Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation
Abstract
:1. Introduction
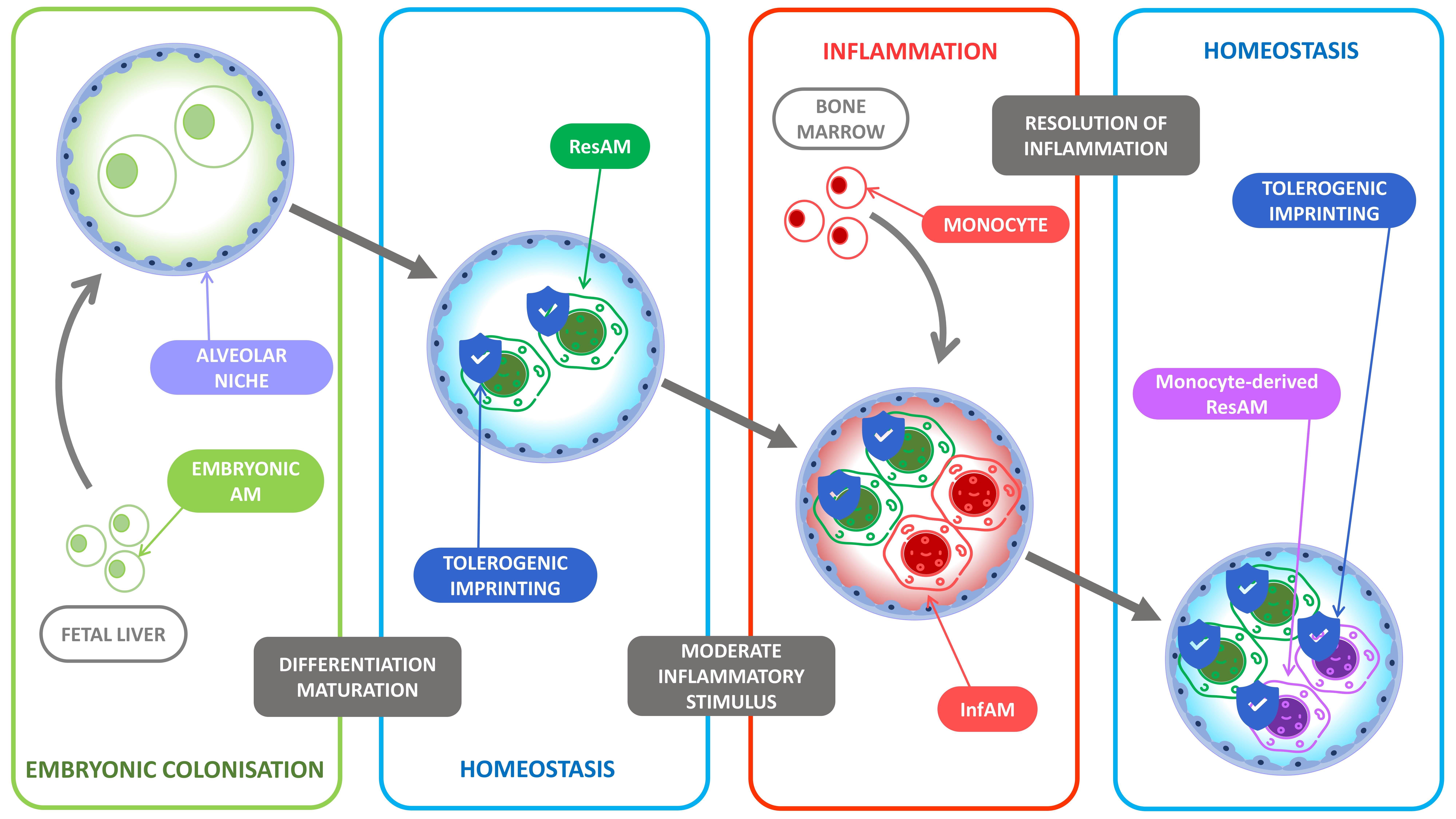
2. Resident Alveolar Macrophages Ontogeny
3. Maintenance of the Alveolar Macrophage Niche during Homeostasis
4. The Role of Alveolar Macrophages to Maintain Lung Homeostasis
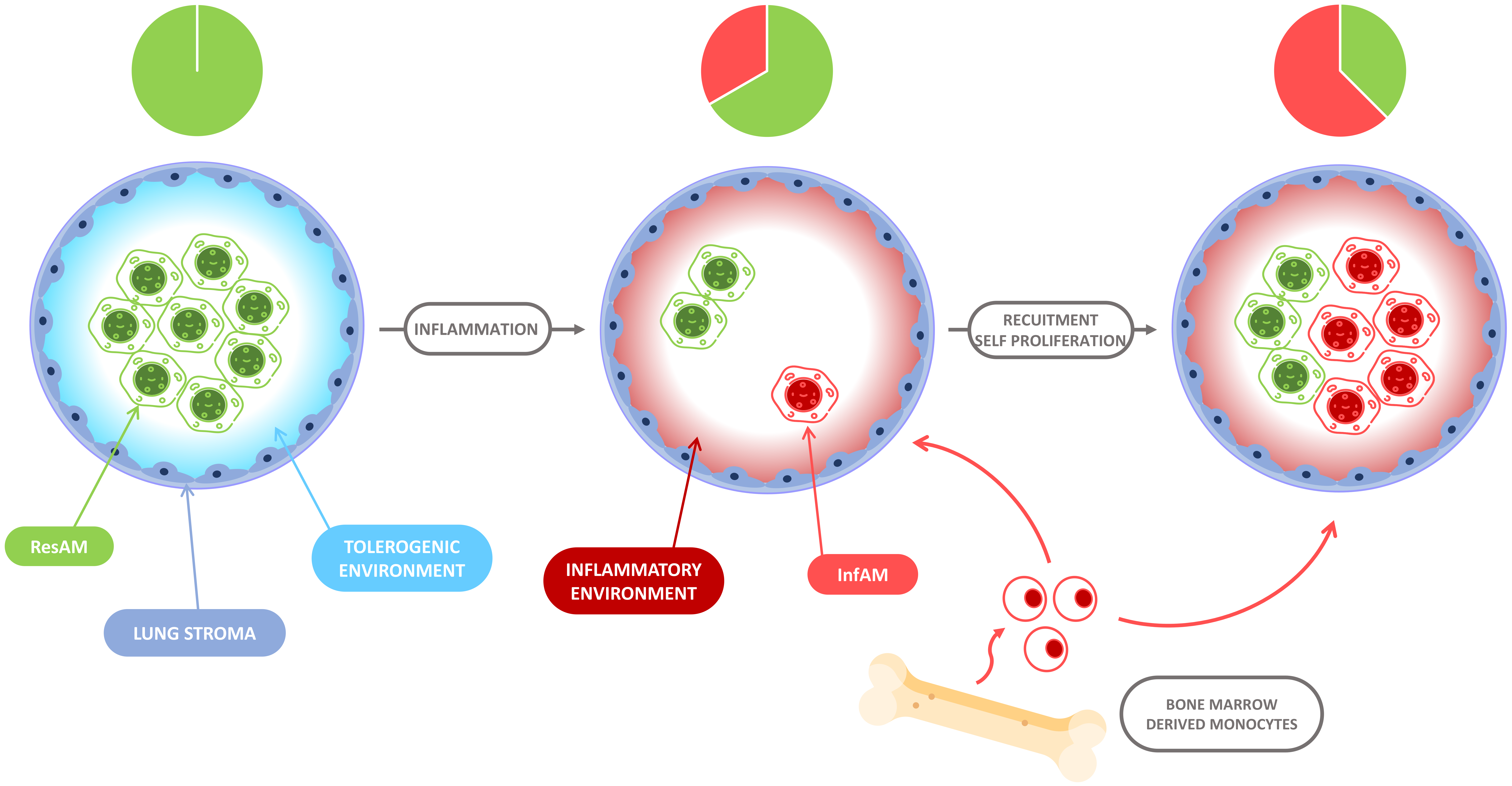
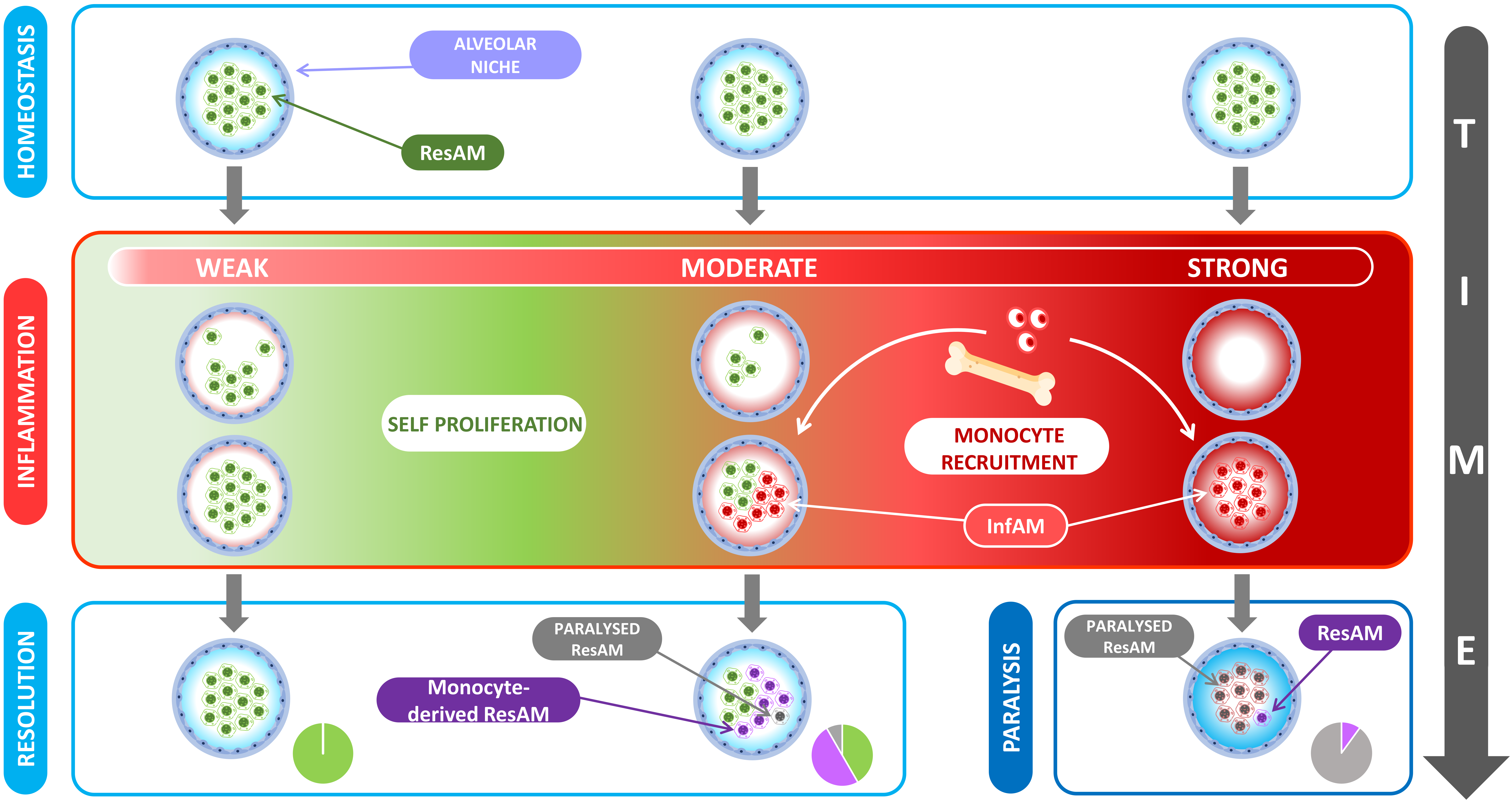
5. The Evolution of Alveolar Macrophage Niche Complexity during Infection and Inflammation
6. Differences between ResAM and InfAM during Inflammation
7. Tissue Repair and Fibrosis
8. The Immunologic Scar Left over by Inflammation: Friend of Foe?
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Charlson, E.S.; Bittinger, K.; Haas, A.R.; Fitzgerald, A.S.; Frank, I.; Yadav, A.; Bushman, F.; Collman, R.G. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 2011, 184, 957–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dranoff, G.; Crawford, A.; Sadelain, M.; Ream, B.; Rashid, A.; Bronson, R.; Dickersin, G.; Bachurski, C.; Mark, E.; Whitsett, J.; et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994, 264, 713–716. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J.; Allen, J.; Biswas, S.K.; Fisher, E.; Gilroy, D.; Goerdt, S.; Gordon, S.; A Hamilton, J.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Guilliams, M.; De Kleer, I.; Henri, S.; Post, S.; Vanhoutte, L.; De Prijck, S.; Deswarte, K.; Malissen, B.; Hammad, H.; Lambrecht, B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013, 210, 1977–1992. [Google Scholar] [CrossRef] [Green Version]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; de Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb + erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012, 42, 3150–3166. [Google Scholar] [CrossRef]
- Gschwend, J.; Sherman, S.P.; Ridder, F.; Feng, X.; Liang, H.-E.; Locksley, R.M.; Becher, B.; Schneider, C. Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J. Exp. Med. 2021, 218, e20210745. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; O Kurrer, M.; Rehrauer, H.; Thiele, C.; Kopf, M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014, 15, 1026–1037. [Google Scholar] [CrossRef]
- Yu, X.; Buttgereit, A.; Lelios, I.; Utz, S.G.; Cansever, D.; Becher, B.; Greter, M. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity 2017, 47, 903–912.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef] [Green Version]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Grifffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Raz, E. Organ-specific regulation of innate immunity. Nat. Immunol. 2007, 8, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Friendly and dangerous signals: Is the tissue in control? Nat. Immunol. 2007, 8, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Chikina, A.S.; Nadalin, F.; Maurin, M.; San-Roman, M.; Thomas-Bonafos, T.; Li, X.V.; Lameiras, S.; Baulande, S.; Henri, S.; Malissen, B.; et al. Macrophages maintain epithelium integrity by limiting fungal product absorption. Cell 2020, 183, 411–428.e16. [Google Scholar] [CrossRef] [PubMed]
- Neupane, A.S.; Willson, M.; Chojnacki, A.K.; Castanheira, F.V.E.S.; Morehouse, C.; Carestia, A.; Keller, A.E.; Peiseler, M.; DiGiandomenico, A.; Kelly, M.M.; et al. Patrolling alveolar macrophages conceal bacteria from the immune system to maintain homeostasis. Cell 2020, 183, 110–125.e11. [Google Scholar] [CrossRef] [PubMed]
- Chelen, C.J.; Fang, Y.; Freeman, G.J.; Secrist, H.; Marshall, J.D.; Hwang, P.T.; Frankel, L.R.; DeKruyff, R.H.; Umetsu, D.T. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J. Clin. Investig. 1995, 95, 1415–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, R.L.; Campbell, D.E.; Hwang, P.; DeKruyff, R.H.; Frankel, L.R.; Umetsu, D.T. Human alveolar macrophages induce functional inactivation in antigen-specific CD4 T cells. J. Allergy Clin. Immunol. 2001, 107, 258–264. [Google Scholar] [CrossRef]
- Snyder, M.E.; Sembrat, J.; Noda, K.; Myerburg, M.M.; Craig, A.; Mitash, N.; Harano, T.; Furukawa, M.; Pilewski, J.; McDyer, J.; et al. Human lung-resident macrophages colocalize with and provide costimulation to PD1hi tissue-resident memory T cells. Am. J. Respir. Crit. Care Med. 2021, 203, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Gautier, E.; the Immunological Genome Consortium; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilliams, M.; Svedberg, F.R. Does tissue imprinting restrict macrophage plasticity? Nat. Immunol. 2021, 22, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Gibbings, S.L.; Goyal, R.; Desch, A.N.; Leach, S.M.; Prabagar, M.; Atif, S.M.; Bratton, D.L.; Janssen, W.J.; Jakubzick, C.V. Transcriptome analysis highlights the conserved difference between embryonic and postnatal-derived alveolar macrophages. Blood 2015, 126, 1357–1366. [Google Scholar] [CrossRef]
- Sieweke, M.H. Waddington’s valleys and Captain Cook’s islands. Cell Stem Cell 2015, 16, 7–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilliams, M.; Scott, C.L. Does niche competition determine the origin of tissue-resident macrophages? Nat. Rev. Immunol. 2017, 17, 451–460. [Google Scholar] [CrossRef]
- Liu, M.; O’Connor, R.S.; Trefely, S.; Graham, K.; Snyder, N.W.; Beatty, G.L. Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal. Nat. Immunol. 2019, 20, 265–275. [Google Scholar] [CrossRef]
- Snelgrove, R.J.; Goulding, J.; Didierlaurent, A.M.; Lyonga, D.; Vekaria, S.; Edwards, L.; Gwyer, E.; Sedgwick, J.D.; Barclay, A.N.; Hussell, T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008, 9, 1074–1083. [Google Scholar] [CrossRef]
- Mayer, A.K.; Bartz, H.; Fey, F.; Schmidt, L.M.; Dalpke, A.H. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur. J. Immunol. 2008, 38, 1689–1699. [Google Scholar] [CrossRef]
- Janssen, W.J.; McPhillips, K.A.; Dickinson, M.G.; Linderman, D.J.; Morimoto, K.; Xiao, Y.Q.; Oldham, K.M.; Vandivier, R.W.; Henson, P.M.; Gardai, S.J. Surfactant Proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRPα. Am. J. Respir. Crit. Care Med. 2008, 178, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.-A.; Gresham, H.D.; Lindberg, F.P. Cd47-signal regulatory protein α (Sirpα) regulates Fcγ and complement receptor–mediated phagocytosis. J. Exp. Med. 2001, 193, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, H.; Motegi, S.-I.; Ohyama, N.; Ohnishi, H.; Tomizawa, T.; Kaneko, Y.; Oldenborg, P.-A.; Ishikawa, O.; Matozaki, T. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J. Immunol. 2005, 174, 2004–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haniffa, M.; Ginhoux, F.; Wang, X.-N.; Bigley, V.; Abel, M.; Dimmick, I.; Bullock, S.; Grisotto, M.; Booth, T.; Taub, P.; et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J. Exp. Med. 2009, 206, 371–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mould, K.J.; Barthel, L.; Mohning, M.P.; Thomas, S.M.; McCubbrey, A.L.; Danhorn, T.; Leach, S.M.; Fingerlin, T.E.; O’Connor, B.P.; Reisz, J.A.; et al. Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am. J. Respir. Cell Mol. Biol. 2017, 57, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Mould, K.J.; Jackson, N.D.; Henson, P.M.; Seibold, M.; Janssen, W.J. Single cell RNA sequencing identifies unique inflammatory airspace macrophage subsets. JCI Insight 2019, 4, 126556. [Google Scholar] [CrossRef] [PubMed]
- Machiels, B.; Dourcy, M.; Xiao, X.; Javaux, J.; Mesnil, C.; Sabatel, C.; Desmecht, D.; Lallemand, F.; Martinive, P.; Hammad, H.; et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 2017, 18, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Kulikauskaite, J.; Crotta, S.; Patel, H.; Kelly, G.; Hessel, E.M.; Mack, M.; Beinke, S.; Wack, A. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 2020, 21, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; Van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef] [Green Version]
- Knapp, S.; Leemans, J.C.; Florquin, S.; Branger, J.; Maris, N.A.; Pater, J.; Van Rooijen, N.; Van Der Poll, T. Alveolar macrophages have a protective antiinflammatory role during murine Pneumococcal Pneumonia. Am. J. Respir. Crit. Care Med. 2003, 167, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archambaud, C.; Salcedo, S.; Lelouard, H.; Devilard, E.; De Bovis, B.; Van Rooijen, N.; Gorvel, J.-P.; Malissen, B. Contrasting roles of macrophages and dendritic cells in controlling initial pulmonary Brucella infection. Eur. J. Immunol. 2010, 40, 3458–3471. [Google Scholar] [CrossRef]
- Uderhardt, S.; Martins, A.J.; Tsang, J.S.; Lämmermann, T.; Germain, R.N. Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 2019, 177, 541–555.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, Y.; Takeuchi, O.; Kato, H.; Kumar, H.; Matsui, K.; Morii, E.; Aozasa, K.; Kawai, T.; Akira, S. Alveolar macrophages are the primary interferon-α producer in pulmonary infection with RNA viruses. Immunity 2007, 27, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Yang, C.; Fung, K.Y.; Bachem, A.; Bourges, D.; Bedoui, S.; Hartland, E.L.; Van Driel, I.R. Cooperation between Monocyte-derived Cells and Lymphoid Cells in the acute response to a bacterial lung pathogen. PLoS Pathog. 2016, 12, e1005691. [Google Scholar] [CrossRef]
- Askenase, M.H.; Han, S.-J.; Byrd, A.L.; da Fonseca, D.M.; Bouladoux, N.; Wilhelm, C.; Konkel, J.E.; Hand, T.W.; Lacerda-Queiroz, N.; Su, X.; et al. Bone-marrow-resident NK cells prime monocytes for regulatory function during infection. Immunity 2015, 42, 1130–1142. [Google Scholar] [CrossRef] [Green Version]
- Goritzka, M.; Makris, S.; Kausar, F.; Durant, L.; Pereira, C.; Kumagai, Y.; Culley, F.; Mack, M.; Akira, S.; Johansson, C. Alveolar macrophage–derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015, 212, 699–714. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Watanabe, S.; Verma, R.; Jabloski, R.P.; Chen, C.-I.; Cheresh, P.; Markov, N.S.; Reffman, P.A.; McQuattie-Pimentel, A.C.; Sichizya, L. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signalling in monocyte-derived alveolar macrophages. Eur. Respir. J. 2020, 55, 1900646. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.; Paine, R.; Christensen, P.J.; Moore, T.A.; Sitterding, S.; Ngan, R.; Wilke, C.A.; Kuziel, W.A.; Toews, G.B. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J. Immunol. 2001, 167, 4368–4377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, M.A.; MacKinnon, A.C.; Ramachandran, P.; Dhaliwal, K.; Duffin, R.; Phythian-Adams, A.; Van Rooijen, N.; Haslett, C.; Howie, S.; Simpson, J.; et al. Ly6ChiMonocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Polosukhin, V.V.; Degryse, A.L.; Newcomb, D.C.; Jones, B.R.; Ware, L.B.; Lee, J.W.; Loyd, J.; Blackwell, T.S.; Lawson, W.E. Intratracheal bleomycin causes airway remodeling and airflow obstruction in mice. Exp. Lung Res. 2012, 38, 135–146. [Google Scholar] [CrossRef]
- Tanjore, H.; Xu, X.C.; Polosukhin, V.V.; Degryse, A.L.; Li, B.; Han, W.; Sherrill, T.P.; Plieth, D.; Neilson, E.G.; Blackwell, T.S.; et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2009, 180, 657–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Reyfman, P.A.; Cuda, C.; Walter, J.M.; McQuattie-Pimentel, A.C.; Chen, C.-I.; Anekalla, K.; Joshi, N.; Williams, K.J.; et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017, 214, 2387–2404. [Google Scholar] [CrossRef] [Green Version]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Goplen, N.P.; Huang, S.; Zhu, B.; Cheon, I.S.; Son, Y.M.; Wang, Z.; Li, C.; Dai, Q.; Jiang, L.; Sun, J. Tissue-resident macrophages limit pulmonary CD8 resident memory T cell establishment. Front. Immunol. 2019, 10, 2332. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.; Schultze, J.L. Innate and adaptive immune memory: An evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Novakovic, B.; Habibi, E.; Wang, S.-Y.; Arts, R.J.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef] [Green Version]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Novakovic, B.; Li, Y.; Scicluna, B.P.; Gresnigt, M.; Arts, R.J.; Oosting, M.; Moorlag, S.J.; Groh, L.A.; Zwaag, J.; et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab. 2019, 29, 211–220.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roquilly, A.; McWilliam, H.E.; Jacqueline, C.; Tian, Z.; Cinotti, R.; Rimbert, M.; Wakim, L.; Caminschi, I.; Lahoud, M.; Belz, G.; et al. Local Modulation of Antigen-Presenting Cell Development after Resolution of Pneumonia Induces Long-Term Susceptibility to Secondary Infections. Immunity 2017, 47, 135–147.e5. [Google Scholar] [CrossRef] [Green Version]
- Roquilly, A.; Jacqueline, C.; Davieau, M.; Mollé, A.; Sadek, A.; Fourgeux, C.; Rooze, P.; Broquet, A.; Misme-Aucouturier, B.; Chaumette, T.; et al. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat. Immunol. 2020, 21, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jeyanathan, M.; Haddadi, S.; Barra, N.G.; Vaseghi-Shanjani, M.; Damjanovic, D.; Lai, R.; Afkhami, S.; Chen, Y.; Dvorkin-Gheva, A.; et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 2018, 175, 1634–1650. [Google Scholar] [CrossRef] [Green Version]
- McQuattie-Pimentel, A.C.; Ren, Z.; Joshi, N.; Watanabe, S.; Stoeger, T.; Chi, M.; Lu, Z.; Sichizya, L.; Aillon, R.P.; Chen, C.-I.; et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J. Clin. Investig. 2021, 131, 140299. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.J.; Bremmers, M.; van Crevel, R.; Händler, K.; Picelli, S.; et al. BCG Vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 2020, 28, 322–334.e5. [Google Scholar] [CrossRef]
- Arts, R.J.; Moorlag, S.J.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.; et al. BCG vaccination protects against experimental viral infection in humans through the induction of Cytokines associated with trained immunity. Cell Host Microbe 2018, 23, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.-C.; et al. BCG educates hematopoietic stem cells to generate protective innate immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.-S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018, 172, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.-J.; Harrison, O.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.T.; Byrd, A.L.; Deming, C.; et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, R.S.; Sherwood, E.R. Getting sepsis therapy right. Science 2015, 347, 1201–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in Patients Who Die of Sepsis and Multiple Organ Failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- van Vught, L.A.; Klouwenberg, P.M.C.K.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nurnberg, P.; Bonten, M.J.M.; Cremer, O.L.; et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 2016, 315, 1469–1479. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Kreutmair, S.; Unger, S.; Nunez, N.G.; Ingelfinger, F.; Alberti, C.; De Feo, D.; Krishnarajah, S.; Kauffmann, M.; Friebel, E.; Babei, S.; et al. Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity 2021, 54, 1578–1593.e5. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Krone, B.; Kölmel, K.F.; Grange, J.M. The biography of the immune system and the control of cancer: From St Peregrine to contemporary vaccination strategies. BMC Cancer 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roquilly, A.; Torres, A.; Villadangos, J.; Netea, M.G.; Dickson, R.; Becher, B.; Asehnoune, K. Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir. Med. 2019, 7, 710–720. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Domínguez-Andrés, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.-E.; Damoraki, G.; et al. Activate: Randomized clinical trial of BCG vaccination against infection in the Elderly. Cell 2020, 183, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Pribul, P.K.; Harker, J.; Wang, B.; Wang, H.; Tregoning, J.; Schwarze, J.; Openshaw, P.J.M. Alveolar Macrophages Are a Major Determinant of Early Responses to Viral Lung Infection but Do Not Influence Subsequent Disease Development. J. Virol. 2008, 82, 4441–4448. [Google Scholar] [CrossRef] [Green Version]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.; Romero, P.; et al. Type I interferon inhibits Interleukin-1 production and inflammasome activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, F.P.; Jacqueline, C.; Poschmann, J.; Roquilly, A. Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation. Cells 2021, 10, 2720. https://doi.org/10.3390/cells10102720
Martin FP, Jacqueline C, Poschmann J, Roquilly A. Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation. Cells. 2021; 10(10):2720. https://doi.org/10.3390/cells10102720
Chicago/Turabian StyleMartin, Florian Pierre, Cédric Jacqueline, Jeremie Poschmann, and Antoine Roquilly. 2021. "Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation" Cells 10, no. 10: 2720. https://doi.org/10.3390/cells10102720
APA StyleMartin, F. P., Jacqueline, C., Poschmann, J., & Roquilly, A. (2021). Alveolar Macrophages: Adaptation to Their Anatomic Niche during and after Inflammation. Cells, 10(10), 2720. https://doi.org/10.3390/cells10102720





