Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Plasmids and Molecular Cloning
2.3. Expression and Purification of Gαo and Gαi1 Wild-Type and Mutant Proteins
2.4. GTP Binding and Hydrolysis Assays
2.5. Antibodies and Reagents
2.6. Cell Line and Culture Conditions
2.7. Co-Immunoprecipitation
2.8. Immunofluorescence and Microscopy
3. Results
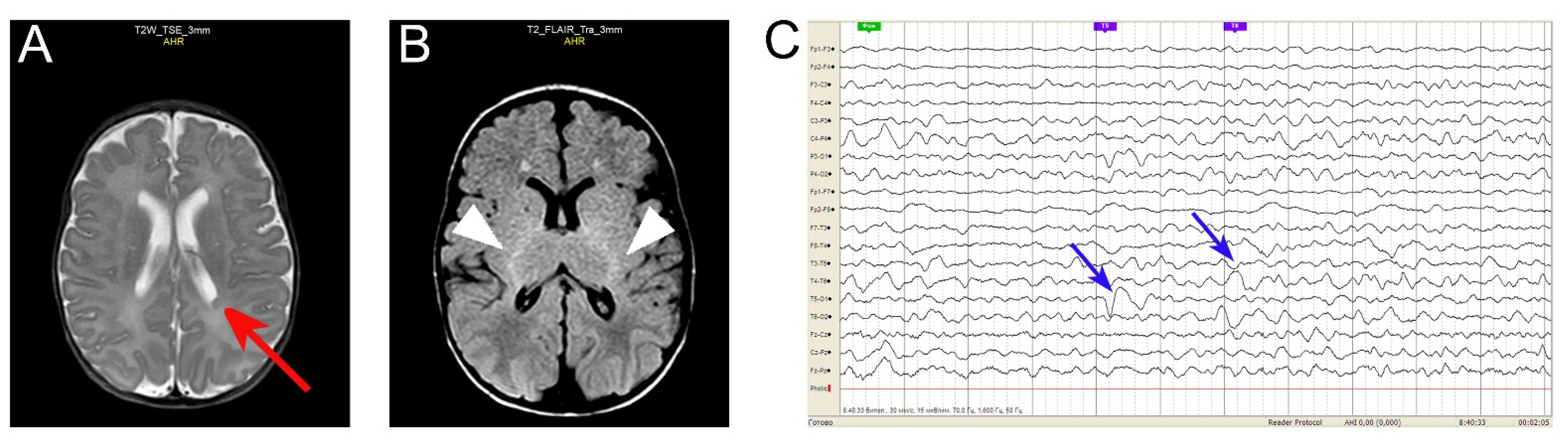
3.1. Case Report: A Gln52Arg GNAO1 Pediatric Encephalopathy Patient
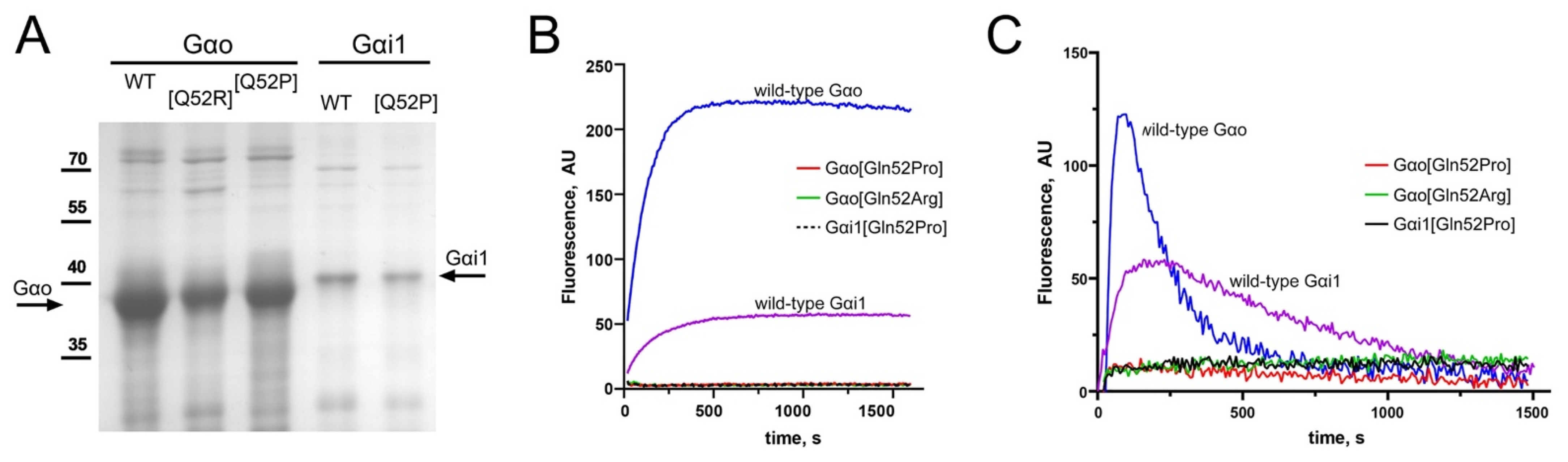
3.2. Biochemical Characterization: Gαo[Gln52Pro], Gαi1[Gln52Pro], and the Novel Gαo[Gln52Arg] Mutants Are Devoid of the Basal GTP Binding Activity
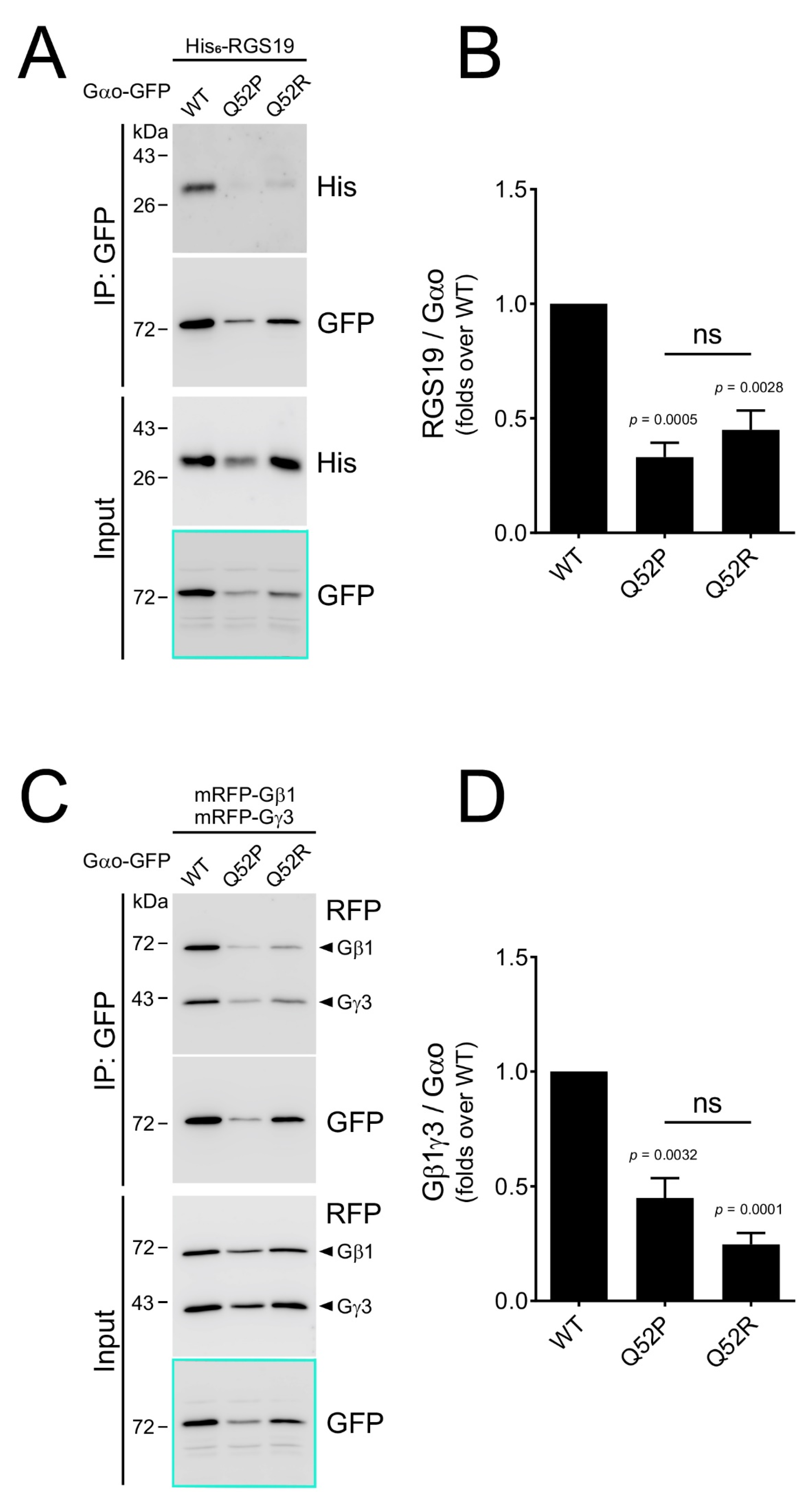
3.3. Cellular Characterization: Gln52 Mutant Proteins Are Deficient in Interaction with Gαo Partner Proteins
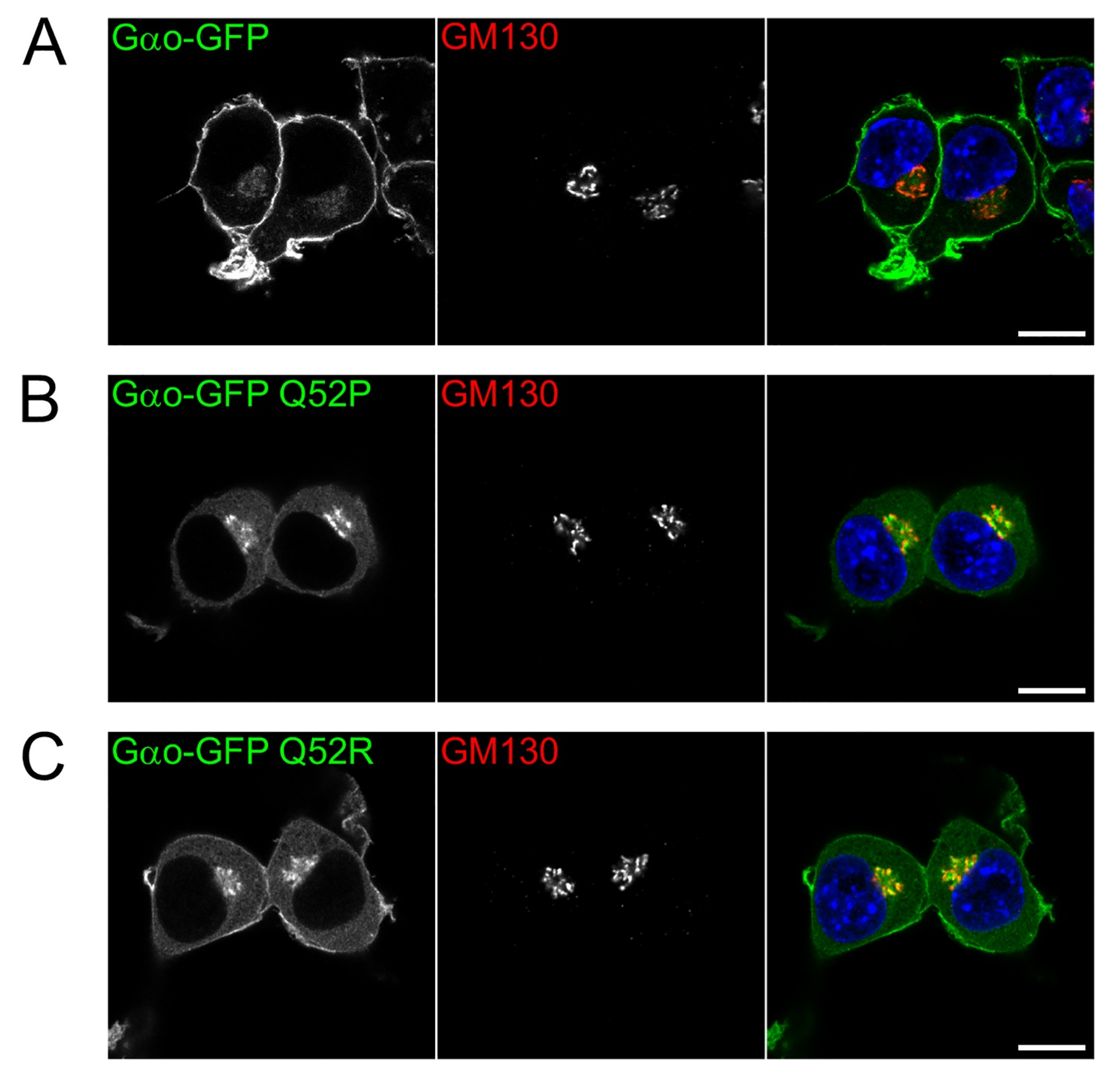
3.4. Subcellular Localization: Severe Loss of Plasma Membrane but Not Golgi Staining by the Gln52 Mutant Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef]
- Lin, C.; Koval, A.; Tishchenko, S.; Gabdulkhakov, A.; Tin, U.; Solis, G.P.; Katanaev, V.L. Double suppression of the Galpha protein activity by RGS proteins. Mol. Cell 2014, 53, 663–671. [Google Scholar] [CrossRef]
- Milligan, G.; Kostenis, E. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S46–S55. [Google Scholar] [CrossRef]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef]
- Katanaev, V.L.; Ponzielli, R.; Semeriva, M.; Tomlinson, A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell 2005, 120, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Koval, A.; Katanaev, V.L. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem. J. 2011, 433, 435–440. [Google Scholar] [CrossRef]
- Luchtenborg, A.M.; Solis, G.P.; Egger-Adam, D.; Koval, A.; Lin, C.; Blanchard, M.G.; Kellenberger, S.; Katanaev, V.L. Heterotrimeric Go protein links Wnt-Frizzled signaling with ankyrins to regulate the neuronal microtubule cytoskeleton. Development 2014, 141, 3399–3409. [Google Scholar] [CrossRef]
- Koval, A.; Ahmed, K.; Katanaev, V.L. Inhibition of Wnt signalling and breast tumour growth by the multi-purpose drug suramin through suppression of heterotrimeric G proteins and Wnt endocytosis. Biochem. J. 2016, 473, 371–381. [Google Scholar] [CrossRef]
- Sternweis, P.C.; Robishaw, J.D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J. Biol. Chem. 1984, 259, 13806–13813. [Google Scholar] [CrossRef]
- Wolfgang, W.J.; Quan, F.; Goldsmith, P.; Unson, C.; Spiegel, A.; Forte, M. Immunolocalization of G protein alpha-subunits in the Drosophila CNS. J. Neurosci. 1990, 10, 1014–1024. [Google Scholar] [CrossRef]
- Bromberg, K.D.; Iyengar, R.; He, J.C. Regulation of neurite outgrowth by G(i/o) signaling pathways. Front. Biosci. 2008, 13, 4544–4557. [Google Scholar] [CrossRef]
- Jiang, M.; Gold, M.S.; Boulay, G.; Spicher, K.; Peyton, M.; Brabet, P.; Srinivasan, Y.; Rudolph, U.; Ellison, G.; Birnbaumer, L. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc. Natl. Acad. Sci. USA 1998, 95, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Greif, G.J.; Sodickson, D.L.; Bean, B.P.; Neer, E.J.; Mende, U. Altered regulation of potassium and calcium channels by GABA(B) and adenosine receptors in hippocampal neurons from mice lacking Galpha(o). J. Neurophysiol. 2000, 83, 1010–1018. [Google Scholar] [CrossRef][Green Version]
- Nakamura, K.; Kodera, H.; Akita, T.; Shiina, M.; Kato, M.; Hoshino, H.; Terashima, H.; Osaka, H.; Nakamura, S.; Tohyama, J.; et al. De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am. J. Hum. Genet. 2013, 93, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Garone, G.; Travaglini, L.; Vasco, G.; Galosi, S.; Rios, L.; Castiglioni, C.; Barassi, C.; Battaglia, D.; Gambardella, M.L.; et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat. Disord. 2019, 61, 19–25. [Google Scholar] [CrossRef]
- Morrison-Levy, N.; Borlot, F.; Jain, P.; Whitney, R. Early-Onset Developmental and Epileptic Encephalopathies of Infancy: An Overview of the Genetic Basis and Clinical Features. Pediatr. Neurol. 2021, 116, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, D.; Thurman, D.J.; Gwinn-Hardy, K.; Mohamed, M.; Chaudhuri, A.R.; Zalutsky, R. How common are the “common” neurologic disorders? Neurology 2007, 68, 326–337. [Google Scholar] [CrossRef]
- Kerr, M.P. The impact of epilepsy on patients’ lives. Acta Neurol. Scand. Suppl. 2012, 126, 1–9. [Google Scholar] [CrossRef]
- Kehrl, J.M.; Sahaya, K.; Dalton, H.M.; Charbeneau, R.A.; Kohut, K.T.; Gilbert, K.; Pelz, M.C.; Parent, J.; Neubig, R.R. Gain-of-function mutation in Gnao1: A murine model of epileptiform encephalopathy (EIEE17)? Mamm. Genome Off. J. Int. Mamm. Genome Soc. 2014, 25, 202–210. [Google Scholar] [CrossRef][Green Version]
- Savitsky, M.; Solis, G.P.; Kryuchkov, M.; Katanaev, V.L. Humanization of Drosophila Gαo to Model GNAO1 Paediatric Encephalopathies. Biomedicines 2020, 8, 395. [Google Scholar] [CrossRef]
- Muntean, B.S.; Masuho, I.; Dao, M.; Sutton, L.P.; Zucca, S.; Iwamoto, H.; Patil, D.N.; Wang, D.; Birnbaumer, L.; Blakely, R.D.; et al. Gαo is a major determinant of cAMP signaling in the pathophysiology of movement disorders. Cell Rep. 2021, 34, 108718. [Google Scholar] [CrossRef] [PubMed]
- Pineda, V.V.; Athos, J.I.; Wang, H.; Celver, J.; Ippolito, D.; Boulay, G.; Birnbaumer, L.; Storm, D.R. Removal of G(ialpha1) constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron 2004, 41, 153–163. [Google Scholar] [CrossRef]
- Muir, A.M.; Gardner, J.F.; van Jaarsveld, R.H.; de Lange, I.M.; van der Smagt, J.J.; Wilson, G.N.; Dubbs, H.; Goldberg, E.M.; Zitano, L.; Bupp, C.; et al. Variants in GNAI1 cause a syndrome associated with variable features including developmental delay, seizures, and hypotonia. Genet. Med. 2021, 23, 881–887. [Google Scholar] [CrossRef]
- Rim, J.H.; Kim, S.H.; Hwang, I.S.; Kwon, S.S.; Kim, J.; Kim, H.W.; Cho, M.J.; Ko, A.; Youn, S.E.; Kim, J.; et al. Efficient strategy for the molecular diagnosis of intractable early-onset epilepsy using targeted gene sequencing. BMC Med. Genom. 2018, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, I.; Gautam, N. A fluorescence resonance energy transfer-based sensor indicates that receptor access to a G protein is unrestricted in a living mammalian cell. J. Biol. Chem. 2004, 279, 27709–27718. [Google Scholar] [CrossRef]
- Thaler, C.; Koushik, S.V.; Blank, P.S.; Vogel, S.S. Quantitative multiphoton spectral imaging and its use for measuring resonance energy transfer. Biophys. J. 2005, 89, 2736–2749. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.P.; Bilousov, O.; Koval, A.; Luchtenborg, A.M.; Lin, C.; Katanaev, V.L. Golgi-Resident Galphao Promotes Protrusive Membrane Dynamics. Cell 2017, 170, 939–955. [Google Scholar] [CrossRef]
- Egger-Adam, D.; Katanaev, V.L. The trimeric G protein Go inflicts a double impact on axin in the Wnt/frizzled signaling pathway. Dev. Dyn. 2010, 239, 168–183. [Google Scholar] [CrossRef]
- Koval, A.; Kopein, D.; Purvanov, V.; Katanaev, V.L. Europium-labeled GTP as a general nonradioactive substitute for [(35)S]GTPgammaS in high-throughput G protein studies. Anal. Biochem. 2010, 397, 202–207. [Google Scholar] [CrossRef]
- Katoh, Y.; Nozaki, S.; Hartanto, D.; Miyano, R.; Nakayama, K. Architectures of multisubunit complexes revealed by a visible immunoprecipitation assay using fluorescent fusion proteins. J. Cell Sci. 2015, 128, 2351–2362. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Purvanov, V.; Koval, A.; Katanaev, V.L. A direct and functional interaction between Go and Rab5 during G protein-coupled receptor signaling. Sci. Signal. 2010, 3, ra65. [Google Scholar] [CrossRef] [PubMed]
- Kopein, D.; Katanaev, V.L. Drosophila GoLoco-protein pins is a target of Galpha(o)-mediated G protein-coupled receptor signaling. Mol. Biol. Cell 2009, 20, 3865–3877. [Google Scholar] [CrossRef]
- Seguin, L.; Odouard, S.; Corlazzoli, F.; Haddad, S.A.; Moindrot, L.; Calvo Tardón, M.; Yebra, M.; Koval, A.; Marinari, E.; Bes, V.; et al. Macropinocytosis requires Gal-3 in a subset of patient-derived glioblastoma stem cells. Commun. Biol. 2021, 4, 718. [Google Scholar] [CrossRef]
- Chinn, I.K.; Xie, Z.; Chan, E.C.; Nagata, B.M.; Koval, A.; Chen, W.-S.; Zhang, F.; Ganesan, S.; Hong, D.N.; Suzuki, M.; et al. Short stature and combined immunodeficiency associated with mutations in RGS10. Sci. Signal. 2021, 14, eabc1940. [Google Scholar] [CrossRef]
- Katanayeva, N.; Kopein, D.; Portmann, R.; Hess, D.; Katanaev, V.L. Competing activities of heterotrimeric G proteins in Drosophila wing maturation. PLoS ONE 2010, 5, e12331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Solis, G.P.; Katanaev, V.L. Galphao (GNAO1) encephalopathies: Plasma membrane vs. Golgi functions. Oncotarget 2018, 9, 23846–23847. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Liao, J. Deciphering the concepts behind “Epileptic encephalopathy” and “Developmental and epileptic encephalopathy”. Eur. J. Paediatr. Neurol. 2020, 24, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Dzinovic, I.; Škorvánek, M.; Necpál, J.; Boesch, S.; Švantnerová, J.; Wagner, M.; Havránková, P.; Pavelekova, P.; Haň, V.; Janzarik, W.G.; et al. Dystonia as a prominent presenting feature in developmental and epileptic encephalopathies: A case series. Parkinsonism Relat. Disord. 2021, 90, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Degtyarev, M.Y.; Spiegel, A.M.; Jones, T.L. Palmitoylation of a G protein alpha i subunit requires membrane localization not myristoylation. J. Biol. Chem. 1994, 269, 30898–30903. [Google Scholar] [CrossRef]
- Fishburn, C.S.; Herzmark, P.; Morales, J.; Bourne, H.R. Gβγ and Palmitate Target Newly Synthesized Gαzto the Plasma Membrane. J. Biol. Chem. 1999, 274, 18793–18800. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Manning, D.R. Regulation of G proteins by covalent modification. Oncogene 2001, 20, 1643–1652. [Google Scholar] [CrossRef]
- Solis, G.P.; Kazemzadeh, A.; Valnohova, J.; Abrami, L.; Alvarez, C.; van der Goot, F.G.; Katanaev, V.L. Local and substrate-specific S-palmitoylation determines subcellular localization of Gαo. bioRxiv 2020. preprint: 2020.08.25.266692. [Google Scholar] [CrossRef]
- Huang, Y.; Thathiah, A. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 2015, 589, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Larasati, Y.; Savitsky, M.; Koval, A.; Solis, G.P.; Katanaev, V.L. Restoration of the GTPase activity of Gαo mutants by Zn2+ in GNAO1 encephalopathy models. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Larrivee, C.L.; Feng, H.; Quinn, J.A.; Shaw, V.S.; Leipprandt, J.R.; Demireva, E.Y.; Xie, H.; Neubig, R.R. Mice with GNAO1 R209H Movement Disorder Variant Display Hyperlocomotion Alleviated by Risperidone. J. Pharmacol. Exp. Ther. 2020, 373, 24–33. [Google Scholar] [CrossRef]
- Knight, K.M.; Ghosh, S.; Campbell, S.L.; Lefevre, T.J.; Olsen, R.H.J.; Smrcka, A.V.; Valentin, N.H.; Yin, G.; Vaidehi, N.; Dohlman, H.G. A universal allosteric mechanism for G protein activation. Mol. Cell 2021, 81, 1384–1396. [Google Scholar] [CrossRef]
| Patient Characteristics | GNAO1 Gln52Pro * | GNAI1 Gln52Pro | GNAO1 Gln52Arg |
|---|---|---|---|
| Gender | not reported | Male | Male |
| Mutation | c.155A > C | c.155A > C | c.155A > G |
| Inheritance | de novo | de novo | de novo |
| Neurodevelopment and neurological features | not reported | Severe intellectual disability, autism spectrum disorder, hypotonia, lower limb hypertonia | Severe developmental delay **, dystonia in limbs |
| Extra neurological findings | not reported | Mild tricuspid regurgitation, severe constipation, asthma | Sleep disorders |
| Epilepsy | Onset <3 years, spasms | Onset age 6 years, | Onset age 1.5 weeks, focal spasms |
| EEG | Multifocal epileptiform discharges with slow background activity | Focal right posterior slowing | Multifocal epileptic activity (1 month) |
| Anti-seizure medication | not reported | Valproic acid | Valproic acid and levetiracetam. |
| MRI | Normal | Enlarged pericerebral spaces. Fronto-temporal atrophy (1 year), persistent at 3 years | Enlarged pericerebral spaces and ventricles. Left posterior periventricular nodular heterotopia (PVNH). PLIC (posterior limb internal capsulae) hyperintensity (38 days). Immature myelinization (32 months) |
| Reference | Rim et al., 2018 [24] * | Muir et al., 2021 [23] | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis, G.P.; Kozhanova, T.V.; Koval, A.; Zhilina, S.S.; Mescheryakova, T.I.; Abramov, A.A.; Ishmuratov, E.V.; Bolshakova, E.S.; Osipova, K.V.; Ayvazyan, S.O.; et al. Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease. Cells 2021, 10, 2749. https://doi.org/10.3390/cells10102749
Solis GP, Kozhanova TV, Koval A, Zhilina SS, Mescheryakova TI, Abramov AA, Ishmuratov EV, Bolshakova ES, Osipova KV, Ayvazyan SO, et al. Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease. Cells. 2021; 10(10):2749. https://doi.org/10.3390/cells10102749
Chicago/Turabian StyleSolis, Gonzalo P., Tatyana V. Kozhanova, Alexey Koval, Svetlana S. Zhilina, Tatyana I. Mescheryakova, Aleksandr A. Abramov, Evgeny V. Ishmuratov, Ekaterina S. Bolshakova, Karina V. Osipova, Sergey O. Ayvazyan, and et al. 2021. "Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease" Cells 10, no. 10: 2749. https://doi.org/10.3390/cells10102749
APA StyleSolis, G. P., Kozhanova, T. V., Koval, A., Zhilina, S. S., Mescheryakova, T. I., Abramov, A. A., Ishmuratov, E. V., Bolshakova, E. S., Osipova, K. V., Ayvazyan, S. O., Lebon, S., Kanivets, I. V., Pyankov, D. V., Troccaz, S., Silachev, D. N., Zavadenko, N. N., Prityko, A. G., & Katanaev, V. L. (2021). Pediatric Encephalopathy: Clinical, Biochemical and Cellular Insights into the Role of Gln52 of GNAO1 and GNAI1 for the Dominant Disease. Cells, 10(10), 2749. https://doi.org/10.3390/cells10102749









