lesions: A Clinicopathological Correlation. Eur. J. Dermatol. 2016, 26, 566–571. [Google Scholar] [CrossRef]
- Bahalı, A.G.; Su, O.; Cengiz, F.P.; Emiroğlu, N.; Ozkaya, D.B.; Onsun, N. Prognostic Factors of Patients with Mycosis Fungoides. Postepy Derm. Alergol. 2020, 37, 796–799. [Google Scholar] [CrossRef]
- Kural, Y.B.; Su, O.; Onsun, N.; Uras, A.R. Atopy, IgE and Eosinophilic Cationic Protein Concentration, Specific IgE Positivity, Eosinophil Count in Cutaneous T Cell Lymphoma. Int. J. Dermatol. 2010, 49, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Tancrède-Bohin, E.; Ionescu, M.A.; de La Salmonière, P.; Dupuy, A.; Rivet, J.; Rybojad, M.; Dubertret, L.; Bachelez, H.; Lebbé, C.; Morel, P. Prognostic Value of Blood Eosinophilia in Primary Cutaneous T-Cell Lymphomas. Arch. Dermatol. 2004, 140, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.A.; Rivet, J.; Daneshpouy, M.; Briere, J.; Morel, P.; Janin, A. In Situ Eosinophil Activation in 26 Primary Cutaneous T-Cell Lymphomas with Blood Eosinophilia. J. Am. Acad. Dermatol. 2005, 52, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, S.; Gjerdrum, L.M.R.; Willerslev-Olsen, A.; Petersen, D.L.; Nielsen, I.Ø.; Kauczok, C.-S.; Wobser, M.; Ralfkiaer, U.; Bonefeld, C.M.; Wasik, M.A.; et al. STAT3 Activation and Infiltration of Eosinophil Granulocytes in Mycosis Fungoides. Anticancer Res. 2014, 10, 5277–5286. [Google Scholar]
- Vonderheid, E.C.; Kantor, G.R.; Telang, G.H.; Bujanouskas, P.; Kadin, M.E. A Histo-Immunopathologic and Prognostic Study of Erythrodermic Cutaneous T-Cell Lymphoma. J. Cutan. Pathol. 2019, 46, 913–924. [Google Scholar] [CrossRef]
- Terada, T. Mycosis Fungoides in Plaque Stage with Pronounced Eosinophilic Infiltration, Folliculotropism, and Concomitant Invasive Squamous Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2013, 6, 749–756. [Google Scholar]
- Boone, S.L.; Guitart, J.; Gerami, P. Follicular Mycosis Fungoides: A Histopathologic, Immunohistochemical, and Genotypic Review. G. Ital. Derm. Venereol. 2008, 143, 409–414. [Google Scholar]
- Chang, N.-S. Transforming Growth Factor-Β1 Blocks the Enhancement of Tumor Necrosis Factor Cytotoxicity by Hyaluronidase Hyal-2 in L929 Fibroblasts. BMC Cell Biol. 2002, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Owusu, B.Y.; Vaid, M.; Kaler, P.; Klampfer, L. Prognostic and Predictive Significance of Stromal Fibroblasts and Macrophages in Colon Cancer: Supplementary Issue: Biomarkers for Colon Cancer. Biomark. Cancer 2015, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, P.; Suchocki, S.; GyĂśrffy, B.; Gansukh, T.; Wojnar, A.; Maciejczyk, A.; Pudełko, M.; Zabel, M. Stromal Myofibroblasts in Breast Cancer: Relations between Their Occurrence, Tumor Grade and Expression of Some Tumor Markers. Folia Histochem. Cytobiol. 2006, 44, 111–116. [Google Scholar] [PubMed]
- Cheng, Y.; Wang, K.; Ma, W.; Zhang, X.; Song, Y.; Wang, J.; Wang, N.; Song, Q.; Cao, F.; Tan, B.; et al. Cancer-Associated Fibroblasts Are Associated with Poor Prognosis in Esophageal Squamous Cell Carcinoma after Surgery. Int. J. Clin. Exp. Med. 2015, 8, 1896–1903. [Google Scholar]
- Lohr, M.; Schmidt, C.; Ringel, J.; Kluth, M. Transforming Growth Factor-β1 Induces Desmoplasia in an Experimental Model of Human Pancreatic Carcinoma. Cancer Res. 2001, 61, 550–555. [Google Scholar]
- Aoyagi, Y.; Oda, T.; Kinoshita, T.; Nakahashi, C.; Hasebe, T.; Ohkohchi, N.; Ochiai, A. Overexpression of TGF-β by Infiltrated Granulocytes Correlates with the Expression of Collagen MRNA in Pancreatic Cancer. Br. J. Cancer 2004, 91, 1316–1326. [Google Scholar] [CrossRef]
- Cirillo, N.; Hassona, Y.; Celentano, A.; Lim, K.P.; Manchella, S.; Parkinson, E.K.; Prime, S.S. Cancer-Associated Fibroblasts Regulate Keratinocyte Cell–Cell Adhesion via TGF-β-Dependent Pathways in Genotype-Specific Oral Cancer. Carcinogenesis 2017, 38, 76–85. [Google Scholar] [CrossRef]
- Kim, S.-A.; Lee, E.K.; Kuh, H.-J. Co-Culture of 3D Tumor Spheroids with Fibroblasts as a Model for Epithelial–Mesenchymal Transition in Vitro. Exp. Cell Res. 2015, 335, 187–196. [Google Scholar] [CrossRef]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in Cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.; Liu, B.; DeFilippis, R.A.; Chang, H.; Rabban, J.T.; Karnezis, A.N.; Tjoe, J.A.; Marx, J.; Parvin, B.; Tlsty, T.D. Breast Fibroblasts Modulate Early Dissemination, Tumorigenesis, and Metastasis through Alteration of Extracellular Matrix Characteristics. Neoplasia 2013, 15, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Elenbaas, B.; Weinberg, R.A. Heterotypic Signaling between Epithelial Tumor Cells and Fibroblasts in Carcinoma Formation. Exp. Cell Res. 2001, 264, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Ishii, G.; Ochiai, A.; Neri, S. Phenotypic and Functional Heterogeneity of Cancer-Associated Fibroblast within the Tumor Microenvironment. Adv. Drug Deliv. Rev. 2016, 99, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-KappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Aronovich, A.; Moyal, L.; Gorovitz, B.; Amitay-Laish, I.; Naveh, H.P.; Forer, Y.; Maron, L.; Knaneh, J.; Ad-El, D.; Yaacobi, D.; et al. Cancer-Associated Fibroblasts in Mycosis Fungoides Promote Tumor Cell Migration and Drug Resistance through CXCL12/CXCR4. J. Investig. Dermatol. 2021, 141, 619–627.e2. [Google Scholar] [CrossRef]
- Krejsgaard, T.; Lindahl, L.M.; Mongan, N.P.; Wasik, M.A.; Litvinov, I.V.; Iversen, L.; Langhoff, E.; Woetmann, A.; Odum, N. Malignant Inflammation in Cutaneous T-cell Lymphoma—a Hostile Takeover. Semin. Immunopathol. 2017, 39, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Durgin, J.S.; Weiner, D.M.; Wysocka, M.; Rook, A.H. The Immunopathogenesis and Immunotherapy of Cutaneous T Cell Lymphoma: Pathways and Targets for Immune Restoration and Tumor Eradication. J. Am. Acad. Dermatol. 2021, 84, 587–595. [Google Scholar] [CrossRef]
- Jones, C.L.; Degasperi, A.; Grandi, V.; Amarante, T.D.; Genomics England Research Consortium; Mitchell, T.J.; Nik-Zainal, S.; Whittaker, S.J. Spectrum of Mutational Signatures in T-Cell Lymphoma Reveals a Key Role for UV Radiation in Cutaneous T-Cell Lymphoma. Sci. Rep. 2021, 11, 3962. [Google Scholar] [CrossRef]
- Rojansky, R.; Fernandez-Pol, S.; Wang, E.; Rieger, K.E.; Novoa, R.A.; Zehnder, J.L.; Kunder, C.A.; Kim, Y.H.; Khodadoust, M.S.; Brown, R.A. Cutaneous T-Cell Lymphomas with Pathogenic Somatic Mutations and Absence of Detectable Clonal T-Cell Receptor Gene Rearrangement: Two Case Reports. Diagn. Pathol. 2020, 15, 122. [Google Scholar] [CrossRef]
- Mirza, A.-S.; Horna, P.; Teer, J.K.; Song, J.; Akabari, R.; Hussaini, M.; Sokol, L. New Insights Into the Complex Mutational Landscape of Sézary Syndrome. Front. Oncol. 2020, 10, 514. [Google Scholar] [CrossRef]
- Kim, E.J.; Hess, S.; Richardson, S.K.; Newton, S.; Showe, L.C.; Benoit, B.M.; Ubriani, R.; Vittorio, C.C.; Junkins-Hopkins, J.M.; Wysocka, M.; et al. Immunopathogenesis and Therapy of Cutaneous T Cell Lymphoma. J. Clin. Investig. 2005, 115, 798–812. [Google Scholar] [CrossRef]
- Budgin, J.B.; Richardson, S.K.; Newton, S.B.; Wysocka, M.; Zaki, M.H.; Benoit, B.; Rook, A.H. Biological Effects of Bexarotene in Cutaneous T-Cell Lymphoma. Arch. Dermatol. 2005, 141, 315–321. [Google Scholar] [CrossRef]
- Kim, Y.H.; Gratzinger, D.; Harrison, C.; Brody, J.D.; Czerwinski, D.K.; Ai, W.Z.; Morales, A.; Abdulla, F.; Xing, L.; Navi, D.; et al. In Situ Vaccination against Mycosis Fungoides by Intratumoral Injection of a TLR9 Agonist Combined with Radiation: A Phase 1/2 Study. Blood 2012, 119, 355–363. [Google Scholar] [CrossRef]
- Sivanand, A.; Surmanowicz, P.; Alhusayen, R.; Hull, P.; Litvinov, I.V.; Zhou, Y.; Gniadecki, R. Immunotherapy for Cutaneous T-Cell Lymphoma: Current Landscape and Future Developments. J. Cutan. Med. Surg. 2019, 23, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Tun-Kyi, A.; Tassis, A.; Jungius, K.-P.; Burg, G.; Dummer, R.; Nestle, F.O. Vaccination of Patients with Cutaneous T-Cell Lymphoma Using Intranodal Injection of Autologous Tumor-Lysate-Pulsed Dendritic Cells. Blood 2003, 102, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Künzi, V.; Oberholzer, P.A.; Kündig, T.; Naim, H.; Dummer, R. Oncolytic Measles Virus in Cutaneous T-Cell Lymphomas Mounts Antitumor Immune Responses in Vivo and Targets Interferon-Resistant Tumor Cells. Blood 2005, 106, 2287–2294. [Google Scholar] [CrossRef]
- Zic, J.A. The Treatment of Cutaneous T-Cell Lymphoma with Photopheresis. Dermatol. Ther. 2003, 16, 337–346. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef]
- Khodadoust, M.S.; Rook, A.H.; Porcu, P.; Foss, F.; Moskowitz, A.J.; Shustov, A.; Shanbhag, S.; Sokol, L.; Fling, S.P.; Ramchurren, N.; et al. Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study. J. Clin. Oncol. 2020, 38, 20–28. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Bergman, R. Complete Regression of Mycosis Fungoides after Ipilimumab Therapy for Advanced Melanoma. JAAD Case Rep. 2015, 1, 99–100. [Google Scholar] [CrossRef]
- Alcantara, M.; Tesio, M.; June, C.H.; Houot, R. CAR T-Cells for T-Cell Malignancies: Challenges in Distinguishing between Therapeutic, Normal, and Neoplastic T-Cells. Leukemia 2018, 32, 2307–2315. [Google Scholar] [CrossRef]
- Tanita, K.; Fujimura, T.; Sato, Y.; Lyu, C.; Kambayashi, Y.; Ogata, D.; Fukushima, S.; Miyashita, A.; Nakajima, H.; Nakamura, M.; et al. Bexarotene Reduces Production of CCL22 From Tumor-Associated Macrophages in Cutaneous T-Cell Lymphoma. Front. Oncol. 2019, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Tavallaee, M.; Sundram, U.; Salva, K.A.; Wood, G.S.; Li, S.; Rozati, S.; Nagpal, S.; Krathen, M.; Reddy, S.; et al. Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sézary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project. J. Clin. Oncol. 2015, 33, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Suda, K. Tumor-Associated Macrophages-Additional Effectors at Anti-PD-1/PD-L1 Therapy? J. Thorac. Dis. 2017, 9, 4197–4200. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Therapeutic Potential of Toll-like Receptor 9 Activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef]
- Herrera, A.; Cheng, A.; Mimitou, E.P.; Seffens, A.; George, D.D.; Bar-Natan, M.; Heguy, A.; Ruggles, K.V.; Scher, J.U.; Hymes, K.; et al. Multimodal Single-Cell Analysis of Cutaneous T Cell Lymphoma Reveals Distinct Sub-Clonal Tissue-Dependent Signatures. Blood 2021. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pileri, A.; Guglielmo, A.; Grandi, V.; Violetti, S.A.; Fanoni, D.; Fava, P.; Agostinelli, C.; Berti, E.; Quaglino, P.; Pimpinelli, N. The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications. Cells 2021, 10, 2780. https://doi.org/10.3390/cells10102780
Pileri A, Guglielmo A, Grandi V, Violetti SA, Fanoni D, Fava P, Agostinelli C, Berti E, Quaglino P, Pimpinelli N. The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications. Cells. 2021; 10(10):2780. https://doi.org/10.3390/cells10102780
Chicago/Turabian StylePileri, Alessandro, Alba Guglielmo, Vieri Grandi, Silvia Alberti Violetti, Daniele Fanoni, Paolo Fava, Claudio Agostinelli, Emilio Berti, Pietro Quaglino, and Nicola Pimpinelli. 2021. "The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications" Cells 10, no. 10: 2780. https://doi.org/10.3390/cells10102780
APA StylePileri, A., Guglielmo, A., Grandi, V., Violetti, S. A., Fanoni, D., Fava, P., Agostinelli, C., Berti, E., Quaglino, P., & Pimpinelli, N. (2021). The Microenvironment’s Role in Mycosis Fungoides and Sézary Syndrome: From Progression to Therapeutic Implications. Cells, 10(10), 2780. https://doi.org/10.3390/cells10102780








