Challenges and Considerations during In Vitro Production of Porcine Embryos
Abstract
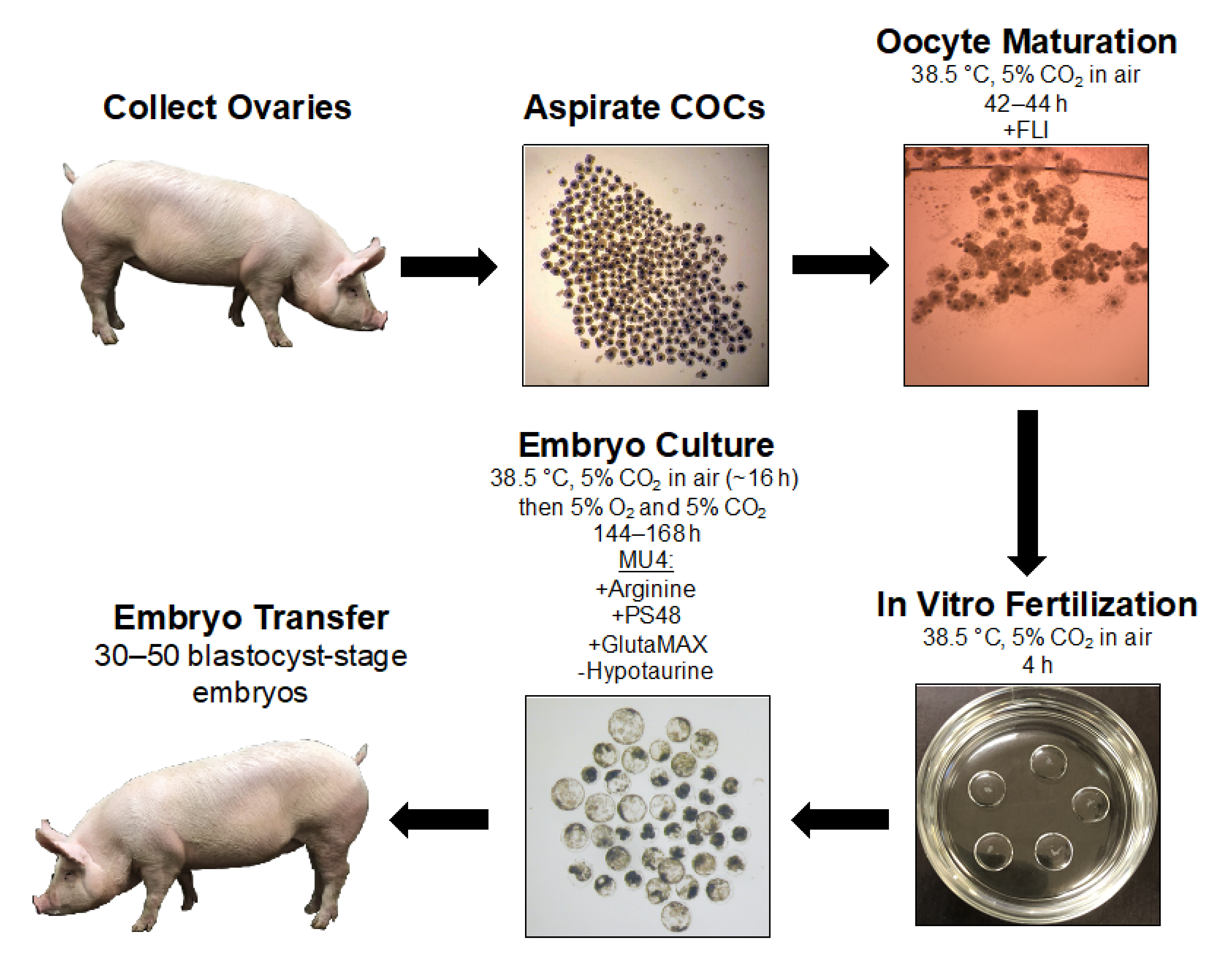
:1. Introduction
2. Oocyte Quality and Maturation
2.1. Selection Criteria
2.2. Supplementation of Growth Factors
3. In Vitro Fertilization
4. Comparing Metabolic Characteristics of Mammalian Embryos
4.1. Carbohydrates
4.2. Amino Acids
4.3. Lipids
5. Strategies for Improving Current Culture Systems
5.1. Media Formulations and Supplements
5.2. Morphological and Chromosomal Quality
5.3. Mitochondrial Function
5.4. Transcriptional Profiling
5.5. Metabolomics
5.6. Microfluidics
5.7. Extended Culture
5.8. Cryopreservation
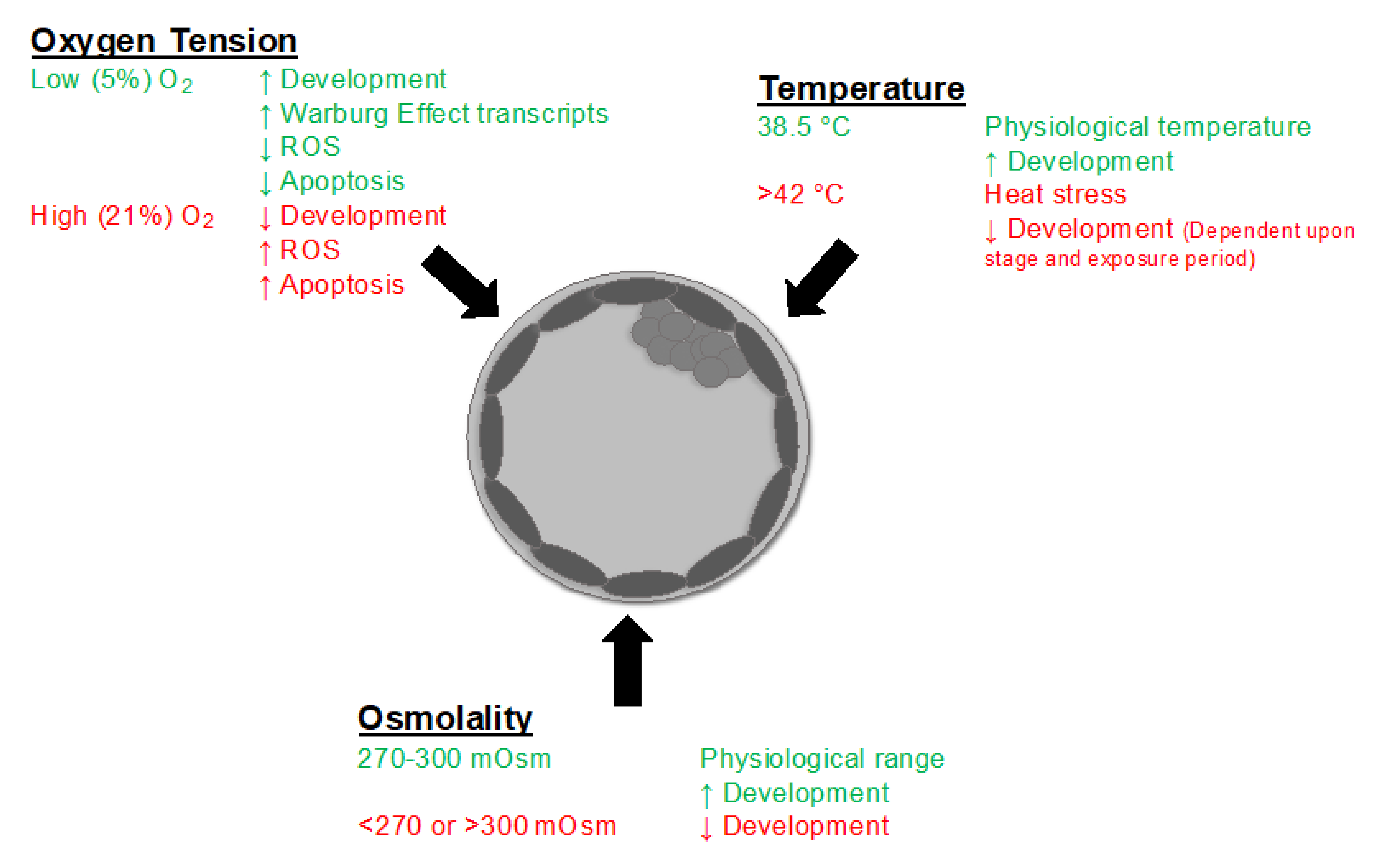
6. Environmental Stressors Arising from the Culture System
6.1. Oxygen Tension and Reactive Oxygen Species
6.2. Temperature
6.3. Osmolality
7. Maternal and Paternal Factors Influencing Development
7.1. Gilt-Versus Sow-Derived Oocytes
7.2. Sperm Quality
7.3. Epigenetics and In Vitro Production of Porcine Embryos
8. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Whyte, J.J.; Meyer, A.E.; Spate, L.D.; Benne, J.A.; Cecil, R.; Samuel, M.S.; Murphy, C.N.; Prather, R.S.; Geisert, R.D. Inactivation of porcine interleukin-1beta results in failure of rapid conceptus elongation. Proc. Natl. Acad. Sci. USA 2018, 115, 307–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.E.; Pfeiffer, C.A.; Brooks, K.E.; Spate, L.D.; Benne, J.A.; Cecil, R.; Samuel, M.S.; Murphy, C.N.; Behura, S.; McLean, M.K.; et al. New perspective on conceptus estrogens in maternal recognition and pregnancy establishment in the pigdagger. Biol. Reprod. 2019, 101, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.A.; Meyer, A.E.; Brooks, K.E.; Chen, P.R.; Milano-Foster, J.; Spate, L.D.; Benne, J.A.; Cecil, R.F.; Samuel, M.S.; Ciernia, L.A.; et al. Ablation of conceptus PTGS2 expression does not alter early conceptus development and establishment of pregnancy in the pigdagger. Biol. Reprod. 2020, 102, 475–488. [Google Scholar] [CrossRef]
- Chen, P.R.; Lucas, C.G.; Cecil, R.F.; Pfeiffer, C.A.; Fudge, M.A.; Samuel, M.S.; Zigo, M.; Seo, H.; Spate, L.D.; Whitworth, K.M.; et al. Disrupting porcine glutaminase does not block preimplantation development and elongation nor decrease mTORC1 activation in conceptuses. Biol. Reprod. 2021, 165. [Google Scholar] [CrossRef]
- Lai, L.; Kang, J.X.; Li, R.; Wang, J.; Witt, W.T.; Yong, H.Y.; Hao, Y.; Wax, D.M.; Murphy, C.N.; Rieke, A.; et al. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 2006, 24, 435–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitworth, K.M.; Rowland, R.R.; Ewen, C.L.; Trible, B.R.; Kerrigan, M.A.; Cino-Ozuna, A.G.; Samuel, M.S.; Lightner, J.E.; McLaren, D.G.; Mileham, A.J.; et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat. Biotechnol. 2016, 34, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, K.M.; Rowland, R.R.R.; Petrovan, V.; Sheahan, M.; Cino-Ozuna, A.G.; Fang, Y.; Hesse, R.; Mileham, A.; Samuel, M.S.; Wells, K.D.; et al. Resistance to coronavirus infection in amino peptidase N-deficient pigs. Transgenic Res. 2019, 28, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.S.; Hao, Y.; Rokhlina, T.; Samuel, M.; Stoltz, D.A.; Li, Y.; Petroff, E.; Vermeer, D.W.; Kabel, A.C.; Yan, Z.; et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Investig. 2008, 118, 1571–1577. [Google Scholar] [CrossRef]
- Koppes, E.A.; Redel, B.K.; Johnson, M.A.; Skvorak, K.J.; Ghaloul-Gonzalez, L.; Yates, M.E.; Lewis, D.W.; Gollin, S.M.; Wu, Y.L.; Christ, S.E.; et al. A porcine model of phenylketonuria generated by CRISPR/Cas9 genome editing. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.W.; Cheong, H.T.; Greenstein, J.L.; Im, G.S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, B.K.; Isom, S.C.; Spate, L.D.; Whitworth, K.M.; Spollen, W.G.; Blake, S.M.; Springer, G.K.; Murphy, C.N.; Prather, R.S. Transcriptional profiling by deep sequencing identifies differences in mRNA transcript abundance in in vivo-derived versus in vitro-cultured porcine blastocyst stage embryos. Biol. Reprod. 2010, 83, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Spate, L.D.; Brown, A.N.; Redel, B.K.; Whitworth, K.M.; Murphy, C.N.; Prather, R.S. Dickkopf-related protein 1 inhibits the WNT signaling pathway and improves pig oocyte maturation. PLoS ONE 2014, 9, e95114. [Google Scholar] [CrossRef]
- Russell, D.L.; Gilchrist, R.B.; Brown, H.M.; Thompson, J.G. Bidirectional communication between cumulus cells and the oocyte: Old hands and new players? Theriogenology 2016, 86, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Norris, R.P.; Ratzan, W.J.; Freudzon, M.; Mehlmann, L.M.; Krall, J.; Movsesian, M.A.; Wang, H.; Ke, H.; Nikolaev, V.O.; Jaffe, L.A. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009, 136, 1869–1878. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, P.; Rizos, D.; Gutierrez-Adan, A.; Fair, T.; Boland, M.P. Oocyte and embryo quality: Effect of origin, culture conditions and gene expression patterns. Reprod. Domest. Anim. 2003, 38, 259–267. [Google Scholar] [CrossRef]
- Armstrong, D.T. Effects of maternal age on oocyte developmental competence. Theriogenology 2001, 55, 1303–1322. [Google Scholar] [CrossRef]
- Lechniak, D.; Warzych, E.; Pers-Kamczyc, E.; Sosnowski, J.; Antosik, P.; Rubes, J. Gilts and sows produce similar rate of diploid oocytes in vitro whereas the incidence of aneuploidy differs significantly. Theriogenology 2007, 68, 755–762. [Google Scholar] [CrossRef]
- Lee, J.B.; Lee, M.G.; Lin, T.; Shin, H.Y.; Lee, J.E.; Kang, J.W.; Jin, D.-I. Effect of oocyte chromatin status in porcine follicles on the embryo development in vitro. Asian-Australas J. Anim. Sci. 2019, 32, 956–965. [Google Scholar] [CrossRef]
- Chen, L.; Russell, P.T.; Larsen, W.J. Functional significance of cumulus expansion in the mouse: Roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol. Reprod. Dev. 1993, 34, 87–93. [Google Scholar] [CrossRef]
- Marchal, R.; Vigneron, C.; Perreau, C.; Bali-Papp, A.; Mermillod, P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology 2002, 57, 1523–1532. [Google Scholar] [CrossRef]
- Costermans, N.G.J.; Soede, N.M.; van Tricht, F.; Blokland, M.; Kemp, B.; Keijer, J.; Teerds, K.J. Follicular fluid steroid profile in sows: Relationship to follicle size and oocyte qualitydagger. Biol. Reprod. 2020, 102, 740–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoo, M.; Kimura, N.; Sato, E. Induction of oocyte maturation by hyaluronan-CD44 interaction in pigs. J. Reprod. Dev. 2010, 56, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevoral, J.; Orsák, M.; Klein, P.; Petr, J.; Dvořáková, M.; Weingartová, I.; Vyskočilová, A.; Zámostná, K.; Krejčová, T.; Jílek, F. Cumulus Cell Expansion, Its Role in Oocyte Biology and Perspectives of Measurement: A Review. Sci. Agric. Bohem. 2015, 45, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Redel, B.K.; Spate, L.D.; Yuan, Y.; Murphy, C.N.; Roberts, R.M.; Prather, R.S. Neither gonadotropin nor cumulus cell expansion is needed for the maturation of competent porcine oocytes in vitro. Biol. Reprod. 2021. [Google Scholar] [CrossRef]
- Nagai, T.; Niwa, K.; Iritani, A. Effect of sperm concentration during preincubation in a defined medium on fertilization in vitro of pig follicular oocytes. J. Reprod. Fertil. 1984, 70, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Abeydeera, L.R.; Day, B.N. In vitro penetration of pig oocytes in a modified Tris-buffered medium: Effect of BSA, caffeine and calcium. Theriogenology 1997, 48, 537–544. [Google Scholar] [CrossRef]
- Evans, J.P. Preventing polyspermy in mammalian eggs-Contributions of the membrane block and other mechanisms. Mol. Reprod. Dev. 2020, 87, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Burkart, A.D.; Xiong, B.; Baibakov, B.; Jimenez-Movilla, M.; Dean, J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. 2012, 197, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Fahrenkamp, E.; Algarra, B.; Jovine, L. Mammalian egg coat modifications and the block to polyspermy. Mol. Reprod. Dev. 2020, 87, 326–340. [Google Scholar] [CrossRef]
- Wang, W.H.; Machaty, Z.; Abeydeera, L.R.; Prather, R.S.; Day, B.N. Parthenogenetic activation of pig oocytes with calcium ionophore and the block to sperm penetration after activation. Biol. Reprod. 1998, 58, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, M.D.; Mondejar, I.; Coy, P.; Betancourt, M.; Gonzalez-Marquez, H.; Jimenez-Movilla, M.; Aviles, M.; Romar, R. Calreticulin from suboolemmal vesicles affects membrane regulation of polyspermy. Reproduction 2014, 147, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funahashi, H.; Day, B.N. Effects of follicular fluid at fertilization in vitro on sperm penetration in pig oocytes. J. Reprod. Fertil. 1993, 99, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Teijeiro, J.M.; Marini, P.E. The effect of oviductal deleted in malignant brain tumor 1 over porcine sperm is mediated by a signal transduction pathway that involves pro-AKAP4 phosphorylation. Reproduction 2012, 143, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coy, P.; Canovas, S.; Mondejar, I.; Saavedra, M.D.; Romar, R.; Grullon, L.; Matas, C.; Aviles, M. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 15809–15814. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Mathialagan, N.; Walters, E.; Mao, J.; Lai, L.; Becker, D.; Li, W.; Critser, J.; Prather, R.S. Osteopontin reduces polyspermy during in vitro fertilization of porcine oocytes. Biol. Reprod. 2006, 75, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Coy, P.; Jimenez-Movilla, M.; Garcia-Vazquez, F.A.; Mondejar, I.; Grullon, L.; Romar, R. Oocytes use the plasminogen-plasmin system to remove supernumerary spermatozoa. Hum. Reprod. 2012, 27, 1985–1993. [Google Scholar] [CrossRef] [Green Version]
- Mondejar, I.; Grullon, L.A.; Garcia-Vazquez, F.A.; Romar, R.; Coy, P. Fertilization outcome could be regulated by binding of oviductal plasminogen to oocytes and by releasing of plasminogen activators during interplay between gametes. Fertil. Steril. 2012, 97, 453–461. [Google Scholar] [CrossRef]
- Umehara, T.; Tsujita, N.; Goto, M.; Tonai, S.; Nakanishi, T.; Yamashita, Y.; Shimada, M. Methyl-beta cyclodextrin and creatine work synergistically under hypoxic conditions to improve the fertilization ability of boar ejaculated sperm. Anim. Sci. J. 2020, 91, e13493. [Google Scholar] [CrossRef]
- Kim, N.H.; Funahashi, H.; Abeydeera, L.R.; Moon, S.J.; Prather, R.S.; Day, B.N. Effects of oviductal fluid on sperm penetration and cortical granule exocytosis during fertilization of pig oocytes in vitro. J. Reprod. Fertil. 1996, 107, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.A.; Stephens, K.K.; Winuthayanon, W. Extracellular Vesicles and the Oviduct Function. Int. J. Mol. Sci. 2020, 21, 8280. [Google Scholar] [CrossRef]
- Zigo, M.; Manaskova-Postlerova, P.; Zuidema, D.; Kerns, K.; Jonakova, V.; Tumova, L.; Bubenickova, F.; Sutovsky, P. Porcine model for the study of sperm capacitation, fertilization and male fertility. Cell Tissue Res. 2020, 380, 237–262. [Google Scholar] [CrossRef]
- Galeati, G.; Modina, S.; Lauria, A.; Mattioli, M. Follicle somatic cells influence pig oocyte penetrability and cortical granule distribution. Mol. Reprod. Dev. 1991, 29, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Probst, S.; Rath, D. Production of piglets using intracytoplasmic sperm injection (ICSI) with flowcytometrically sorted boar semen and artificially activated oocytes. Theriogenology 2003, 59, 961–973. [Google Scholar] [CrossRef]
- Yong, H.Y.; Hao, Y.; Lai, L.; Li, R.; Murphy, C.N.; Rieke, A.; Wax, D.; Samuel, M.; Prather, R.S. Production of a transgenic piglet by a sperm injection technique in which no chemical or physical treatments were used for oocytes or sperm. Mol. Reprod. Dev. 2006, 73, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Ozawa, M.; Maedomari, N.; Noguchi, J.; Kaneko, H.; Ito, J.; Onishi, A.; Kashiwazaki, N.; Kikuchi, K. Delay in cleavage of porcine embryos after intracytoplasmic sperm injection (ICSI) shows poorer embryonic development. J. Reprod. Dev. 2014, 60, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Nakai, M.; Suzuki, S.I.; Ito, J.; Fuchimoto, D.I.; Sembon, S.; Noguchi, J.; Onishi, A.; Kashiwazaki, N.; Kikuchi, K. Efficient pig ICSI using Percoll-selected spermatozoa; evidence for the essential role of phospholipase C-zeta in ICSI success. J. Reprod. Dev. 2016, 62, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Casillas, F.; Betancourt, M.; Cuello, C.; Ducolomb, Y.; Lopez, A.; Juarez-Rojas, L.; Retana-Marquez, S. An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Heal. Manag. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Redel, B.K.; Brown, A.N.; Spate, L.D.; Whitworth, K.M.; Green, J.A.; Prather, R.S. Glycolysis in preimplantation development is partially controlled by the Warburg Effect. Mol. Reprod. Dev. 2012, 79, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L.; Heuberger, A.L.; Paczkowski, M.; Stevens, J.; Pospisil, C.; Prather, R.S.; Sturmey, R.G.; Herrick, J.R.; Schoolcraft, W.B. Applying metabolomic analyses to the practice of embryology: Physiology, development and assisted reproductive technology. Reprod. Fertil. Dev. 2015, 27, 602–620. [Google Scholar] [CrossRef] [PubMed]
- Rieger, D. Relationships between energy metabolism and development of early mammalian embryos. Theriogenology 1992, 37, 75–93. [Google Scholar] [CrossRef]
- Chi, F.; Sharpley, M.S.; Nagaraj, R.; Roy, S.S.; Banerjee, U. Glycolysis-Independent Glucose Metabolism Distinguishes TE from ICM Fate during Mammalian Embryogenesis. Dev. Cell 2020, 53, 9–26.e24. [Google Scholar] [CrossRef]
- Biggers, J.D.; Whittingham, D.G.; Donahue, R.P. The pattern of energy metabolism in the mouse oocyte and zygote. Proc. Natl. Acad. Sci. USA 1967, 58, 560–567. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, A.P.; Lane, M.; Gardner, D.K.; Krisher, R.L. Substrate utilization in porcine embryos cultured in NCSU23 and G1.2/G2.2 sequential culture media. Mol. Reprod. Dev. 2001, 58, 269–275. [Google Scholar] [CrossRef]
- Martin, K.L.; Leese, H.J. Role of developmental factors in the switch from pyruvate to glucose as the major exogenous energy substrate in the preimplantation mouse embryo. Reprod. Fertil. Dev. 1999, 11, 425–433. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Batt, P. Uptake and metabolism of pyruvate and glucose by individual sheep preattachment embryos developed in vivo. Mol. Reprod Dev. 1993, 36, 313–319. [Google Scholar] [CrossRef]
- Leese, H.J.; Barton, A.M. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J. Reprod. Fertil. 1984, 72, 9–13. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Leese, H.J. Energy metabolism in pig oocytes and early embryos. Reproduction 2003, 126, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Swain, J.E.; Bormann, C.L.; Clark, S.G.; Walters, E.M.; Wheeler, M.B.; Krisher, R.L. Use of energy substrates by various stage preimplantation pig embryos produced in vivo and in vitro. Reproduction 2002, 123, 253–260. [Google Scholar] [CrossRef]
- Chatot, C.L.; Ziomek, C.A.; Bavister, B.D.; Lewis, J.L.; Torres, I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 1989, 86, 679–688. [Google Scholar] [CrossRef]
- Schini, S.A.; Bavister, B.D. Two-cell block to development of cultured hamster embryos is caused by phosphate and glucose. Biol. Reprod. 1988, 39, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Conaghan, J.; Hardy, K.; Handyside, A.H.; Winston, R.M.; Leese, H.J. Selection criteria for human embryo transfer: A comparison of pyruvate uptake and morphology. J. Assist. Reprod. Genet. 1993, 10, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; First, N.L. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992, 37, 963–978. [Google Scholar] [CrossRef]
- Thompson, J.G.; Simpson, A.C.; Pugh, P.A.; Tervit, H.R. Requirement for glucose during in vitro culture of sheep preimplantation embryos. Mol. Reprod. Dev. 1992, 31, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Hagen, D.R.; Prather, R.S.; Sims, M.M.; First, N.L. Development of one-cell porcine embryos to the blastocyst stage in simple media. J. Anim. Sci. 1991, 69, 1147–1150. [Google Scholar] [CrossRef]
- Petters, R.M.; Johnson, B.H.; Reed, M.L.; Archibong, A.E. Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro. J. Reprod. Fertil. 1990, 89, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Flood, M.R.; Wiebold, J.L. Glucose metabolism by preimplantation pig embryos. J. Reprod. Fertil. 1988, 84, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, K.; Suzuki, C.; Tanaka, A.; Anas, I.M.; Iwamura, S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol. Reprod. 2002, 66, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.K.; Leese, H.J. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J. Reprod. Fertil. 1990, 88, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugentobler, S.A.; Diskin, M.G.; Leese, H.J.; Humpherson, P.G.; Watson, T.; Sreenan, J.M.; Morris, D.G. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol. Reprod. Dev. 2007, 74, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Whitworth, K.; Lai, L.; Wax, D.; Spate, L.; Murphy, C.N.; Rieke, A.; Isom, C.; Hao, Y.; Zhong, Z.; et al. Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos. Mol. Reprod. Dev. 2007, 74, 1228–1235. [Google Scholar] [CrossRef] [Green Version]
- Van Winkle, L.J. Amino acid transport regulation and early embryo development. Biol. Reprod. 2001, 64, 1–12. [Google Scholar] [CrossRef]
- Prather, R.S.; Peters, M.S.; Van Winkle, L.J. Alanine and leucine transport in unfertilized pig oocytes and early blastocysts. Mol. Reprod. Dev. 1993, 34, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Tesch, J.K.; Shah, A.; Campione, A.L. System B0,+ amino acid transport regulates the penetration stage of blastocyst implantation with possible long-term developmental consequences through adulthood. Hum. Reprod. Updat. 2006, 12, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Prather, R.S.; Peters, M.S.; Van Winkle, L.J. Aspartate and glutamate transport in unfertilized pig oocytes and blastocysts. Mol. Reprod. Dev. 1993, 36, 49–52. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M. Amino acids and ammonium regulate mouse embryo development in culture. Biol. Reprod. 1993, 48, 377–385. [Google Scholar] [CrossRef]
- Pinyopummintr, T.; Bavister, B.D. Effects of amino acids on development in vitro of cleavage-stage bovine embryos into blastocysts. Reprod. Fertil. Dev. 1996, 8, 835–841. [Google Scholar] [CrossRef]
- Steeves, T.E.; Gardner, D.K. Temporal and differential effects of amino acids on bovine embryo development in culture. Biol. Reprod. 1999, 61, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Van Thuan, N.; Harayama, H.; Miyake, M. Characteristics of preimplantational development of porcine parthenogenetic diploids relative to the existence of amino acids in vitro. Biol. Reprod. 2002, 67, 1688–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redel, B.K.; Tessanne, K.J.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Arginine increases development of in vitro-produced porcine embryos and affects the protein arginine methyltransferase-dimethylarginine dimethylaminohydrolase-nitric oxide axis. Reprod. Fertil. Dev. 2015, 27, 655–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranguch, S.; Steuerwald, N.; Huet-Hudson, Y.M. Nitric oxide synthase production and nitric oxide regulation of preimplantation embryo development. Biol. Reprod. 2003, 68, 1538–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redel, B.K.; Spate, L.D.; Lee, K.; Mao, J.; Whitworth, K.M.; Prather, R.S. Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not lead to live births. Mol. Reprod. Dev. 2016, 83, 246–258. [Google Scholar] [CrossRef]
- Carney, E.W.; Bavister, B.D. Stimulatory and inhibitory effects of amino acids on the development of hamster eight-cell embryos in vitro. J. Assist. Reprod. Genet. 1987, 4, 162–167. [Google Scholar] [CrossRef]
- Chen, P.R.; Redel, B.K.; Spate, L.D.; Ji, T.; Salazar, S.R.; Prather, R.S. Glutamine supplementation enhances development of in vitro-produced porcine embryos and increases leucine consumption from the medium. Biol. Reprod. 2018, 99, 938–948. [Google Scholar] [CrossRef]
- Chen, P.R.; Lucas, C.G.; Spate, L.D.; Prather, R.S. Glutaminolysis is involved in the activation of mTORC1 in in vitro-produced porcine embryos. Mol. Reprod. Dev. 2021, 88, 490–499. [Google Scholar] [CrossRef]
- Dunning, K.R.; Cashman, K.; Russell, D.L.; Thompson, J.G.; Norman, R.J.; Robker, R.L. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol. Reprod. 2010, 83, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Hewitson, L.C.; Martin, K.L.; Leese, H.J. Effects of metabolic inhibitors on mouse preimplantation embryo development and the energy metabolism of isolated inner cell masses. Mol. Reprod. Dev. 1996, 43, 323–330. [Google Scholar] [CrossRef]
- Lowe, J.L.; Bathgate, R.; Grupen, C.G. Effect of carbohydrates on lipid metabolism during porcine oocyte IVM. Reprod. Fertil. Dev. 2019, 31, 557–569. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.K.; Paek, S.K.; Kim, J.W.; Kwak, I.P.; Lee, H.J.; Lyu, S.W.; Lee, W.S. Effects and pregnancy outcomes of L-carnitine supplementation in culture media for human embryo development from in vitro fertilization. J. Obstet. Gynaecol. Res. 2018, 44, 2059–2066. [Google Scholar] [CrossRef]
- Somfai, T.; Kaneda, M.; Akagi, S.; Watanabe, S.; Haraguchi, S.; Mizutani, E.; Dang-Nguyen, T.Q.; Geshi, M.; Kikuchi, K.; Nagai, T. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod. Fertil. Dev. 2011, 23, 912–920. [Google Scholar] [CrossRef]
- Knitlova, D.; Hulinska, P.; Jeseta, M.; Hanzalova, K.; Kempisty, B.; Machatkova, M. Supplementation of l-carnitine during in vitro maturation improves embryo development from less competent bovine oocytes. Theriogenology 2017, 102, 16–22. [Google Scholar] [CrossRef]
- Loewenstein, J.E.; Cohen, A.I. Dry Mass, Lipid Content and Protein Content of the Intact and Zona-Free Mouse Ovum. J. Embryol. Exp. Morphol. 1964, 12, 113–121. [Google Scholar]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, H.; Zheng, X.; Marjani, S.L.; Donovan, D.M.; Chen, J.; Tian, X.C. mRNA Levels of Imprinted Genes in Bovine In Vivo Oocytes, Embryos and Cross Species Comparisons with Humans, Mice and Pigs. Sci. Rep. 2015, 5, 17898. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Choi, K.H.; Hwang, J.Y.; Oh, J.N.; Kim, S.H.; Lee, C.K. Stearoyl-coenzyme A desaturase 1 is required for lipid droplet formation in pig embryo. Reproduction 2019, 157, 235–243. [Google Scholar] [CrossRef]
- Romek, M.; Gajda, B.; Krzysztofowicz, E.; Smorag, Z. Lipid content of non-cultured and cultured pig embryo. Reprod. Domest. Anim. 2009, 44, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lai, L.; Wax, D.; Hao, Y.; Murphy, C.N.; Rieke, A.; Samuel, M.; Linville, M.L.; Korte, S.W.; Evans, R.W.; et al. Cloned transgenic swine via in vitro production and cryopreservation. Biol. Reprod. 2006, 75, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Murphy, C.N.; Spate, L.; Wax, D.; Isom, C.; Rieke, A.; Walters, E.M.; Samuel, M.; Prather, R.S. Production of piglets after cryopreservation of embryos using a centrifugation-based method for delipation without micromanipulation. Biol. Reprod.. 2009, 80, 563–571. [Google Scholar] [CrossRef]
- Lowe, J.L.; Bartolac, L.K.; Bathgate, R.; Grupen, C.G. Supplementation of culture medium with L-carnitine improves the development and cryotolerance of in vitro-produced porcine embryos. Repro. Fertil. Dev. 2017, 29, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Kashiwazaki, N.; Ashman, R.J.; Grupen, C.G.; Seamark, R.F.; Nottle, M.B. Removal of cytoplasmic lipid enhances the tolerance of porcine embryos to chilling. Biol. Reprod. 1994, 51, 618–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, L.S.; Day, B.N. Effects of media NaCl concentration and osmolarity on the culture of early-stage porcine embryos and the viability of embryos cultured in a selected superior medium. Theriogenology 1993, 39, 611–622. [Google Scholar] [CrossRef]
- Petters, R.M.; Wells, K.D. Culture of pig embryos. J. Reprod. Fertil. Suppl. 1993, 48, 61–73. [Google Scholar] [PubMed]
- Pollard, J.W.; Plante, C.; Leibo, S.P. Comparison of development of pig zygotes and embryos in simple and complex culture media. J. Reprod. Fertil. 1995, 103, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Dobrinsky, J.R.; Johnson, L.A.; Rath, D. Development of a culture medium (BECM-3) for porcine embryos: Effects of bovine serum albumin and fetal bovine serum on embryo development. Biol. Reprod. 1996, 55, 1069–1074. [Google Scholar] [CrossRef] [Green Version]
- Im, G.S.; Lai, L.; Liu, Z.; Hao, Y.; Wax, D.; Bonk, A.; Prather, R.S. In vitro development of preimplantation porcine nuclear transfer embryos cultured in different media and gas atmospheres. Theriogenology 2004, 61, 1125–1135. [Google Scholar] [CrossRef]
- Spate, L.D.; Redel, B.K.; Brown, A.N.; Murphy, C.N.; Prather, R.S. Replacement of bovine serum albumin with N-methyl-D-aspartic acid and homocysteine improves development, but not live birth. Mol. Reprod. Dev. 2012, 79, 310. [Google Scholar] [CrossRef]
- Spate, L.D.; Brown, A.; Redel, B.K.; Whitworth, K.M.; Prather, R.S. PS48 can replace bovine serum albumin in pig embryo culture medium, and improve in vitro embryo development by phosphorylating AKT. Mol. Reprod. Dev. 2015, 82, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.R.; Spate, L.D.; Leffeler, E.C.; Benne, J.A.; Cecil, R.F.; Hord, T.K.; Prather, R.S. Removal of hypotaurine from porcine embryo culture medium does not impair development of in vitro-fertilized or somatic cell nuclear transfer-derived embryos at low oxygen tension. Mol. Reprod. Dev. 2020. [Google Scholar] [CrossRef]
- Guérin, P.; Mouatassim, S.E.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Machaty, Z.; Day, B.N.; Prather, R.S. Development of early porcine embryos in vitro and in vivo. Biol. Reprod. 1998, 59, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Sanfins, A.; Plancha, C.E.; Albertini, D.F. Pre-implantation developmental potential from in vivo and in vitro matured mouse oocytes: A cytoskeletal perspective on oocyte quality. J. Assist. Reprod. Genet. 2015, 32, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Ushijima, H.; Akiyama, K.; Tajima, T. Transition of cell numbers in bovine preimplantation embryos: In vivo collected and in vitro produced embryos. J. Reprod. Dev. 2008, 54, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Lindner, G.M.; Wright, R.W.J. Morphological evaluation of bovine embryos. Theriogenology 1983, 20, 407–416. [Google Scholar] [CrossRef]
- Sugimura, S.; Akai, T.; Imai, K. Selection of viable in vitro-fertilized bovine embryos using time-lapse monitoring in microwell culture dishes. J. Reprod. Dev. 2017, 63, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Isom, S.C.; Li, R.F.; Whitworth, K.M.; Prather, R.S. Timing of first embryonic cleavage is a positive indicator of the in vitro developmental potential of porcine embryos derived from in vitro fertilization, somatic cell nuclear transfer and parthenogenesis. Mol. Reprod. Dev. 2012, 79, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lundin, K.; Bergh, C.; Hardarson, T. Early embryo cleavage is a strong indicator of embryo quality in human IVF. Hum. Reprod. 2001, 16, 2652–2657. [Google Scholar] [CrossRef]
- Mateusen, B.; Van Soom, A.; Maes, D.G.; Donnay, I.; Duchateau, L.; Lequarre, A.S. Porcine embryo development and fragmentation and their relation to apoptotic markers: A cinematographic and confocal laser scanning microscopic study. Reproduction 2005, 129, 443–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornak, M.; Hulinska, P.; Musilova, P.; Kubickova, S.; Rubes, J. Investigation of chromosome aneuploidies in early porcine embryos using comparative genomic hybridization. Cytogenet. Genome Res. 2009, 126, 210–216. [Google Scholar] [CrossRef] [PubMed]
- McCauley, T.C.; Mazza, M.R.; Didion, B.A.; Mao, J.; Wu, G.; Coppola, G.; Coppola, G.F.; Di Berardino, D.; Day, B.N. Chromosomal abnormalities in Day-6, in vitro-produced pig embryos. Theriogenology 2003, 60, 1569–1580. [Google Scholar] [CrossRef]
- Viuff, D.; Rickords, L.; Offenberg, H.; Hyttel, P.; Avery, B.; Greve, T.; Olsaker, I.; Williams, J.L.; Callesen, H.; Thomsen, P.D. A high proportion of bovine blastocysts produced in vitro are mixoploid. Biol. Reprod. 1999, 60, 1273–1278. [Google Scholar] [CrossRef] [Green Version]
- Tsuiko, O.; Catteeuw, M.; Zamani Esteki, M.; Destouni, A.; Bogado Pascottini, O.; Besenfelder, U.; Havlicek, V.; Smits, K.; Kurg, A.; Salumets, A.; et al. Genome stability of bovine in vivo-conceived cleavage-stage embryos is higher compared to in vitro-produced embryos. Hum. Reprod. 2017, 32, 2348–2357. [Google Scholar] [CrossRef]
- Destouni, A.; Zamani Esteki, M.; Catteeuw, M.; Tsuiko, O.; Dimitriadou, E.; Smits, K.; Kurg, A.; Salumets, A.; Van Soom, A.; Voet, T.; et al. Zygotes segregate entire parental genomes in distinct blastomere lineages causing cleavage-stage chimerism and mixoploidy. Genome Res. 2016, 26, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Sathananthan, A.H.; Trounson, A.O. Mitochondrial morphology during preimplantational human embryogenesis. Hum. Reprod. 2000, 15 (Suppl. 2), 148–159. [Google Scholar] [CrossRef] [Green Version]
- Reers, M.; Smiley, S.T.; Mottola-Hartshorn, C.; Chen, A.; Lin, M.; Chen, L.B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995, 260, 406–417. [Google Scholar] [CrossRef]
- Mordhorst, B.R.; Murphy, S.L.; Ross, R.M.; Benne, J.A.; Samuel, M.S.; Cecil, R.F.; Redel, B.K.; Spate, L.D.; Murphy, C.N.; Wells, K.D.; et al. Pharmacologic treatment of donor cells induced to have a Warburg effect-like metabolism does not alter embryonic development in vitro or survival during early gestation when used in somatic cell nuclear transfer in pigs. Mol. Reprod. Dev. 2018, 85, 290–302. [Google Scholar] [CrossRef]
- Agnello, M.; Morici, G.; Rinaldi, A.M. A method for measuring mitochondrial mass and activity. Cytotechnology 2008, 56, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Miles, J.R.; Blomberg, L.A.; Krisher, R.L.; Everts, R.E.; Sonstegard, T.S.; Van Tassell, C.P.; Zuelke, K.A. Comparative transcriptome analysis of in vivo- and in vitro-produced porcine blastocysts by small amplified RNA-serial analysis of gene expression (SAR-SAGE). Mol. Reprod. Dev. 2008, 75, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, K.M.; Agca, C.; Kim, J.G.; Patel, R.V.; Springer, G.K.; Bivens, N.J.; Forrester, L.J.; Mathialagan, N.; Green, J.A.; Prather, R.S. Transcriptional profiling of pig embryogenesis by using a 15-K member unigene set specific for pig reproductive tissues and embryos. Biol. Reprod. 2005, 72, 1437–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, R.N.; Baltz, J.M.; Lechene, C.P.; Biggers, J.D. Use of ultramicrofluorometric methods for the study of single preimplantation embryos. Poult. Sci. 1989, 68, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.; Hooper, M.A.; Handyside, A.H.; Rutherford, A.J.; Winston, R.M.; Leese, H.J. Non-invasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum. Reprod. 1989, 4, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Booth, P.J.; Humpherson, P.G.; Watson, T.J.; Leese, H.J. Amino acid depletion and appearance during porcine preimplantation embryo development in vitro. Reproduction 2005, 130, 655–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, P.J.; Hawkhead, J.A.; Fawthrop, R.K.; Picton, H.M.; Sharma, V.; Leese, H.J.; Houghton, F.D. Metabolism of human embryos following cryopreservation: Implications for the safety and selection of embryos for transfer in clinical IVF. Hum. Reprod. 2007, 22, 829–835. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, F.; Uppangala, S.; Asampille, G.; Salian, S.R.; Kalthur, G.; Talevi, R.; Atreya, H.S.; Adiga, S.K. Spent embryo culture medium metabolites are related to the in vitro attachment ability of blastocysts. Sci. Rep. 2018, 8, 17025. [Google Scholar] [CrossRef] [Green Version]
- Seli, E.; Botros, L.; Sakkas, D.; Burns, D.H. Noninvasive metabolomic profiling of embryo culture media using proton nuclear magnetic resonance correlates with reproductive potential of embryos in women undergoing in vitro fertilization. Fertil. Steril. 2008, 90, 2183–2189. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Yi, X.; Zhou, X. Microfluidic method reduces osmotic stress injury to oocytes during cryoprotectant addition and removal processes in porcine oocytes. Cryobiology 2019, 90, 63–70. [Google Scholar] [CrossRef]
- Zeringue, H.C.; Wheeler, M.B.; Beebe, D.J. A microfluidic method for removal of the zona pellucida from mammalian embryos. Lab. Chip. 2005, 5, 108–110. [Google Scholar] [CrossRef]
- Sano, H.; Matsuura, K.; Naruse, K.; Funahashi, H. Application of a microfluidic sperm sorter to the in-vitro fertilization of porcine oocytes reduced the incidence of polyspermic penetration. Theriogenology 2010, 74, 863–870. [Google Scholar] [CrossRef]
- Clark, S.G.; Haubert, K.; Beebe, D.J.; Ferguson, C.E.; Wheeler, M.B. Reduction of polyspermic penetration using biomimetic microfluidic technology during in vitro fertilization. Lab Chip 2005, 5, 1229–1232. [Google Scholar] [CrossRef]
- Krisher, R.L.; Wheeler, M.B. Towards the use of microfluidics for individual embryo culture. Reprod. Fertil. Dev. 2010, 22, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.B.; Walters, E.M.; Beebe, D.J. Toward culture of single gametes: The development of microfluidic platforms for assisted reproduction. Theriogenology 2007, 68 (Suppl. 1), S178–S189. [Google Scholar] [CrossRef] [PubMed]
- Sargus-Patino, C.N.; Wright, E.C.; Plautz, S.A.; Miles, J.R.; Vallet, J.L.; Pannier, A.K. In vitro development of preimplantation porcine embryos using alginate hydrogels as a three-dimensional extracellular matrix. Reprod. Fertil. Dev. 2014, 26, 943–953. [Google Scholar] [CrossRef]
- Laughlin, T.D.; Miles, J.R.; Wright-Johnson, E.C.; Rempel, L.A.; Lents, C.A.; Pannier, A.K. Development of pre-implantation porcine blastocysts cultured within alginate hydrogel systems either supplemented with secreted phosphoprotein 1 or conjugated with Arg-Gly-Asp Peptide. Reprod. Fertil. Dev. 2017, 29, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Cunha, E.R.; Blume, G.R.; Malaquias, J.V.; Bao, S.N.; Martins, C.F. Cryopreservation of boar sperm comparing different cryoprotectants associated in media based on powdered coconut water, lactose and trehalose. Cryobiology 2015, 70, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, H.; Kashiwazaki, N.; Ashman, R.J.; Grupen, C.G.; Nottle, M.B. Cryopreservation of porcine embryos. Nature 1995, 374, 416. [Google Scholar] [CrossRef] [PubMed]
- Spate, L.D.; Murphy, C.N.; Prather, R.S. High-throughput cryopreservation of in vivo-derived swine embryos. PLoS ONE 2013, 8, e65545. [Google Scholar] [CrossRef] [Green Version]
- Somfai, T.; Kikuchi, K. Vitrification of Porcine Oocytes and Zygotes in Microdrops on a Solid Metal Surface or Liquid Nitrogen. Methods Mol. Biol. 2021, 2180, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, K.S.; Ortega, M.S.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PLoS ONE 2021, 16, e0243727. [Google Scholar] [CrossRef]
- Tajima, S.; Motoyama, S.; Wakiya, Y.; Uchikura, K.; Misawa, H.; Takishita, R.; Hirayama, Y.; Kikuchi, K. Piglet production by non-surgical transfer of vitrified embryos, transported to commercial swine farms and warmed on site. Anim. Sci. J. 2020, 91, e13476. [Google Scholar] [CrossRef]
- Hirayama, Y.; Takishita, R.; Misawa, H.; Kikuchi, K.; Misumi, K.; Egawa, S.; Motoyama, S.; Hasuta, Y.; Nakamura, Y.; Hashiyada, Y. Non-surgical transfer of vitrified porcine embryos using a catheter designed for a proximal site of the uterus. Anim. Sci. J. 2020, 91, e13457. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalvit, G.C.; Cetica, P.D.; Pintos, L.N.; Beconi, M.T. Reactive oxygen species in bovine embryo in vitro production. Biocell 2005, 29, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.G.; Lee, S.; Jeong, P.S.; Kim, M.J.; Park, S.H.; Joo, Y.E.; Park, S.H.; Song, B.S.; Kim, S.U.; Kim, M.K.; et al. Lycopene Improves In Vitro Development of Porcine Embryos by Reducing Oxidative Stress and Apoptosis. Antioxidants 2021, 10, 230. [Google Scholar] [CrossRef]
- Yang, S.G.; Park, H.J.; Kim, J.W.; Jung, J.M.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Kang, M.J.; Wee, G.; Yang, H.Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef]
- Berthelot, F.; Terqui, M. Effects of oxygen, CO2/pH and medium on the in vitro development of individually cultured porcine one- and two-cell embryos. Reprod. Nutr. Dev. 1996, 36, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Rinaudo, P.F.; Giritharan, G.; Talbi, S.; Dobson, A.T.; Schultz, R.M. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil. Steril. 2006, 86, 1265.e1–1265.e36. [Google Scholar] [CrossRef] [PubMed]
- Jeseta, M.; Cela, A.; Zakova, J.; Madr, A.; Crha, I.; Glatz, Z.; Kempisty, B.; Ventruba, P. Metabolic Activity of Human Embryos after Thawing Differs in Atmosphere with Different Oxygen Concentrations. J. Clin. Med. 2020, 9, 2609. [Google Scholar] [CrossRef]
- Yin, C.; Liu, J.; He, B.; Jia, L.; Gong, Y.; Guo, H.; Zhao, R. Heat stress induces distinct responses in porcine cumulus cells and oocytes associated with disrupted gap junction and trans-zonal projection colocalization. J. Cell. Physiol. 2019, 234, 4787–4798. [Google Scholar] [CrossRef]
- Do, L.T.; Luu, V.V.; Morita, Y.; Taniguchi, M.; Nii, M.; Peter, A.T.; Otoi, T. Astaxanthin present in the maturation medium reduces negative effects of heat shock on the developmental competence of porcine oocytes. Reprod. Biol. 2015, 15, 86–93. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, Z.; Yi, J.; He, C.; Wang, F.; Tian, X.; Yang, M.; Song, Y.; He, P.; et al. Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J. Anim. Sci. Biotechnol 2016, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, Z.; He, C.; Zhu, K.; Xu, Z.; Ma, T.; Tao, J.; Liu, G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal. Res. 2015, 59, 365–375. [Google Scholar] [CrossRef]
- Kojima, T.; Udagawa, K.; Onishi, A.; Iwahashi, H.; Komatsu, Y. Effect of heat stress on development in vitro and in vivo and on synthesis of heat shock proteins in porcine embryos. Mol. Reprod. Dev. 1996, 43, 452–457. [Google Scholar] [CrossRef]
- Isom, S.C.; Lai, L.; Prather, R.S.; Rucker, E.B., 3rd. Heat shock of porcine zygotes immediately after oocyte activation increases viability. Mol. Reprod. Dev. 2009, 76, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Isom, S.C.; Prather, R.S.; Rucker Iii, E.B. Enhanced developmental potential of heat-shocked porcine parthenogenetic embryos is related to accelerated mitogen-activated protein kinase dephosphorylation. Reprod. Fertil. Dev. 2009, 21, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Park, M.R.; Moon, H.J.; Shim, J.H.; Kim, D.H.; Yang, B.C.; Ko, Y.G.; Yang, B.S.; Cheong, H.T.; Im, G.S. Osmolarity at early culture stage affects development and expression of apoptosis related genes (Bax-alpha and Bcl-xl) in pre-implantation porcine NT embryos. Mol. Reprod. Dev. 2008, 75, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Marchal, R.; Feugang, J.M.; Perreau, C.; Venturi, E.; Terqui, M.; Mermillod, P. Meiotic and developmental competence of prepubertal and adult swine oocytes. Theriogenology 2001, 56, 17–29. [Google Scholar] [CrossRef]
- Bagg, M.A.; Nottle, M.B.; Grupen, C.G.; Armstrong, D.T. Effect of dibutyryl cAMP on the cAMP content, meiotic progression, and developmental potential of in vitro matured pre-pubertal and adult pig oocytes. Mol. Reprod. Dev. 2006, 73, 1326–1332. [Google Scholar] [CrossRef]
- Bagg, M.A.; Nottle, M.B.; Armstrong, D.T.; Grupen, C.G. Relationship between follicle size and oocyte developmental competence in prepubertal and adult pigs. Reprod. Fertil. Dev. 2007, 19, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Lee, G.S.; Kim, D.Y.; Kim, H.S.; Lee, S.H.; Kim, S.; Lee, E.S.; Lim, J.M.; Kang, S.K.; Lee, B.C.; et al. Effect of maturation media and oocytes derived from sows or gilts on the development of cloned pig embryos. Theriogenology 2003, 59, 1641–1649. [Google Scholar] [CrossRef]
- Amann, R.P.; Waberski, D. Computer-assisted sperm analysis (CASA): Capabilities and potential developments. Theriogenology 2014, 81, 5–17. [Google Scholar] [CrossRef]
- Vazquez, J.M.; Martinez, E.; Pastor, L.M.; Roca, J.; Matas, C.; Calvo, A. Lectin histochemistry during in vitro capacitation and acrosome reaction in boar spermatozoa: New lectins for evaluating acrosomal status of boar spermatozoa. Acta Histochem. 1996, 98, 93–100. [Google Scholar] [CrossRef]
- Garner, D.L.; Pinkel, D.; Johnson, L.A.; Pace, M.M. Assessment of spermatozoal function using dual fluorescent staining and flow cytometric analyses. Biol. Reprod. 1986, 34, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nature Communications 2018, 9, 2061. [Google Scholar] [CrossRef] [Green Version]
- Huo, L.-J.; Yue, K.-Z.; Yang, Z.-M. Characterization of viability, mitochondrial activity, acrosomal integrity and capacitation status in boar sperm during in vitro storage at different ambient temperatures. Reprod. Fertil. Dev. 2002, 14, 509. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Siqueira, A.P.; Wallgren, M.; Rodriguez-Martinez, H. Spermatozoa in the sperm-peak-fraction of the boar ejaculate show a lower flow of Ca2+ under capacitation conditions post-thaw which might account for their higher membrane stability after cryopreservation. Anim. Reprod. Sci. 2011, 128, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Kerns, K.; Jankovitz, J.; Robinson, J.; Minton, A.; Kuster, C.; Sutovsky, P. Relationship between the Length of Sperm Tail Mitochondrial Sheath and Fertility Traits in Boars Used for Artificial Insemination. Antioxidants 2020, 9, 1033. [Google Scholar] [CrossRef]
- Lovercamp, K.W.; Safranski, T.J.; Fischer, K.A.; Manandhar, G.; Sutovsky, M.; Herring, W.; Sutovsky, P. Arachidonate 15-lipoxygenase and ubiquitin as fertility markers in boars. Theriogenology 2007, 67, 704–718. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry1. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [Green Version]
- Brouwers, J.F.; Silva, P.F.N.; Gadella, B.M. New assays for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze–thawing. Theriogenology 2005, 63, 458–469. [Google Scholar] [CrossRef]
- Evenson, D.P.; Thompson, L.; Jost, L. Flow cytometric evaluation of boar semen by the sperm chromatin structure assay as related to cryopreservation and fertility. Theriogenology 1994, 41, 637–651. [Google Scholar] [CrossRef]
- Parrilla, I.; Vazquez, J.; Oliver-Bonet, M.; Navarro, J.; Yelamos, J.; Roca, J.; Martinez, E. Fluorescence in situ hybridization in diluted and flow cytometrically sorted boar spermatozoa using specific DNA direct probes labelled by nick translation. Reproduction 2003, 126, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Steele, H.; Makri, D.; Maalouf, W.E.; Reese, S.; Kölle, S. Bovine Sperm Sexing Alters Sperm Morphokinetics and Subsequent Early Embryonic Development. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, T.K.; Schenk, J.L.; Seidel, G.E. High pressure flow cytometric sorting damages sperm. Theriogenology 2005, 64, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Umezu, K.; Hiradate, Y.; Numabe, T.; Hara, K.; Tanemura, K. Effects on glycocalyx structures of frozen-thawed bovine sperm induced by flow cytometry and artificial capacitation. J. Reprod. Dev. 2017, 63, 473–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLCζ: A sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef]
- Wu, A.T.; Sutovsky, P.; Manandhar, G.; Xu, W.; Katayama, M.; Day, B.N.; Park, K.W.; Yi, Y.J.; Xi, Y.W.; Prather, R.S.; et al. PAWP, a Sperm-specific WW Domain-binding Protein, Promotes Meiotic Resumption and Pronuclear Development during Fertilization. J. Biol. Chem. 2007, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeler, J.F.; Sharma, A.; Zaluzec, N.J.; Bleher, R.; Lai, B.; Schultz, E.G.; Hoffman, B.M.; Labonne, C.; Woodruff, T.K.; O’Halloran, T.V. Metal ion fluxes controlling amphibian fertilization. Nat. Chem. 2021, 13, 683–691. [Google Scholar] [CrossRef]
- Que, E.L.; Duncan, F.E.; Lee, H.C.; Hornick, J.E.; Vogt, S.; Fissore, R.A.; O’Halloran, T.V.; Woodruff, T.K. Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation. Theriogenology 2019, 127, 41–48. [Google Scholar] [CrossRef]
- Kim, A.M.; Bernhardt, M.L.; Kong, B.Y.; Ahn, R.W.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Bio 2011, 6, 716–723. [Google Scholar] [CrossRef]
- Lee, K.; Davis, A.; Zhang, L.; Ryu, J.; Spate, L.D.; Park, K.-W.; Samuel, M.S.; Walters, E.M.; Murphy, C.N.; Machaty, Z.; et al. Pig oocyte activation using a Zn2+ chelator, TPEN. Theriogenology 2015, 84, 1024–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm Cohort-Specific Zinc Signature Acquisition and Capacitation-Induced Zinc Flux Regulate Sperm-Oviduct and Sperm-Zona Pellucida Interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Que, E.L.; Duncan, F.E.; Bayer, A.R.; Philips, S.J.; Roth, E.W.; Bleher, R.; Gleber, S.C.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. 2017, 9, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonk, A.J.; Cheong, H.T.; Li, R.; Lai, L.; Hao, Y.; Liu, Z.; Samuel, M.; Fergason, E.A.; Whitworth, K.M.; Murphy, C.N.; et al. Correlation of developmental differences of nuclear transfer embryos cells to the methylation profiles of nuclear transfer donor cells in Swine. Epigenetics 2007, 2, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonk, A.J.; Li, R.; Lai, L.; Hao, Y.; Liu, Z.; Samuel, M.; Fergason, E.A.; Whitworth, K.M.; Murphy, C.N.; Antoniou, E.; et al. Aberrant DNA methylation in porcine in vitro-, parthenogenetic-, and somatic cell nuclear transfer-produced blastocysts. Mol. Reprod. Dev. 2008, 75, 250–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.; Canovas, S.; Garcia-Martinez, S.; Romar, R.; Lopes, J.S.; Rizos, D.; Sanchez-Calabuig, M.J.; Krueger, F.; Andrews, S.; Perez-Sanz, F.; et al. DNA methylation changes during preimplantation development reveal inter-species differences and reprogramming events at imprinted genes. Clin. Epigenetics 2020, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Jost, J.P.; Saluz, H.P.; McEwan, I.; Feavers, I.M.; Hughes, M.; Reiber, S.; Liang, H.M.; Vaccaro, M. Tissue specific expression of avian vitellogenin gene is correlated with DNA hypomethylation and in vivo specific protein-DNA interactions. Philos Trans. R Soc. Lond. B Biol. Sci. 1990, 326, 231–240. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Horikoshi, S.; Yamada, Y. DNA methylation and collagen IV gene expression in F9 teratocarcinoma cells. J. Biol. Chem. 1990, 265, 4839–4843. [Google Scholar] [CrossRef]
- Knust, B.; Bruggemann, U.; Doerfler, W. Reactivation of a methylation-silenced gene in adenovirus-transformed cells by 5-azacytidine or by E1A trans activation. J. Virol. 1989, 63, 3519–3524. [Google Scholar] [CrossRef] [Green Version]
- Gerondakis, S.; Boyd, A.; Bernard, O.; Webb, E.; Adams, J.M. Activation of immunoglobulin mu gene expression involves stepwise demethylation. EMBO J. 1984, 3, 3013–3021. [Google Scholar] [CrossRef]
- Zhao, J.; Ross, J.W.; Hao, Y.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol. Reprod. 2009, 81, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Jeong, P.S.; Sim, B.W.; Park, S.H.; Kim, M.J.; Kang, H.G.; Nanjidsuren, T.; Lee, S.; Song, B.S.; Koo, D.B.; Kim, S.U. Chaetocin Improves Pig Cloning Efficiency by Enhancing Epigenetic Reprogramming and Autophagic Activity. Int. J. Mol. Sci 2020, 21, 4836. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, J.; Zhou, R.; He, X.; Yang, H.; Wu, Z. DZNep and UNC0642 enhance in vitro developmental competence of cloned pig embryos. Reproduction 2018, 157, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Qi, X.; Zhang, L.; Ning, W.; Gao, D.; Xu, T.; Ma, Y.; Knott, J.G.; Sathanawongs, A.; Cao, Z.; et al. Dynamic reprogramming and function of RNA N(6)-methyladenosine modification during porcine early embryonic development. Zygote 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Whyte, J.; Prather, R.S. Effect of epigenetic regulation during swine embryogenesis and on cloning by nuclear transfer. Cell Tissue Res. 2010, 341, 13–21. [Google Scholar] [CrossRef]
- Li, G.; Jia, Q.; Zhao, J.; Li, X.; Yu, M.; Samuel, M.S.; Zhao, S.; Prather, R.S.; Li, C. Dysregulation of genome-wide gene expression and DNA methylation in abnormal cloned piglets. BMC Genom. 2014, 15, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitworth, K.M.; Mao, J.; Lee, K.; Spollen, W.G.; Samuel, M.S.; Walters, E.M.; Spate, L.D.; Prather, R.S. Transcriptome Analysis of Pig In Vivo, In Vitro-Fertilized, and Nuclear Transfer Blastocyst-Stage Embryos Treated with Histone Deacetylase Inhibitors Postfusion and Activation Reveals Changes in the Lysosomal Pathway. Cell. Reprogram 2015, 17, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Zhao, M.T.; Whitworth, K.M.; Spate, L.D.; Walters, E.M.; O’Gorman, C.; Lee, K.; Samuel, M.S.; Murphy, C.N.; Wells, K.; et al. Oxamflatin treatment enhances cloned porcine embryo development and nuclear reprogramming. Cell. Reprogram 2015, 17, 28–40. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.R.; Redel, B.K.; Kerns, K.C.; Spate, L.D.; Prather, R.S. Challenges and Considerations during In Vitro Production of Porcine Embryos. Cells 2021, 10, 2770. https://doi.org/10.3390/cells10102770
Chen PR, Redel BK, Kerns KC, Spate LD, Prather RS. Challenges and Considerations during In Vitro Production of Porcine Embryos. Cells. 2021; 10(10):2770. https://doi.org/10.3390/cells10102770
Chicago/Turabian StyleChen, Paula R., Bethany K. Redel, Karl C. Kerns, Lee D. Spate, and Randall S. Prather. 2021. "Challenges and Considerations during In Vitro Production of Porcine Embryos" Cells 10, no. 10: 2770. https://doi.org/10.3390/cells10102770
APA StyleChen, P. R., Redel, B. K., Kerns, K. C., Spate, L. D., & Prather, R. S. (2021). Challenges and Considerations during In Vitro Production of Porcine Embryos. Cells, 10(10), 2770. https://doi.org/10.3390/cells10102770





