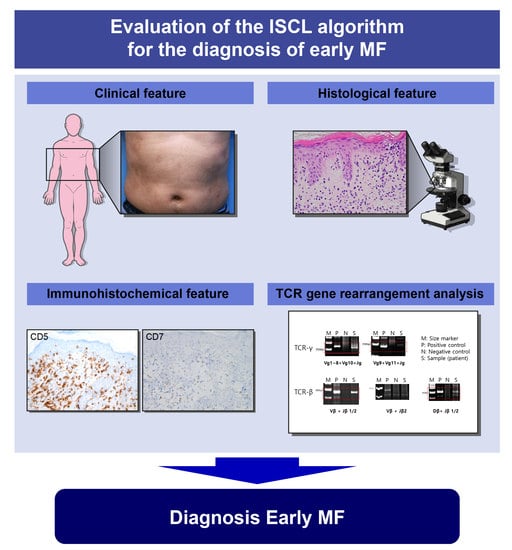
Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. IHC Staining
2.3. DNA Extraction and TCR Gene Rearrangement Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinicopathologic Features of Early MF
3.2. Determinative Immunopathologic Markers of Early MF
3.3. Molecular Features of Early MF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerroni, L.; Sander, C.A.; Smoller, B.R.; Willemze, R. World Health Organization Classification of Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Benner, M.F.; Jansen, P.M.; Vermeer, M.H.; Willemze, R. Prognostic factors in transformed mycosis fungoides: A retrospective analysis of 100 cases. Blood 2012, 119, 1643–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Doorn, R.; van Haselen, C.W.; van Voorst Vader, P.C.; Geerts, M.L.; Heule, F.; de Rie, M.; Steijlen, P.M.; Dekker, S.K.; van Vloten, W.A.; Willemze, R. Mycosis fungoides: Disease evolution and prognosis of 309 Dutch patients. Arch. Dermatol. 2000, 136, 504–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gru, A.A.; Schaffer, A. Hematopathology of the Skin: Clinical & Pathological Approach, 1st ed.; Wolters Kluwer Heath: Philadelphia, PA, USA, 2017. [Google Scholar]
- Pimpinelli, N.; Olsen, E.A.; Santucci, M.; Vonderheid, E.; Haeffner, A.C.; Stevens, S.; Burg, G.; Cerroni, L.; Dreno, B.; Glusac, E.; et al. Defining early mycosis fungoides. J. Am. Acad. Dermatol. 2005, 53, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.; Vonderheid, E.; Pimpinelli, N.; Willemze, R.; Kim, Y.; Knobler, R.; Zackheim, H.; Duvic, M.; Estrach, T.; Lamberg, S.; et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 1713–1722. [Google Scholar] [PubMed] [Green Version]
- Vandergriff, T.; Nezafati, K.A.; Susa, J.; Karai, L.; Sanguinetti, A.; Hynan, L.S.; Ambruzs, J.M.; Oliver, D.H.; Pandya, A.G. Defining early mycosis fungoides: Validation of a diagnostic algorithm proposed by the International Society for Cutaneous Lymphomas. J. Cutan. Pathol. 2015, 42, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Amorim, G.M.; Quintella, D.C.; Niemeyer-Corbellini, J.P.; Ferreira, L.C.; Ramos, E.S.M.; Cuzzi, T. Validation of an algorithm based on clinical, histopathological and immunohistochemical data for the diagnosis of early-stage mycosis fungoides. An. Bras. Dermatol. 2020, 95, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Lindae, M.L.; Abel, E.A.; Hoppe, R.T.; Wood, G.S. Poikilodermatous mycosis fungoides and atrophic large-plaque parapsoriasis exhibit similar abnormalities of T-cell antigen expression. Arch. Dermatol. 1988, 124, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Smoller, B.R.; Santucci, M.; Wood, G.S.; Whittaker, S.J. Histopathology and genetics of cutaneous T-cell lymphoma. Hematol. Oncol. Clin. N. Am. 2003, 17, 1277–1311. [Google Scholar] [CrossRef]
- Wood, G.S.; Hong, S.R.; Sasaki, D.T.; Abel, E.A.; Hoppe, R.T.; Warnke, R.A.; Morhenn, V.B. Leu-8/CD7 antigen expression by CD3+ T cells: Comparative analysis of skin and blood in mycosis fungoides/Sezary syndrome relative to normal blood values. J. Am. Acad. Dermatol. 1990, 22, 602–607. [Google Scholar] [CrossRef]
- Michie, S.A.; Abel, E.A.; Hoppe, R.T.; Warnke, R.A.; Wood, G.S. Discordant expression of antigens between intraepidermal and intradermal T cells in mycosis fungoides. Am. J. Pathol. 1990, 137, 1447–1451. [Google Scholar] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Muller, M.P.; Tomlinson, G.; Marrie, T.J.; Tang, P.; McGeer, A.; Low, D.E.; Detsky, A.S.; Gold, W.L. Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community-acquired pneumonia? Clin. Infect. Dis. 2005, 40, 1079–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, S.R.; Ke, M.S.; Birol, A.; Terhune, M.H.; Parry, E.J.; Ross, C.; Mostow, E.N.; Gilliam, A.C.; Cooper, K.D.; Interdisciplinary Cutaneous Lymphoma Program. A simple clinical scoring system to improve the sensitivity and standardization of the diagnosis of mycosis fungoides type cutaneous T-cell lymphoma: Logistic regression of clinical and laboratory data. Br. J. Dermatol. 2003, 149, 513–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, P.E.; Pinto, F.J. The histologic spectrum of mycosis fungoides/Sezary syndrome (cutaneous T-cell lymphoma). A review of 222 biopsies, including newly described patterns and the earliest pathologic changes. Am. J. Surg. Pathol. 1994, 18, 645–667. [Google Scholar] [CrossRef]
- Sanchez, J.L.; Ackerman, A.B. The patch stage of mycosis fungoides. Criteria for histologic diagnosis. Am. J. Dermatopathol. 1979, 1, 5–26. [Google Scholar] [CrossRef]
- Delfau-Larue, M.H.; Laroche, L.; Wechsler, J.; Lepage, E.; Lahet, C.; Asso-Bonnet, M.; Bagot, M.; Farcet, J.P. Diagnostic value of dominant T-cell clones in peripheral blood in 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. Blood 2000, 96, 2987–2992. [Google Scholar] [CrossRef]
- Plaza, J.A.; Morrison, C.; Magro, C.M. Assessment of TCR-beta clonality in a diverse group of cutaneous T-Cell infiltrates. J. Cutan. Pathol. 2008, 35, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Guitart, J.; Magro, C. Cutaneous T-cell lymphoid dyscrasia: A unifying term for idiopathic chronic dermatoses with persistent T-cell clones. Arch. Dermatol. 2007, 143, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.W. Weedon’s Skin Pathology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 114–115. [Google Scholar]
- Lazar, A.P.; Caro, W.A.; Roenigk, H.H., Jr.; Pinski, K.S. Parapsoriasis and mycosis fungoides: The Northwestern University experience, 1970 to 1985. J. Am. Acad. Dermatol. 1989, 21, 919–923. [Google Scholar] [CrossRef]
- Kikuchi, A.; Naka, W.; Harada, T.; Sakuraoka, K.; Harada, R.; Nishikawa, T. Parapsoriasis en plaques: Its potential for progression to malignant lymphoma. J. Am. Acad. Dermatol. 1993, 29, 419–422. [Google Scholar] [CrossRef]
- Kempf, W.; Burg, G.; Massone, C.; Cerroni, L.; Pulitzer, M.; Crowson, A.N.; Magro, C.M. Barnhill’s Dermatopathology, 4th ed.; Barnhill, R.L., Ed.; Mc Graw Hill: New York, NY, USA, 2020; pp. 1184–1185. [Google Scholar]



| Early MF (n = 38) | Non-MF (n = 22) | p-Value | |
|---|---|---|---|
| Sex (male:female) | 22:16 | 10:12 | 0.4254 |
| Age (years, mean ± SD) | 43.13 ± 2.858 | 51.91 ± 3.896 | 0.0717 |
| Total ISCL score (mean ± SD) | 4.868 ± 0.1261 | 2.0 ± 0.1861 | <0.0001 |
| Clinical points | <0.0001 | ||
| 0 1 2 | 1 7 30 | 9 8 5 | |
| Histopathologic points | 0.0500 | ||
| 0 1 2 | 3 15 20 | 5 12 5 | |
| Molecular/biologic points | <0.0001 | ||
| 0 1 | 2 34 | 16 4 | |
| Immunopathologic points | <0.0001 | ||
| 0 1 | 11 25 | 20 0 |
| Early MF (n = 38) | Non-MF (n = 22) | p-Value | |
|---|---|---|---|
| IHC (%, ± SEM) CD2 CD3 CD5 CD7 | |||
| 84.08 ± 3.155 91.84 ± 1.722 81.18 ± 3.438 17.45 ± 2.648 | 92.27 ± 1.174 93.41 ± 0.764 92.50 ± 1.175 42.95 ± 4.763 | 0.0590 0.5065 0.0174 <0.0001 | |
| Epidermal discordance CD2 CD3 CD5 CD7 | 0 0 2 15 | 0 0 0 0 | >0.9999 >0.9999 0.5402 0.0010 |
| IHC | AUC | Muller’s Value | SE | 95% Confidence Interval | p-Value | Cut-Off (%) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| CD2 CD3 CD5 CD7 | 0.669 0.531 0.718 0.837 | Poor Fail Fair Good | 0.071 0.077 0.067 0.050 | 0.529 0.379 0.587 0.739 | 0.808 0.682 0.849 0.934 | 0.031 0.696 0.005 0.000 | 92.5 92.5 92.5 22.5 | 60.5 28.9 65.8 73.7 | 68.2 77.3 72.7 77.3 |
| Early MF (n = 38) | Non-MF (n = 22) | p-Value | |
|---|---|---|---|
| TCR gene rearrangement TCR-γ TCR-δ TCR-β | <0.0001 | ||
| 33 (86.8%) 6 (15.8%) 16 (42.1%) | 4 (18.2%) 3 (13.6%) 0 (0.0%) | <0.0001 >0.9999 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-J.; Kim, S.-I.; Jang, H.-O.; Kim, S.-H.; Oh, S.-H.; Park, S.; Kim, S.-K. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides. Cells 2021, 10, 2758. https://doi.org/10.3390/cells10102758
Ryu H-J, Kim S-I, Jang H-O, Kim S-H, Oh S-H, Park S, Kim S-K. Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides. Cells. 2021; 10(10):2758. https://doi.org/10.3390/cells10102758
Chicago/Turabian StyleRyu, Hyang-Joo, Sun-Il Kim, Hyung-Ook Jang, Se-Hoon Kim, Sang-Ho Oh, Sujin Park, and Sang-Kyum Kim. 2021. "Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides" Cells 10, no. 10: 2758. https://doi.org/10.3390/cells10102758
APA StyleRyu, H.-J., Kim, S.-I., Jang, H.-O., Kim, S.-H., Oh, S.-H., Park, S., & Kim, S.-K. (2021). Evaluation of the International Society for Cutaneous Lymphoma Algorithm for the Diagnosis of Early Mycosis Fungoides. Cells, 10(10), 2758. https://doi.org/10.3390/cells10102758