Vascular Endothelial Cells: Heterogeneity and Targeting Approaches
Abstract
1. Overview
2. Physiological Endothelial Heterogeneity
2.1. Endothelial Heterogeneity along the Vascular Tree
2.2. Endothelial Heterogeneity within the Capillary Network
2.2.1. Continuous Endothelium
2.2.2. Fenestrated Endothelium
2.2.3. Discontinuous Endothelium
2.2.4. High Endothelial Venules
3. Endothelial Heterogeneity in Response to Stress
3.1. Inflammation
3.2. Ischemia/Hypoxia
3.3. Cancer
4. Selective Targeting of Distinct EC Subpopulations
4.1. EC Targeting by Antibodies and Nanobodies
4.2. EC Targeting by Phage-Displayed Peptides
4.3. EC Targeting by Viral Vectors
4.3.1. Adenoviral Targeting of ECs
4.3.2. Retroviral Targeting of ECs
4.3.3. Adeno-Associated Viral Targeting of ECs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| ACE | angiotensin converting enzyme |
| AMD | age-related macular degeneration |
| BBB | blood–brain barrier |
| bFGF | basic fibroblast growth factor |
| BMAEC(s) | bone marrow arterial endothelial cell(s) |
| BMSEC(s) | bone marrow sinusoidal endothelial cell(s) |
| cREC(s) | cortical renal endothelial cell(s) |
| EC | endothelial cell(s) |
| eNOS | endothelial nitric oxide synthase |
| gCap(s) | general capillary cell(s) |
| gREC(s) | glomeruli renal endothelial cell(s) |
| GVB | gut vascular barrier |
| HAEC(s) | human aortic endothelial cell(s) |
| HCAEC(s) | human coronary artery endothelial cell(s) |
| HEV(s) | high endothelial venule(s) |
| HIF | hypoxia-induced factor |
| HSVEC(s) | human saphenous vein endothelial cell(s) |
| HUVEC(s) | human umbilical vein endothelial cell(s) |
| MAdCAM | mucosal vascular addressin cell adhesion molecule |
| MLV | murine leukemia virus |
| mREC(s) | medulla renal endothelial cell(s) |
| NO | nitric oxide |
| PAEC(s) | porcine aortic endothelial cell(s) |
| PECAM-1 | platelet endothelial cell adhesion molecule 1 |
| PEG | polyethylene glycol |
| PVLAP | plasmalemmal vesicle-associated protein |
| REC(s) | renal endothelial cell(s) |
| VCAM1 | vascular cell adhesion molecule 1 |
| VEGF(R) | vascular endothelial growth factor (receptor) |
| VSV | vesicular stromatitis virus |
| vWF | von Willebrand Factor |
References
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Augustin, H.G.; Kozian, D.H.; Johnson, R.C. Differentiation of endothelial cells: Analysis of the constitutive and activated endothelial cell phenotypes. Bioessays 1994, 16, 901–906. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. The endothelial surface layer. Pflug. Arch. 2000, 440, 653–666. [Google Scholar] [CrossRef]
- Jaffe, E.A. Cell biology of endothelial cells. Hum. Pathol. 1987, 18, 234–239. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Afsar, B.; Ortiz, A.; Kanbay, M. The role of endothelial glycocalyx in health and disease. Clin. Kidney J. 2019, 12, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb Haemost 2019, 17, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kalucka, J.; de Rooij, L.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779. [Google Scholar] [CrossRef]
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H.; et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7, 575–583. [Google Scholar] [CrossRef]
- Gillich, A.; Zhang, F.; Farmer, C.G.; Travaglini, K.J.; Tan, S.Y.; Gu, M.; Zhou, B.; Feinstein, J.A.; Krasnow, M.A.; Metzger, R.J. Capillary cell-type specialization in the alveolus. Nature 2020, 586, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357. [Google Scholar] [CrossRef]
- Minami, T.; Muramatsu, M.; Kume, T. Organ/Tissue-Specific Vascular Endothelial Cell Heterogeneity in Health and Disease. Biol. Pharm. Bull. 2019, 42, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shen, W.; Veizades, S.; Liang, G.; Sayed, N.; Nguyen, P.K. Single-Cell Transcriptional Profiling Reveals Sex and Age Diversity of Gene Expression in Mouse Endothelial Cells. Front. Genet. 2021, 12, 590377. [Google Scholar] [CrossRef]
- Song, H.W.; Foreman, K.L.; Gastfriend, B.D.; Kuo, J.S.; Palecek, S.P.; Shusta, E.V. Transcriptomic comparison of human and mouse brain microvessels. Sci Rep. 2020, 10, 12358. [Google Scholar] [CrossRef] [PubMed]
- Monahan-Earley, R.; Dvorak, A.M.; Aird, W.C. Evolutionary origins of the blood vascular system and endothelium. J. Thromb Haemost 2013, 11 (Suppl. 1), 46–66. [Google Scholar] [CrossRef]
- Bundgaard, M.; Abbott, N.J. All vertebrates started out with a glial blood-brain barrier 4–500 million years ago. Glia 2008, 56, 699–708. [Google Scholar] [CrossRef]
- O’Brown, N.M.; Pfau, S.J.; Gu, C. Bridging barriers: A comparative look at the blood-brain barrier across organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef]
- Rosen, P.; Du, X.; Sui, G.Z. Molecular mechanisms of endothelial dysfunction in the diabetic heart. Adv. Exp. Med. Biol. 2001, 498, 75–86. [Google Scholar]
- Balletshofer, B.M.; Rittig, K.; Rett, K.; Haring, H.U.; Nawroth, P.P. Flow associated (endothelium dependent) vasodilation and TSH-levels in young normotensive and normoglycemic subjects. Vasa 2001, 30, 97–100. [Google Scholar] [CrossRef]
- McIntyre, T.M.; Zimmerman, G.A.; Satoh, K.; Prescott, S.M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J. Clin. Investig. 1985, 76, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Vukelic, S.; Griendling, K.K. Angiotensin II, from vasoconstrictor to growth factor: A paradigm shift. Circ. Res. 2014, 114, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S. Endothelial dysfunction: Does it matter? Is it reversible? J. Am. Coll Cardiol. 1997, 30, 325–333. [Google Scholar] [CrossRef]
- Lilly, B. We have contact: Endothelial cell-smooth muscle cell interactions. Physiology 2014, 29, 234–241. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect Med. 2012, 2, a006429. [Google Scholar] [CrossRef]
- Burns, A.R.; Bowden, R.A.; Abe, Y.; Walker, D.C.; Simon, S.I.; Entman, M.L.; Smith, C.W. P-selectin mediates neutrophil adhesion to endothelial cell borders. J. Leukoc. Biol. 1999, 65, 299–306. [Google Scholar] [CrossRef]
- Bevilacqua, M.P.; Nelson, R.M.; Mannori, G.; Cecconi, O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev. Med. 1994, 45, 361–378. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell Physiol 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Aird, W.C. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 2003, 101, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Riedl Khursigara, M.; Schlam, D.; Noone, D.G.; Bruno, V.; Ortiz-Sandoval, C.G.; Pluthero, F.G.; Kahr, W.H.A.; Bowman, M.L.; James, P.; Grinstein, S.; et al. Vascular endothelial cells evade complement-mediated membrane injury via Weibel-Palade body mobilization. J. Thromb. Haemost. 2020, 18, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.P.; Childs, S.; Leu, J.P.; Fishman, M.C. Gridlock signalling pathway fashions the first embryonic artery. Nature 2001, 414, 216–220. [Google Scholar] [CrossRef]
- dela Paz, N.G.; D’Amore, P.A. Arterial versus venous endothelial cells. Cell Tissue Res. 2009, 335, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, J.A. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J. Ultrastruct. Res. 1968, 25, 452–500. [Google Scholar] [CrossRef]
- Rahman, M.; Siddik, A.B. Anatomy, Arterioles; StatPearls LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Reiser, J.; Altintas, M.M. Podocytes. F1000Research 2016, 5, 114. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef]
- Gustavsson, C.; Agardh, C.D.; Zetterqvist, A.V.; Nilsson, J.; Agardh, E.; Gomez, M.F. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PLoS ONE 2010, 5, e12699. [Google Scholar] [CrossRef] [PubMed]
- Mukouyama, Y.S.; Shin, D.; Britsch, S.; Taniguchi, M.; Anderson, D.J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 2002, 109, 693–705. [Google Scholar] [CrossRef]
- Villa, N.; Walker, L.; Lindsell, C.E.; Gasson, J.; Iruela-Arispe, M.L.; Weinmaster, G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 2001, 108, 161–164. [Google Scholar] [CrossRef]
- Lawson, N.D.; Scheer, N.; Pham, V.N.; Kim, C.H.; Chitnis, A.B.; Campos-Ortega, J.A.; Weinstein, B.M. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 2001, 128, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yun, J.; Oh, S.P. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 2003, 93, 682–689. [Google Scholar] [CrossRef]
- Yuan, L.; Moyon, D.; Pardanaud, L.; Breant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Brachtendorf, G.; Kurth, F.; Sonntag, M.; Samulowitz, U.; Metze, D.; Vestweber, D. Expression of endomucin, a novel endothelial sialomucin, in normal and diseased human skin. J. Investig. Derm. 2002, 119, 1388–1393. [Google Scholar] [CrossRef]
- Liu, C.; Shao, Z.M.; Zhang, L.; Beatty, P.; Sartippour, M.; Lane, T.; Livingston, E.; Nguyen, M. Human endomucin is an endothelial marker. Biochem. Biophys. Res. Commun. 2001, 288, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wigle, J.T.; Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef]
- Srinivasan, R.S.; Oliver, G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011, 25, 2187–2197. [Google Scholar] [CrossRef]
- Francois, M.; Caprini, A.; Hosking, B.; Orsenigo, F.; Wilhelm, D.; Browne, C.; Paavonen, K.; Karnezis, T.; Shayan, R.; Downes, M.; et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 2008, 456, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.S.; Dillard, M.E.; Lagutin, O.V.; Lin, F.J.; Tsai, S.; Tsai, M.J.; Samokhvalov, I.M.; Oliver, G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007, 21, 2422–2432. [Google Scholar] [CrossRef]
- Podgrabinska, S.; Braun, P.; Velasco, P.; Kloos, B.; Pepper, M.S.; Skobe, M. Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16069–16074. [Google Scholar] [CrossRef] [PubMed]
- Skalak, T.C.; Price, R.J. The role of mechanical stresses in microvascular remodeling. Microcirculation 1996, 3, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.; Eskin, S.G. Effects of fluid shear stress on gene regulation of vascular cells. Biotechnol. Prog. 1997, 13, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewicz, A.; Secomb, T.W.; Pries, A.R. Angioadaptation: Keeping the vascular system in shape. News Physiol. Sci. 2002, 17, 197–201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Resnick, N.; Yahav, H.; Shay-Salit, A.; Shushy, M.; Schubert, S.; Zilberman, L.C.; Wofovitz, E. Fluid shear stress and the vascular endothelium: For better and for worse. Prog. Biophys. Mol. Biol. 2003, 81, 177–199. [Google Scholar] [CrossRef]
- Wragg, J.W.; Durant, S.; McGettrick, H.M.; Sample, K.M.; Egginton, S.; Bicknell, R. Shear stress regulated gene expression and angiogenesis in vascular endothelium. Microcirculation 2014, 21, 290–300. [Google Scholar] [CrossRef]
- Chiu, J.J.; Lee, P.L.; Chang, S.F.; Chen, L.J.; Lee, C.I.; Lin, K.M.; Usami, S.; Chien, S. Shear stress regulates gene expression in vascular endothelial cells in response to tumor necrosis factor-alpha: A study of the transcription profile with complementary DNA microarray. J. Biomed. Sci. 2005, 12, 481–502. [Google Scholar] [CrossRef]
- Ohura, N.; Yamamoto, K.; Ichioka, S.; Sokabe, T.; Nakatsuka, H.; Baba, A.; Shibata, M.; Nakatsuka, T.; Harii, K.; Wada, Y.; et al. Global analysis of shear stress-responsive genes in vascular endothelial cells. J. Atheroscler. Thromb. 2003, 10, 304–313. [Google Scholar] [CrossRef]
- Zhuang, X.; Cross, D.; Heath, V.L.; Bicknell, R. Shear stress, tip cells and regulators of endothelial migration. Biochem. Soc. Trans. 2011, 39, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Moonen, J.R.; Chappell, J.; Shi, M.; Shinohara, T.; Li, D.; Mumbach, M.R.; Zhang, F.; Nasser, J.; Mai, D.H.; Taylor, S.; et al. KLF4 Recruits SWI/SNF to Increase Chromatin Acessability and Reprogram the Endothelial Enhancer Landscape under Laminar Shear Stress. bioRxiv 2021. [Google Scholar] [CrossRef]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef]
- Adams, R.H.; Wilkinson, G.A.; Weiss, C.; Diella, F.; Gale, N.W.; Deutsch, U.; Risau, W.; Klein, R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: Demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999, 13, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Gerety, S.S.; Anderson, D.J. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 2002, 129, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, O.; Melton, D.A. Endothelial signaling during development. Nat. Med. 2003, 9, 661–668. [Google Scholar] [CrossRef]
- Dumas, S.J.; Meta, E.; Borri, M.; Luo, Y.; Li, X.; Rabelink, T.J.; Carmeliet, P. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat. Rev. Nephrol. 2021, 17, 441–464. [Google Scholar] [CrossRef]
- Aird, W.C.; Edelberg, J.M.; Weiler-Guettler, H.; Simmons, W.W.; Smith, T.W.; Rosenberg, R.D. Vascular bed-specific expression of an endothelial cell gene is programmed by the tissue microenvironment. J. Cell Biol. 1997, 138, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, A.M.; Nassiri, M.; Stitt, M.S.; Wasserloos, K.; Watkins, S.C.; Pitt, B.R.; Jahroudi, N. Sequences in intron 51 of the von Willebrand factor gene target promoter activation to a subset of lung endothelial cells in transgenic mice. J. Biol. Chem. 2008, 283, 2741–2750. [Google Scholar] [CrossRef]
- Nassiri, M.; Liu, J.; Kulak, S.; Uwiera, R.R.; Aird, W.C.; Ballermann, B.J.; Jahroudi, N. Repressors NFI and NFY participate in organ-specific regulation of von Willebrand factor promoter activity in transgenic mice. Arter. Thromb Vasc. Biol. 2010, 30, 1423–1429. [Google Scholar] [CrossRef]
- Minami, T.; Aird, W.C. Endothelial cell gene regulation. Trends Cardiovasc. Med. 2005, 15, 174–184. [Google Scholar] [CrossRef]
- Grasshoff, H.; Muller-Fielitz, H.; Dogbevia, G.K.; Korbelin, J.; Bannach, J.; Vahldieck, C.M.; Kusche-Vihrog, K.; Johren, O.; Muller, O.J.; Nogueiras, R.; et al. Short regulatory DNA sequences to target brain endothelial cells for gene therapy. J. Cereb. Blood Flow Metab. 2021. [Google Scholar] [CrossRef]
- Ridder, D.A.; Lang, M.F.; Salinin, S.; Roderer, J.P.; Struss, M.; Maser-Gluth, C.; Schwaninger, M. TAK1 in brain endothelial cells mediates fever and lethargy. J. Exp. Med. 2011, 208, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 6 September 2021).
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moises, F.P.; Macias-Islas, M.A.; Flores-Alvarado, L.J.; Mireles-Ramirez, M.A.; Gonzalez-Renovato, E.D.; Hernandez-Navarro, V.E.; Sanchez-Lopez, A.L.; Alatorre-Jimenez, M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PLoS ONE 2010, 5, e13741. [Google Scholar] [CrossRef]
- Mazzoni, J.; Smith, J.R.; Shahriar, S.; Cutforth, T.; Ceja, B.; Agalliu, D. The Wnt Inhibitor Apcdd1 Coordinates Vascular Remodeling and Barrier Maturation of Retinal Blood Vessels. Neuron 2017, 96, 1055–1069. [Google Scholar] [CrossRef]
- Roberts, L.M.; Woodford, K.; Zhou, M.; Black, D.S.; Haggerty, J.E.; Tate, E.H.; Grindstaff, K.K.; Mengesha, W.; Raman, C.; Zerangue, N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 2008, 149, 6251–6261. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Faiz, A.; Moshage, H.; Schubert, R.; Schilling, L.; Kamps, J.A. Comparative transcriptome analysis of inner blood-retinal barrier and blood-brain barrier in rats. Sci. Rep. 2021, 11, 12151. [Google Scholar] [CrossRef]
- Rhett, J.M.; Jourdan, J.; Gourdie, R.G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell 2011, 22, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Kovacs-Oller, T.; Sagdullaev, B.T. Vascular Pericyte Impairment and Connexin43 Gap Junction Deficit Contribute to Vasomotor Decline in Diabetic Retinopathy. J. Neurosci. 2017, 37, 7580–7594. [Google Scholar] [CrossRef] [PubMed]
- Niethamer, T.K.; Stabler, C.T.; Leach, J.P.; Zepp, J.A.; Morley, M.P.; Babu, A.; Zhou, S.; Morrisey, E.E. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Sayner, S.L. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L667–L678. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Cao, A.; Li, C.G.; Shi, M.; Mienert, J.; Miyagawa, K.; Korbelin, J.; Marciano, D.P.; Chen, P.I.; Roughley, M.; et al. PPARgamma-p53-Mediated Vasculoregenerative Program to Reverse Pulmonary Hypertension. Circ. Res. 2021, 128, 401–418. [Google Scholar] [CrossRef]
- Diebold, I.; Hennigs, J.K.; Miyagawa, K.; Li, C.G.; Nickel, N.P.; Kaschwich, M.; Cao, A.; Wang, L.; Reddy, S.; Chen, P.I.; et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015, 21, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Chavala, S.H.; Kim, Y.; Tudisco, L.; Cicatiello, V.; Milde, T.; Kerur, N.; Claros, N.; Yanni, S.; Guaiquil, V.H.; Hauswirth, W.W.; et al. Retinal angiogenesis suppression through small molecule activation of p53. J. Clin. Investig. 2013, 123, 4170–4181. [Google Scholar] [CrossRef]
- Gogiraju, R.; Xu, X.; Bochenek, M.L.; Steinbrecher, J.H.; Lehnart, S.E.; Wenzel, P.; Kessel, M.; Zeisberg, E.M.; Dobbelstein, M.; Schafer, K. Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J. Am. Heart Assoc. 2015, 4, e001770. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Gonelli, A.; Dell’Eva, R.; Vitale, M.; Capitani, S.; Albini, A.; Zauli, G. Antiangiogenic activity of the MDM2 antagonist nutlin-3. Circ. Res. 2007, 100, 61–69. [Google Scholar] [CrossRef]
- Alastalo, T.P.; Li, M.; Perez Vde, J.; Pham, D.; Sawada, H.; Wang, J.K.; Koskenvuo, M.; Wang, L.; Freeman, B.A.; Chang, H.Y.; et al. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J. Clin. Investig. 2011, 121, 3735–3746. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Kivela, R. Cardiomyocyte-Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front. Cardiovasc. Med. 2018, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Colliva, A.; Braga, L.; Giacca, M.; Zacchigna, S. Endothelial cell-cardiomyocyte crosstalk in heart development and disease. J. Physiol. 2020, 598, 2923–2939. [Google Scholar] [CrossRef] [PubMed]
- Lother, A.; Bergemann, S.; Deng, L.; Moser, M.; Bode, C.; Hein, L. Cardiac Endothelial Cell Transcriptome. Arter. Thromb. Vasc. Biol 2018, 38, 566–574. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Tran, K.V.; Gealekman, O.; Frontini, A.; Zingaretti, M.C.; Morroni, M.; Giordano, A.; Smorlesi, A.; Perugini, J.; De Matteis, R.; Sbarbati, A.; et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab 2012, 15, 222–229. [Google Scholar] [CrossRef]
- Frontini, A.; Giordano, A.; Cinti, S. Endothelial cells of adipose tissues: A niche of adipogenesis. Cell Cycle 2012, 11, 2765–2766. [Google Scholar] [CrossRef]
- Gogg, S.; Nerstedt, A.; Boren, J.; Smith, U. Human adipose tissue microvascular endothelial cells secrete PPARgamma ligands and regulate adipose tissue lipid uptake. JCI Insight 2019, 4, e125914. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Marcu, R.; Choi, Y.J.; Xue, J.; Fortin, C.L.; Wang, Y.; Nagao, R.J.; Xu, J.; MacDonald, J.W.; Bammler, T.K.; Murry, C.E.; et al. Human Organ-Specific Endothelial Cell Heterogeneity. iScience 2018, 4, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Fretz, J.A.; Nelson, T.; Velazquez, H.; Xi, Y.; Moeckel, G.W.; Horowitz, M.C. Early B-cell factor 1 is an essential transcription factor for postnatal glomerular maturation. Kidney Int. 2014, 85, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.T.; Potter, S.S. Atlas of Hox gene expression in the developing kidney. Dev. Dyn. 2004, 229, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Narlis, M.; Grote, D.; Gaitan, Y.; Boualia, S.K.; Bouchard, M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J. Am. Soc. Nephrol. 2007, 18, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef]
- Dumas, S.J.; Meta, E.; Borri, M.; Goveia, J.; Rohlenova, K.; Conchinha, N.V.; Falkenberg, K.; Teuwen, L.A.; de Rooij, L.; Kalucka, J.; et al. Single-Cell RNA Sequencing Reveals Renal Endothelium Heterogeneity and Metabolic Adaptation to Water Deprivation. J. Am. Soc. Nephrol. 2020, 31, 118–138. [Google Scholar] [CrossRef]
- Guerci, P.; Ergin, B.; Ince, C. The macro- and microcirculation of the kidney. Best Pr. Res. Clin. Anaesthesiol. 2017, 31, 315–329. [Google Scholar] [CrossRef]
- Satchell, S.C.; Braet, F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef]
- Ichimura, K.; Stan, R.V.; Kurihara, H.; Sakai, T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J. Am. Soc. Nephrol. 2008, 19, 1463–1471. [Google Scholar] [CrossRef]
- Bearer, E.L.; Orci, L. Endothelial fenestral diaphragms: A quick-freeze, deep-etch study. J. Cell Biol. 1985, 100, 418–428. [Google Scholar] [CrossRef]
- Brunskill, E.W.; Sequeira-Lopez, M.L.; Pentz, E.S.; Lin, E.; Yu, J.; Aronow, B.J.; Potter, S.S.; Gomez, R.A. Genes that confer the identity of the renin cell. J. Am. Soc. Nephrol. 2011, 22, 2213–2225. [Google Scholar] [CrossRef]
- Karaiskos, N.; Rahmatollahi, M.; Boltengagen, A.; Liu, H.; Hoehne, M.; Rinschen, M.; Schermer, B.; Benzing, T.; Rajewsky, N.; Kocks, C.; et al. A Single-Cell Transcriptome Atlas of the Mouse Glomerulus. J. Am. Soc. Nephrol. 2018, 29, 2060–2068. [Google Scholar] [CrossRef]
- Takemoto, M.; He, L.; Norlin, J.; Patrakka, J.; Xiao, Z.; Petrova, T.; Bondjers, C.; Asp, J.; Wallgard, E.; Sun, Y.; et al. Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J. 2006, 25, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, S.; He, Y.; Gutsol, A.; Wight, A.; Hebert, R.L.; Vilmundarson, R.O.; Makrigiannis, A.P.; Chalmers, J.; Hamet, P.; Tremblay, J.; et al. Endothelial Gata5 transcription factor regulates blood pressure. Nat. Commun. 2015, 6, 8835. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.M.; McMillan, E.A.; Kunar, B.; Lis, R.; Zhang, T.; Lu, T.; Daniel, E.; Yokoyama, M.; Gomez-Salinero, J.M.; Sureshbabu, A.; et al. Molecular determinants of nephron vascular specialization in the kidney. Nat. Commun. 2019, 10, 5705. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.O.; Santelli, A.; Abumoawad, A.; Eirin, A.; Ferguson, C.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Loss of Renal Peritubular Capillaries in Hypertensive Patients Is Detectable by Urinary Endothelial Microparticle Levels. Hypertension 2018, 72, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Sol, M.; Kamps, J.; van den Born, J.; van den Heuvel, M.C.; van der Vlag, J.; Krenning, G.; Hillebrands, J.L. Glomerular Endothelial Cells as Instigators of Glomerular Sclerotic Diseases. Front. Pharm. 2020, 11, 573557. [Google Scholar] [CrossRef]
- Loeven, M.A.; Rops, A.L.; Lehtinen, M.J.; van Kuppevelt, T.H.; Daha, M.R.; Smith, R.J.; Bakker, M.; Berden, J.H.; Rabelink, T.J.; Jokiranta, T.S.; et al. Mutations in Complement Factor H Impair Alternative Pathway Regulation on Mouse Glomerular Endothelial Cells in Vitro. J. Biol. Chem. 2016, 291, 4974–4981. [Google Scholar] [CrossRef]
- Schmidley, J.W.; Wissig, S.L. Anionic sites on the luminal surface of fenestrated and continuous capillaries of the CNS. Brain Res. 1986, 363, 265–271. [Google Scholar] [CrossRef]
- Kratzer, I.; Liddelow, S.A.; Saunders, N.R.; Dziegielewska, K.M.; Strazielle, N.; Ghersi-Egea, J.F. Developmental changes in the transcriptome of the rat choroid plexus in relation to neuroprotection. Fluids Barriers CNS 2013, 10, 25. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Coppola, G.; Gao, F.; Puga, R.; Brentani, H.; Geschwind, D.H.; Sousa, N.; Correia-Neves, M.; Palha, J.A. Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS 2011, 8, 10. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, Y.; Lee, D.; Webster, M.J. Transcriptome sequencing of the choroid plexus in schizophrenia. Transl. Psychiatry 2016, 6, e964. [Google Scholar] [CrossRef] [PubMed]
- Stan, R.V.; Kubitza, M.; Palade, G.E. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl. Acad. Sci. USA 1999, 96, 13203–13207. [Google Scholar] [CrossRef] [PubMed]
- Stucker, S.; De Angelis, J.; Kusumbe, A.P. Heterogeneity and Dynamics of Vasculature in the Endocrine System During Aging and Disease. Front. Physiol. 2021, 12, 624928. [Google Scholar] [CrossRef] [PubMed]
- Kamba, T.; Tam, B.Y.; Hashizume, H.; Haskell, A.; Sennino, B.; Mancuso, M.R.; Norberg, S.M.; O’Brien, S.M.; Davis, R.B.; Gowen, L.C.; et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H560–H576. [Google Scholar] [CrossRef] [PubMed]
- Amar, A.P.; Weiss, M.H. Pituitary anatomy and physiology. Neurosurg. Clin. N. Am. 2003, 14, 11–23. [Google Scholar] [CrossRef]
- Geraud, C.; Evdokimov, K.; Straub, B.K.; Peitsch, W.K.; Demory, A.; Dorflinger, Y.; Schledzewski, K.; Schmieder, A.; Schemmer, P.; Augustin, H.G.; et al. Unique cell type-specific junctional complexes in vascular endothelium of human and rat liver sinusoids. PLoS ONE 2012, 7, e34206. [Google Scholar]
- Braet, F.; Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: A review. Comp. Hepatol. 2002, 1, 1. [Google Scholar] [CrossRef]
- Martin-Llahi, M.; Albillos, A.; Banares, R.; Berzigotti, A.; Garcia-Criado, M.A.; Genesca, J.; Hernandez-Gea, V.; Llop-Herrera, E.; Masnou-Ridaura, H.; Mateo, J.; et al. Vascular diseases of the liver. Clinical Guidelines from the Catalan Society of Digestology and the Spanish Association for the Study of the Liver. Gastroenterol. Hepatol. 2017, 40, 538–580. [Google Scholar] [CrossRef]
- Maslak, E.; Gregorius, A.; Chlopicki, S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharm. Rep. 2015, 67, 689–694. [Google Scholar] [CrossRef]
- Wisse, E.; De Zanger, R.B.; Charels, K.; Van Der Smissen, P.; McCuskey, R.S. The liver sieve: Considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology 1985, 5, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, L.; Wang, X.; DeLeve, L.D. Isolation of periportal, midlobular, and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1204–G1210. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef]
- Strauss, O.; Phillips, A.; Ruggiero, K.; Bartlett, A.; Dunbar, P.R. Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Sci. Rep. 2017, 7, 44356. [Google Scholar] [CrossRef] [PubMed]
- Taichman, R.S. Blood and bone: Two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 2005, 105, 2631–2639. [Google Scholar] [CrossRef]
- Kang, C.; Jia, X.; Liu, H. Development and validation of a RNA binding protein gene pair-associated prognostic signature for prediction of overall survival in hepatocellular carcinoma. Biomed. Eng. Online 2020, 19, 68. [Google Scholar] [CrossRef]
- Prendergast, A.M.; Kuck, A.; van Essen, M.; Haas, S.; Blaszkiewicz, S.; Essers, M.A. IFNalpha-mediated remodeling of endothelial cells in the bone marrow niche. Haematologica 2017, 102, 445–453. [Google Scholar] [CrossRef]
- Tikhonova, A.N.; Dolgalev, I.; Hu, H.; Sivaraj, K.K.; Hoxha, E.; Cuesta-Dominguez, A.; Pinho, S.; Akhmetzyanova, I.; Gao, J.; Witkowski, M.; et al. The bone marrow microenvironment at single-cell resolution. Nature 2019, 569, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Gao, X.; Wei, Q.; Nakahara, F.; Zimmerman, S.E.; Mar, J.; Frenette, P.S. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat. Commun. 2018, 9, 2449. [Google Scholar] [CrossRef]
- Drayton, D.L.; Ying, X.; Lee, J.; Lesslauer, W.; Ruddle, N.H. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J. Exp. Med. 2003, 197, 1153–1163. [Google Scholar] [CrossRef]
- Ruddle, N.H. High Endothelial Venules and Lymphatic Vessels in Tertiary Lymphoid Organs: Characteristics, Functions, and Regulation. Front. Immunol. 2016, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Brulois, K.; Rajaraman, A.; Szade, A.; Nordling, S.; Bogoslowski, A.; Dermadi, D.; Rahman, M.; Kiefel, H.; O’Hara, E.; Koning, J.J.; et al. A molecular map of murine lymph node blood vascular endothelium at single cell resolution. Nat. Commun. 2020, 11, 3798. [Google Scholar] [CrossRef] [PubMed]
- Ager, A.; May, M.J. Understanding high endothelial venules: Lessons for cancer immunology. Oncoimmunology 2015, 4, e1008791. [Google Scholar] [CrossRef]
- Ager, A. High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Front. Immunol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Hjelmstrom, P.; Fjell, J.; Nakagawa, T.; Sacca, R.; Cuff, C.A.; Ruddle, N.H. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am. J. Pathol. 2000, 156, 1133–1138. [Google Scholar] [CrossRef]
- Kratz, A.; Campos-Neto, A.; Hanson, M.S.; Ruddle, N.H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 1996, 183, 1461–1472. [Google Scholar] [CrossRef]
- Mionnet, C.; Sanos, S.L.; Mondor, I.; Jorquera, A.; Laugier, J.P.; Germain, R.N.; Bajenoff, M. High endothelial venules as traffic control points maintaining lymphocyte population homeostasis in lymph nodes. Blood 2011, 118, 6115–6122. [Google Scholar] [CrossRef]
- Blanchard, L.; Girard, J.P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 2021, 719–731. [Google Scholar] [CrossRef]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Egan, K.; FitzGerald, G.A. Eicosanoids and the vascular endothelium. Handb. Exp. Pharmacol. 2006, 176 Pt 1, 189–211. [Google Scholar]
- Lorant, D.E.; Patel, K.D.; McIntyre, T.M.; McEver, R.P.; Prescott, S.M.; Zimmerman, G.A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: A juxtacrine system for adhesion and activation of neutrophils. J. Cell Biol. 1991, 115, 223–234. [Google Scholar] [CrossRef]
- Pober, J.S. The Endothelium: A Comprehensive Reference; Cambrigde University Press: Cambrigde, UK, 2007; Volume 1. [Google Scholar]
- Ley, K.; Reutershan, J. Leucocyte-endothelial interactions in health and disease. Handb. Exp. Pharmacol. 2006, 176 Pt 2, 97–133. [Google Scholar]
- Munro, J.M.; Pober, J.S.; Cotran, R.S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am. J. Pathol. 1989, 135, 121–133. [Google Scholar]
- Austrup, F.; Vestweber, D.; Borges, E.; Lohning, M.; Brauer, R.; Herz, U.; Renz, H.; Hallmann, R.; Scheffold, A.; Radbruch, A.; et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature 1997, 385, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V.; Martinelli, R. T Lymphocyte-Endothelial Interactions: Emerging Understanding of Trafficking and Antigen-Specific Immunity. Front. Immunol. 2015, 6, 603. [Google Scholar] [CrossRef] [PubMed]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 2020, 9, e51413. [Google Scholar] [CrossRef] [PubMed]
- Schuenke, P.; Windschuh, J.; Roeloffs, V.; Ladd, M.E.; Bachert, P.; Zaiss, M. Simultaneous mapping of water shift and B1 (WASABI)-Application to field-Inhomogeneity correction of CEST MRI data. Magn. Reson. Med. 2017, 77, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 3865–3873. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, S.; Munoz-Vega, M.; Contreras, W.E.; Crespo-Avilan, G.E.; Rodriguez-Montesinos, J.; Arias-Carrion, O.; Perez-Mendez, O.; Boisvert, W.A.; Preissner, K.T.; Cabrera-Fuentes, H.A. Responses of Endothelial Cells Towards Ischemic Conditioning Following Acute Myocardial Infarction. Cond. Med. 2018, 1, 247–258. [Google Scholar] [PubMed]
- Ladilov, Y.; Schafer, C.; Held, A.; Schafer, M.; Noll, T.; Piper, H.M. Mechanism of Ca(2+) overload in endothelial cells exposed to simulated ischemia. Cardiovasc. Res. 2000, 47, 394–403. [Google Scholar] [CrossRef]
- Sandow, S.L.; Senadheera, S.; Grayson, T.H.; Welsh, D.G.; Murphy, T.V. Calcium and endothelium-mediated vasodilator signaling. Adv. Exp. Med. Biol. 2012, 740, 811–831. [Google Scholar]
- Kumar, S.; Kasseckert, S.; Kostin, S.; Abdallah, Y.; Schafer, C.; Kaminski, A.; Reusch, H.P.; Piper, H.M.; Steinhoff, G.; Ladilov, Y. Ischemic acidosis causes apoptosis in coronary endothelial cells through activation of caspase-12. Cardiovasc. Res. 2007, 73, 172–180. [Google Scholar] [CrossRef][Green Version]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef]
- Sommer, N.; Dietrich, A.; Schermuly, R.T.; Ghofrani, H.A.; Gudermann, T.; Schulz, R.; Seeger, W.; Grimminger, F.; Weissmann, N. Regulation of hypoxic pulmonary vasoconstriction: Basic mechanisms. Eur. Respir. J. 2008, 32, 1639–1651. [Google Scholar] [CrossRef]
- Nishiyama, A.; Miyatake, A.; Aki, Y.; Fukui, T.; Rahman, M.; Kimura, S.; Abe, Y. Adenosine A(1) receptor antagonist KW-3902 prevents hypoxia-induced renal vasoconstriction. J. Pharm. Exp. Ther. 1999, 291, 988–993. [Google Scholar]
- Vio, C.P.; Salas, D.; Cespedes, C.; Diaz-Elizondo, J.; Mendez, N.; Alcayaga, J.; Iturriaga, R. Imbalance in Renal Vasoactive Enzymes Induced by Mild Hypoxia: Angiotensin-Converting Enzyme Increases While Neutral Endopeptidase Decreases. Front. Physiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Winn, R.K.; Ramamoorthy, C.; Vedder, N.B.; Sharar, S.R.; Harlan, J.M. Leukocyte-endothelial cell interactions in ischemia-reperfusion injury. Ann. N. Y. Acad. Sci. 1997, 832, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Nata, T.; Morishita, R.; Matsushita, H.; Nakagami, H.; Yamamoto, K.; Yamazaki, K.; Nakabayashi, M.; Ogihara, T.; Kaneda, Y. Endothelial apoptosis induced by oxidative stress through activation of NF-kappaB: Antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension 2001, 38, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nagl, L.; Horvath, L.; Pircher, A.; Wolf, D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment—New Findings and Future Perspectives. Front. Cell Dev. Biol. 2020, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Alitalo, K. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 1999, 5, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef]
- Ohga, N.; Ishikawa, S.; Maishi, N.; Akiyama, K.; Hida, Y.; Kawamoto, T.; Sadamoto, Y.; Osawa, T.; Yamamoto, K.; Kondoh, M.; et al. Heterogeneity of tumor endothelial cells: Comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am. J. Pathol. 2012, 180, 1294–1307. [Google Scholar] [CrossRef]
- Al-Soudi, A.; Kaaij, M.H.; Tas, S.W. Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmun Rev. 2017, 16, 951–962. [Google Scholar] [CrossRef]
- De Sanctis, F.; Ugel, S.; Facciponte, J.; Facciabene, A. The dark side of tumor-associated endothelial cells. Semin. Immunol. 2018, 35, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Goveia, J.; Rohlenova, K.; Taverna, F.; Treps, L.; Conradi, L.C.; Pircher, A.; Geldhof, V.; de Rooij, L.; Kalucka, J.; Sokol, L.; et al. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell 2020, 37, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, C.Y.; Lawson, D.A.; Kwek, S.; Velozo, H.G.; Owyong, M.; Lai, M.D.; Fong, L.; Wilson, M.; Su, H.; et al. Single-cell RNA sequencing reveals gene expression signatures of breast cancer-associated endothelial cells. Oncotarget 2018, 9, 10945–10961. [Google Scholar] [CrossRef] [PubMed]
- St Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montgomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; et al. Genes expressed in human tumor endothelium. Science 2000, 289, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.S.; Argani, P.; Cook, B.P.; Liangfeng, H.; Chartrand, S.D.; Zhang, M.; Saha, S.; Bardelli, A.; Jiang, Y.; St Martin, T.B.; et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 2004, 64, 7857–7866. [Google Scholar] [CrossRef]
- Madden, S.L.; Cook, B.P.; Nacht, M.; Weber, W.D.; Callahan, M.R.; Jiang, Y.; Dufault, M.R.; Zhang, X.; Zhang, W.; Walter-Yohrling, J.; et al. Vascular gene expression in nonneoplastic and malignant brain. Am. J. Pathol. 2004, 165, 601–608. [Google Scholar] [CrossRef]
- Zhang, L.; Hoffman, J.A.; Ruoslahti, E. Molecular profiling of heart endothelial cells. Circulation 2005, 112, 1601–1611. [Google Scholar] [CrossRef]
- Fan, X.; Venegas, R.; Fey, R.; van der Heyde, H.; Bernard, M.A.; Lazarides, E.; Woods, C.M. An in vivo approach to structure activity relationship analysis of peptide ligands. Pharm. Res. 2007, 24, 868–879. [Google Scholar] [CrossRef]
- Korbelin, J.; Dogbevia, G.; Michelfelder, S.; Ridder, D.A.; Hunger, A.; Wenzel, J.; Seismann, H.; Lampe, M.; Bannach, J.; Pasparakis, M.; et al. A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med. 2016, 8, 609–625. [Google Scholar] [CrossRef]
- Dogbevia, G.K.; Tollner, K.; Korbelin, J.; Broer, S.; Ridder, D.A.; Grasshoff, H.; Brandt, C.; Wenzel, J.; Straub, B.K.; Trepel, M.; et al. Gene therapy decreases seizures in a model of Incontinentia pigmenti. Ann. Neurol. 2017, 82, 93–104. [Google Scholar] [CrossRef]
- Dogbevia, G.; Grasshoff, H.; Othman, A.; Penno, A.; Schwaninger, M. Brain endothelial specific gene therapy improves experimental Sandhoff disease. J. Cereb. Blood Flow Metab. 2020, 40, 1338–1350. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, M.; Davidson, B.L. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat. Med. 2009, 15, 1215–1218. [Google Scholar] [CrossRef]
- Work, L.M.; Buning, H.; Hunt, E.; Nicklin, S.A.; Denby, L.; Britton, N.; Leike, K.; Odenthal, M.; Drebber, U.; Hallek, M.; et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol. Ther. 2006, 13, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, K.; Ding, B.S.; Gottstein, C.; Cines, D.B.; Muzykantov, V.R. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J. Pharm. Exp. Ther. 2007, 321, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.H.F.; Staquicini, F.I.; Teixeira, A.A.R.; Michaloski, J.S.; Namiyama, G.M.; Taniwaki, N.N.; Setubal, J.C.; da Silva, A.M.; Sidman, R.L.; Pasqualini, R.; et al. A ligand motif enables differential vascular targeting of endothelial junctions between brain and retina. Proc. Natl. Acad. Sci. USA 2019, 116, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Kolonin, M.G.; Trepel, M.; Lahdenranta, J.; Cardo-Vila, M.; Giordano, R.J.; Mintz, P.J.; Ardelt, P.U.; Yao, V.J.; Vidal, C.I.; et al. Steps toward mapping the human vasculature by phage display. Nat. Med. 2002, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.; Rots, M.G.; Kok, R.J.; Moorlag, H.E.; Van Loenen, A.M.; Meijer, D.K.; Haisma, H.J.; Molema, G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004, 15, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, B.; Uehara, M.; Jiang, L.; Ordikhani, F.; Banouni, N.; Ichimura, T.; Solhjou, Z.; Furtmuller, G.J.; Brandacher, G.; Alvarez, D.; et al. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J. Clin. Investig. 2018, 128, 4770–4786. [Google Scholar] [CrossRef]
- Rosen, S.D.; Tsay, D.; Singer, M.S.; Hemmerich, S.; Abraham, W.M. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am. J. Pathol. 2005, 166, 935–944. [Google Scholar] [CrossRef]
- Broisat, A.; Hernot, S.; Toczek, J.; De Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ. Res. 2012, 110, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Kiseleva, R.Y.; Arguiri, E.; Villa, C.H.; Muro, S.; Christofidou-Solomidou, M.; Stan, R.V.; Muzykantov, V.R. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J. Control. Release 2018, 272, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Senders, M.L.; Hernot, S.; Carlucci, G.; van de Voort, J.C.; Fay, F.; Calcagno, C.; Tang, J.; Alaarg, A.; Zhao, Y.; Ishino, S.; et al. Nanobody-Facilitated Multiparametric PET/MRI Phenotyping of Atherosclerosis. JACC Cardiovasc. Imaging 2019, 12, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, M.; Xu, L.; Ochoa-Espinosa, A.; Kosareva, A.; Wolff, T.; Murtaja, A.; Broisat, A.; Devoogdt, N.; Kaufmann, B.A. Ultrasound Molecular Imaging of Atherosclerosis With Nanobodies: Translatable Microbubble Targeting Murine and Human VCAM (Vascular Cell Adhesion Molecule) 1. Arter. Thromb. Vasc. Biol. 2019, 39, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Shaish, A.; Rofe, K.; Feige, E.; Varda-Bloom, N.; Afek, A.; Barshack, I.; Bangio, L.; Hodish, I.; Greenberger, S.; et al. Endothelial-targeted gene transfer of hypoxia-inducible factor-1alpha augments ischemic neovascularization following systemic administration. Mol. Ther. 2008, 16, 1927–1936. [Google Scholar] [CrossRef]
- Mahanivong, C.; Kruger, J.A.; Bian, D.; Reisfeld, R.A.; Huang, S. A simplified cloning strategy for the generation of an endothelial cell selective recombinant adenovirus vector. J. Virol. Methods 2006, 135, 127–135. [Google Scholar] [CrossRef]
- Rajotte, D.; Arap, W.; Hagedorn, M.; Koivunen, E.; Pasqualini, R.; Ruoslahti, E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Investig. 1998, 102, 430–437. [Google Scholar] [CrossRef]
- Rajotte, D.; Ruoslahti, E. Membrane dipeptidase is the receptor for a lung-targeting peptide identified by in vivo phage display. J. Biol. Chem. 1999, 274, 11593–11598. [Google Scholar] [CrossRef]
- Korbelin, J.; Sieber, T.; Michelfelder, S.; Lunding, L.; Spies, E.; Hunger, A.; Alawi, M.; Rapti, K.; Indenbirken, D.; Muller, O.J.; et al. Pulmonary Targeting of Adeno-associated Viral Vectors by Next-generation Sequencing-guided Screening of Random Capsid Displayed Peptide Libraries. Mol. Ther. 2016, 24, 1050–1061. [Google Scholar] [CrossRef]
- Reynolds, P.N.; Zinn, K.R.; Gavrilyuk, V.D.; Balyasnikova, I.V.; Rogers, B.E.; Buchsbaum, D.J.; Wang, M.H.; Miletich, D.J.; Grizzle, W.E.; Douglas, J.T.; et al. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol. Ther. 2000, 2, 562–578. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Holmes, M.D.; Danilov, S.M.; Reynolds, P.N. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur. Respir. J. 2012, 39, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Brosnan, M.J.; Graham, D.; Nicol, C.G.; Morecroft, I.; Channon, K.M.; Danilov, S.M.; Reynolds, P.N.; Baker, A.H.; Dominiczak, A.F. Targeting endothelial cells with adenovirus expressing nitric oxide synthase prevents elevation of blood pressure in stroke-prone spontaneously hypertensive rats. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 12, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.N.; Nicklin, S.A.; Kaliberova, L.; Boatman, B.G.; Grizzle, W.E.; Balyasnikova, I.V.; Baker, A.H.; Danilov, S.M.; Curiel, D.T. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 2001, 19, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Weih, S.; Metzger, R.; Albrecht, R.F., 2nd; Post, S.; Hohenberger, P.; Gebhard, M.M.; Danilov, S.M. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L162–L169. [Google Scholar] [CrossRef]
- Nowak, K.; Hanusch, C.; Nicksch, K.; Metzger, R.P.; Beck, G.; Gebhard, M.M.; Hohenberger, P.; Danilov, S.M. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur. J. Cardiothorac. Surg. 2010, 37, 859–863. [Google Scholar] [CrossRef]
- Marchetti, G.M.; Burwell, T.J.; Peterson, N.C.; Cann, J.A.; Hanna, R.N.; Li, Q.; Ongstad, E.L.; Boyd, J.T.; Kennedy, M.A.; Zhao, W.; et al. Targeted drug delivery via caveolae-associated protein PV1 improves lung fibrosis. Commun. Biol. 2019, 2, 92. [Google Scholar] [CrossRef]
- Shuvaev, V.V.; Christofidou-Solomidou, M.; Bhora, F.; Laude, K.; Cai, H.; Dikalov, S.; Arguiri, E.; Solomides, C.C.; Albelda, S.M.; Harrison, D.G.; et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharm. Exp. Ther. 2009, 331, 404–411. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Scherpereel, A.; Wiewrodt, R.; Ng, K.; Sweitzer, T.; Arguiri, E.; Shuvaev, V.; Solomides, C.C.; Albelda, S.M.; Muzykantov, V.R. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L283–L292. [Google Scholar] [CrossRef]
- Ding, B.S.; Hong, N.; Christofidou-Solomidou, M.; Gottstein, C.; Albelda, S.M.; Cines, D.B.; Fisher, A.B.; Muzykantov, V.R. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am. J. Respir. Crit. Care Med. 2009, 180, 247–256. [Google Scholar] [CrossRef]
- Ding, B.S.; Gottstein, C.; Grunow, A.; Kuo, A.; Ganguly, K.; Albelda, S.M.; Cines, D.B.; Muzykantov, V.R. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood 2005, 106, 4191–4198. [Google Scholar] [CrossRef]
- Ding, B.S.; Hong, N.; Murciano, J.C.; Ganguly, K.; Gottstein, C.; Christofidou-Solomidou, M.; Albelda, S.M.; Fisher, A.B.; Cines, D.B.; Muzykantov, V.R. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood 2008, 111, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Kozower, B.D.; Christofidou-Solomidou, M.; Sweitzer, T.D.; Muro, S.; Buerk, D.G.; Solomides, C.C.; Albelda, S.M.; Patterson, G.A.; Muzykantov, V.R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003, 21, 392–398. [Google Scholar] [CrossRef]
- Preissler, G.; Loehe, F.; Huff, I.V.; Ebersberger, U.; Shuvaev, V.V.; Bittmann, I.; Hermanns, I.; Kirkpatrick, J.C.; Fischer, K.; Eichhorn, M.E.; et al. Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death. Transplantation 2011, 92, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Millikan, R.E.; Christianson, D.R.; Cardo-Vila, M.; Driessen, W.H.; Giordano, R.J.; Hajitou, A.; Hoang, A.G.; Wen, S.; Barnhart, K.F.; et al. Targeting the interleukin-11 receptor alpha in metastatic prostate cancer: A first-in-man study. Cancer 2015, 121, 2411–2421. [Google Scholar] [CrossRef]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, N.K.; Kang, S.K.; Choi, S.H.; Kim, D.; Park, K.; Choi, K.; Choi, Y.J.; Jung, D.H. Identification of tissue-specific targeting peptide. J. Comput. Aided Mol. Des. 2012, 26, 1267–1275. [Google Scholar] [CrossRef]
- Denby, L.; Work, L.M.; Seggern, D.J.; Wu, E.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Development of renal-targeted vectors through combined in vivo phage display and capsid engineering of adenoviral fibers from serotype 19p. Mol. Ther. 2007, 15, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Koivunen, E.; Ruoslahti, E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat. Biotechnol 1997, 15, 542–546. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Ding, N.; Zou, Z.; Sha, H.; Su, S.; Qian, H.; Meng, F.; Chen, F.; Du, S.; Zhou, S.; Chen, H.; et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat. Commun. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Huang, Y.; Chen, Y. Co-administration of iRGD with peptide HPRP-A1 to improve anticancer activity and membrane penetrability. Sci. Rep. 2018, 8, 2274. [Google Scholar] [CrossRef] [PubMed]
- Hajitou, A.; Trepel, M.; Lilley, C.E.; Soghomonyan, S.; Alauddin, M.M.; Marini, F.C., 3rd; Restel, B.H.; Ozawa, M.G.; Moya, C.A.; Rangel, R.; et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006, 125, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Hajitou, A.; Rangel, R.; Trepel, M.; Soghomonyan, S.; Gelovani, J.G.; Alauddin, M.M.; Pasqualini, R.; Arap, W. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat. Protoc. 2007, 2, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Bayo-Puxan, N.; Gimenez-Alejandre, M.; Lavilla-Alonso, S.; Gros, A.; Cascallo, M.; Hemminki, A.; Alemany, R. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum. Gene Ther. 2009, 20, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Puig-Saus, C.; Rojas, L.A.; Laborda, E.; Figueras, A.; Alba, R.; Fillat, C.; Alemany, R. iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination and antitumor efficacy. Gene Ther. 2014, 21, 767–774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Palma, M.; Venneri, M.A.; Naldini, L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum. Gene Ther. 2003, 14, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Nicklin, S.A.; Buning, H.; Brosnan, M.J.; Leike, K.; Papadakis, E.D.; Hallek, M.; Baker, A.H. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 2004, 109, 513–519. [Google Scholar] [CrossRef]
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef]
- Vincke, C.; Muyldermans, S. Introduction to heavy chain antibodies and derived Nanobodies. Methods Mol. Biol. 2012, 911, 15–26. [Google Scholar]
- Guo, L.; Zhang, H.; Hou, Y.; Wei, T.; Liu, J. Plasmalemma vesicle-associated protein: A crucial component of vascular homeostasis. Exp. Ther. Med. 2016, 12, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Carson-Walter, E.B.; Hampton, J.; Shue, E.; Geynisman, D.M.; Pillai, P.K.; Sathanoori, R.; Madden, S.L.; Hamilton, R.L.; Walter, K.A. Plasmalemmal vesicle associated protein-1 is a novel marker implicated in brain tumor angiogenesis. Clin. Cancer Res. 2005, 11, 7643–7650. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carson-Walter, E.B.; Cooper, A.; Winans, B.N.; Johnson, M.D.; Walter, K.A. Vascular gene expression patterns are conserved in primary and metastatic brain tumors. J. Neurooncol. 2010, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mozer, A.B.; Whittemore, S.R.; Benton, R.L. Spinal microvascular expression of PV-1 is associated with inflammation, perivascular astrocyte loss, and diminished EC glucose transport potential in acute SCI. Curr Neurovasc. Res. 2010, 7, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shue, E.H.; Carson-Walter, E.B.; Liu, Y.; Winans, B.N.; Ali, Z.S.; Chen, J.; Walter, K.A. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood-brain barrier disruption in rodent models. BMC Neurosci. 2008, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10. [Google Scholar]
- Behdani, M.; Zeinali, S.; Khanahmad, H.; Karimipour, M.; Asadzadeh, N.; Azadmanesh, K.; Khabiri, A.; Schoonooghe, S.; Habibi Anbouhi, M.; Hassanzadeh-Ghassabeh, G.; et al. Generation and characterization of a functional Nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol. Immunol. 2012, 50, 35–41. [Google Scholar] [CrossRef]
- Ma, L.; Gu, K.; Zhang, C.H.; Chen, X.T.; Jiang, Y.; Melcher, K.; Zhang, J.; Wang, M.; Xu, H.E. Generation and characterization of a human nanobody against VEGFR-2. Acta Pharm. Sin. 2016, 37, 857–864. [Google Scholar] [CrossRef]
- Mashayekhi, V.; Xenaki, K.T.; van Bergen En Henegouwen, P.M.P.; Oliveira, S. Dual Targeting of Endothelial and Cancer Cells Potentiates In Vitro Nanobody-Targeted Photodynamic Therapy. Cancers 2020, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Papadopoulos, Z. Aflibercept: A review of its effect on the treatment of exudative age-related macular degeneration. Eur J. Ophthalmol. 2019, 29, 368–378. [Google Scholar] [CrossRef]
- Stewart, M.W. A Review of Ranibizumab for the Treatment of Diabetic Retinopathy. Ophthalmol. Ther. 2017, 6, 33–47. [Google Scholar] [CrossRef]
- Paz, K.; Zhu, Z. Development of angiogenesis inhibitors to vascular endothelial growth factor receptor 2. Current status and future perspective. Front. Biosci. 2005, 10, 1415–1439. [Google Scholar] [CrossRef] [PubMed]
- Robert, B.; Zhao, X.; Abrahamson, D.R. Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am. J. Physiol. Ren. Physiol. 2000, 279, F275–F282. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004, 65, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Paulus, Y.M.; Sodhi, A. Anti-angiogenic Therapy for Retinal Disease. Handb Exp. Pharm. 2017, 242, 271–307. [Google Scholar]
- Nixon, A.E.; Sexton, D.J.; Ladner, R.C. Drugs derived from phage display: From candidate identification to clinical practice. MAbs 2014, 6, 73–85. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Suwan, K.; Yata, T.; Waramit, S.; Przystal, J.M.; Stoneham, C.A.; Bentayebi, K.; Asavarut, P.; Chongchai, A.; Pothachareon, P.; Lee, K.Y.; et al. Next-generation of targeted AAVP vectors for systemic transgene delivery against cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 18571–18577. [Google Scholar] [CrossRef]
- Diaz Bessone, M.I.; Simon-Gracia, L.; Scodeller, P.; Ramirez, M.L.A.; Lago Huvelle, M.A.; Soler-Illia, G.; Simian, M. iRGD-guided tamoxifen polymersomes inhibit estrogen receptor transcriptional activity and decrease the number of breast cancer cells with self-renewing capacity. J. Nanobiotechnol. 2019, 17, 120. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar]
- Curnis, F.; Sacchi, A.; Borgna, L.; Magni, F.; Gasparri, A.; Corti, A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat. Biotechnol. 2000, 18, 1185–1190. [Google Scholar] [CrossRef]
- Curnis, F.; Gasparri, A.; Sacchi, A.; Cattaneo, A.; Magni, F.; Corti, A. Targeted delivery of IFNgamma to tumor vessels uncouples antitumor from counterregulatory mechanisms. Cancer Res. 2005, 65, 2906–2913. [Google Scholar] [CrossRef]
- Lee, T.Y.; Lin, C.T.; Kuo, S.Y.; Chang, D.K.; Wu, H.C. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007, 67, 10958–10965. [Google Scholar] [CrossRef]
- El-Sheikh, A.; Liu, C.; Huang, H.; Edgington, T.S. A novel vascular endothelial growth factor heparin-binding domain substructure binds to glycosaminoglycans in vivo and localizes to tumor microvascular endothelium. Cancer Res. 2002, 62, 7118–7123. [Google Scholar]
- Koivunen, E.; Ranta, T.M.; Annila, A.; Taube, S.; Uppala, A.; Jokinen, M.; van Willigen, G.; Ihanus, E.; Gahmberg, C.G. Inhibition of beta(2) integrin-mediated leukocyte cell adhesion by leucine-leucine-glycine motif-containing peptides. J. Cell Biol. 2001, 153, 905–916. [Google Scholar] [CrossRef]
- Cardo-Vila, M.; Zurita, A.J.; Giordano, R.J.; Sun, J.; Rangel, R.; Guzman-Rojas, L.; Anobom, C.D.; Valente, A.P.; Almeida, F.C.; Lahdenranta, J.; et al. A ligand peptide motif selected from a cancer patient is a receptor-interacting site within human interleukin-11. PLoS ONE 2008, 3, e3452. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Hensen, L.C.M.; Hoeben, R.C.; Bots, S.T.F. Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 6828. [Google Scholar] [CrossRef]
- Leopold, P.L.; Crystal, R.G. Intracellular trafficking of adenovirus: Many means to many ends. Adv. Drug Deliv. Rev. 2007, 59, 810–821. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; Cai, Y. An overview of development in gene therapeutics in China. Gene Ther. 2020, 27, 338–348. [Google Scholar] [CrossRef]
- European Medicines Agency. Zabdeno. Ebola Vaccine (Ad26.ZEBOV-GP). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zabdeno (accessed on 6 September 2021).
- Chung, J.; Kim, K.H.; An, S.H.; Lee, S.; Lim, B.K.; Kang, S.W.; Kwon, K. Coxsackievirus and adenovirus receptor mediates the responses of endothelial cells to fluid shear stress. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. alphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Verna, L.; Hardy, S.; Forsayeth, J.; Zhu, Y.; Stemerman, M.B. Adenovirus-mediated overexpression of c-Jun and c-Fos induces intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human endothelial cells. Arter. Thromb. Vasc. Biol. 1999, 19, 2078–2084. [Google Scholar] [CrossRef][Green Version]
- Erzurum, S.C.; Lemarchand, P.; Rosenfeld, M.A.; Yoo, J.H.; Crystal, R.G. Protection of human endothelial cells from oxidant injury by adenovirus-mediated transfer of the human catalase cDNA. Nucleic Acids Res. 1993, 21, 1607–1612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ivanciu, L.; Gerard, R.D.; Tang, H.; Lupu, F.; Lupu, C. Adenovirus-mediated expression of tissue factor pathway inhibitor-2 inhibits endothelial cell migration and angiogenesis. Arter. Thromb. Vasc. Biol. 2007, 27, 310–316. [Google Scholar] [CrossRef]
- Jornot, L.; Morris, M.A.; Petersen, H.; Moix, I.; Rochat, T. N-acetylcysteine augments adenovirus-mediated gene expression in human endothelial cells by enhancing transgene transcription and virus entry. J. Gene Med. 2002, 4, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, C.J.; Hofer-Warbinek, R.; Moll, T.; Eytner, R.; Bach, F.H.; de Martin, R. Inhibition of endothelial cell activation by adenovirus-mediated expression of I kappa B alpha, an inhibitor of the transcription factor NF-kappa B. J. Exp. Med. 1996, 183, 1013–1022. [Google Scholar] [CrossRef]
- Soares, M.P.; Muniappan, A.; Kaczmarek, E.; Koziak, K.; Wrighton, C.J.; Steinhauslin, F.; Ferran, C.; Winkler, H.; Bach, F.H.; Anrather, J. Adenovirus-mediated expression of a dominant negative mutant of p65/RelA inhibits proinflammatory gene expression in endothelial cells without sensitizing to apoptosis. J. Immunol. 1998, 161, 4572–4582. [Google Scholar]
- Lee, K.U.; Lee, I.K.; Han, J.; Song, D.K.; Kim, Y.M.; Song, H.S.; Kim, H.S.; Lee, W.J.; Koh, E.H.; Song, K.H.; et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ. Res. 2005, 96, 1200–1207. [Google Scholar] [CrossRef]
- Lin, S.J.; Shyue, S.K.; Liu, P.L.; Chen, Y.H.; Ku, H.H.; Chen, J.W.; Tam, K.B.; Chen, Y.L. Adenovirus-mediated overexpression of catalase attenuates oxLDL-induced apoptosis in human aortic endothelial cells via AP-1 and C-Jun N-terminal kinase/extracellular signal-regulated kinase mitogen-activated protein kinase pathways. J. Mol. Cell Cardiol. 2004, 36, 129–139. [Google Scholar] [CrossRef]
- Qiu, K.; Su, Y.; Block, E.R. Use of recombinant calpain-2 siRNA adenovirus to assess calpain-2 modulation of lung endothelial cell migration and proliferation. Mol. Cell Biochem. 2006, 292, 69–78. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, X.; Scott, G.I.; Esberg, L.B.; Ren, B.H.; Culver, B.; Chen, A.F. Adenovirus gene transfer of recombinant endothelial nitric oxide synthase enhances contractile function in ventricular myocytes. J. Cardiovasc. Pharm. 2004, 43, 171–177. [Google Scholar] [CrossRef][Green Version]
- Kang, H.; Yang, P.Y.; Rui, Y.C. Adenovirus viral interleukin-10 inhibits adhesion molecule expressions induced by hypoxia/reoxygenation in cerebrovascular endothelial cells. Acta Pharm. Sin. 2008, 29, 50–56. [Google Scholar] [CrossRef]
- Ooboshi, H.; Chu, Y.; Rios, C.D.; Faraci, F.M.; Davidson, B.L.; Heistad, D.D. Altered vascular function after adenovirus-mediated overexpression of endothelial nitric oxide synthase. Am. J. Physiol. 1997, 273, H265–H270. [Google Scholar] [CrossRef]
- Fennell, J.P.; Brosnan, M.J.; Frater, A.J.; Hamilton, C.A.; Alexander, M.Y.; Nicklin, S.A.; Heistad, D.D.; Baker, A.H.; Dominiczak, A.F. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther. 2002, 9, 110–117. [Google Scholar] [CrossRef]
- Yan, J.; Tang, G.L.; Wang, R.; Messina, L.M. Optimization of adenovirus-mediated endothelial nitric oxide synthase delivery in rat hindlimb ischemia. Gene Ther. 2005, 12, 1640–1650. [Google Scholar] [CrossRef]
- Kholova, I.; Koota, S.; Kaskenpaa, N.; Leppanen, P.; Narvainen, J.; Kavec, M.; Rissanen, T.T.; Hazes, T.; Korpisalo, P.; Grohn, O.; et al. Adenovirus-mediated gene transfer of human vascular endothelial growth factor-d induces transient angiogenic effects in mouse hind limb muscle. Hum. Gene Ther. 2007, 18, 232–244. [Google Scholar] [CrossRef]
- Roy, H.; Bhardwaj, S.; Babu, M.; Jauhiainen, S.; Herzig, K.H.; Bellu, A.R.; Haisma, H.J.; Carmeliet, P.; Alitalo, K.; Yla-Herttuala, S. Adenovirus-mediated gene transfer of placental growth factor to perivascular tissue induces angiogenesis via upregulation of the expression of endogenous vascular endothelial growth factor-A. Hum. Gene Ther. 2005, 16, 1422–1428. [Google Scholar] [CrossRef]
- Sharma, A.; Li, X.; Bangari, D.S.; Mittal, S.K. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009, 143, 184–194. [Google Scholar] [CrossRef]
- Hayashi, H.; Ohba, M. Immobilization of the periplasmic maltose-binding protein of Escherichia coli. Ann. Microbiol. 1982, 133A, 195–197. [Google Scholar]
- Reynolds, A.M.; Xia, W.; Holmes, M.D.; Hodge, S.J.; Danilov, S.; Curiel, D.T.; Morrell, N.W.; Reynolds, P.N. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1182–L1192. [Google Scholar] [CrossRef]
- Nicklin, S.A.; White, S.J.; Watkins, S.J.; Hawkins, R.E.; Baker, A.H. Selective targeting of gene transfer to vascular endothelial cells by use of peptides isolated by phage display. Circulation 2000, 102, 231–237. [Google Scholar] [CrossRef]
- Nicklin, S.A.; Von Seggern, D.J.; Work, L.M.; Pek, D.C.; Dominiczak, A.F.; Nemerow, G.R.; Baker, A.H. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol. Ther. 2001, 4, 534–542. [Google Scholar] [CrossRef]
- Nicklin, S.A.; White, S.J.; Nicol, C.G.; Von Seggern, D.J.; Baker, A.H. In vitro and in vivo characterisation of endothelial cell selective adenoviral vectors. J. Gene Med. 2004, 6, 300–308. [Google Scholar] [CrossRef]
- Seimetz, D.; Heller, K.; Richter, J. Approval of First CAR-Ts: Have we Solved all Hurdles for ATMPs? Cell Med. 2019, 11, 2155179018822781. [Google Scholar] [CrossRef]
- Vogt, V.M. Retroviral Virions and Genomes. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor: New York, NY, USA, 1997. [Google Scholar]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Primo, L.; Roca, C.; Ferrandi, C.; Lanfrancone, L.; Bussolino, F. Human endothelial cells expressing polyoma middle T induce tumors. Oncogene 2000, 19, 3632–3641. [Google Scholar] [CrossRef][Green Version]
- Inaba, M.; Toninelli, E.; Vanmeter, G.; Bender, J.R.; Conte, M.S. Retroviral gene transfer: Effects on endothelial cell phenotype. J. Surg Res. 1998, 78, 31–36. [Google Scholar] [CrossRef]
- Zheng, L.; Dengler, T.J.; Kluger, M.S.; Madge, L.A.; Schechner, J.S.; Maher, S.E.; Pober, J.S.; Bothwell, A.L. Cytoprotection of human umbilical vein endothelial cells against apoptosis and CTL-mediated lysis provided by caspase-resistant Bcl-2 without alterations in growth or activation responses. J. Immunol. 2000, 164, 4665–4671. [Google Scholar] [CrossRef]
- Batzlsperger, C.A.; Achatz, S.; Spreng, J.; Riegger, G.A.; Griese, D.P. Evidence for a possible inhibitory interaction between the HO-1/CO- and Akt/NO-pathways in human endothelial cells. Cardiovasc. Drugs Ther. 2007, 21, 347–355. [Google Scholar] [CrossRef]
- Finkelshtein, D.; Werman, A.; Novick, D.; Barak, S.; Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2013, 110, 7306–7311. [Google Scholar] [CrossRef]
- Koren, B.; Weisz, A.; Fischer, L.; Gluzman, Z.; Preis, M.; Avramovitch, N.; Cohen, T.; Cosset, F.L.; Lewis, B.S.; Flugelman, M.Y. Efficient transduction and seeding of human endothelial cells onto metallic stents using bicistronic pseudo-typed retroviral vectors encoding vascular endothelial growth factor. Cardiovasc. Revasc. Med. 2006, 7, 173–178. [Google Scholar] [CrossRef]
- Landers, M.C.; Dugger, N.; Quadros, M.; Hoffman, P.M.; Gaulton, G.N. Neuropathogenic murine leukemia virus TR1.3 induces selective syncytia formation of brain capillary endothelium. Virology 2004, 321, 57–64. [Google Scholar] [CrossRef][Green Version]
- Valtink, M.; Stanke, N.; Knels, L.; Engelmann, K.; Funk, R.H.; Lindemann, D. Pseudotyping and culture conditions affect efficiency and cytotoxicity of retroviral gene transfer to human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6807–6813. [Google Scholar] [CrossRef]
- Cefai, D.; Simeoni, E.; Ludunge, K.M.; Driscoll, R.; von Segesser, L.K.; Kappenberger, L.; Vassalli, G. Multiply attenuated, self-inactivating lentiviral vectors efficiently transduce human coronary artery cells in vitro and rat arteries in vivo. J. Mol. Cell Cardiol. 2005, 38, 333–344. [Google Scholar] [CrossRef]
- Morizono, K.; Bristol, G.; Xie, Y.M.; Kung, S.K.; Chen, I.S. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001, 75, 8016–8020. [Google Scholar] [CrossRef]
- Pariente, N.; Mao, S.H.; Morizono, K.; Chen, I.S. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J. Gene Med. 2008, 10, 242–248. [Google Scholar] [CrossRef]
- Ahani, R.; Roohvand, F.; Cohan, R.A.; Etemadzadeh, M.H.; Mohajel, N.; Behdani, M.; Shahosseini, Z.; Madani, N.; Azadmanesh, K. Sindbis Virus-Pseudotyped Lentiviral Vectors Carrying VEGFR2-Specific Nanobody for Potential Transductional Targeting of Tumor Vasculature. Mol. Biotechnol. 2016, 58, 738–747. [Google Scholar] [CrossRef]
- Yang, L.; Bailey, L.; Baltimore, D.; Wang, P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 11479–11484. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Summerford, C.; Samulski, R.J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef]
- Asokan, A.; Hamra, J.B.; Govindasamy, L.; Agbandje-McKenna, M.; Samulski, R.J. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J. Virol. 2006, 80, 8961–8969. [Google Scholar] [CrossRef]
- Qing, K.; Mah, C.; Hansen, J.; Zhou, S.; Dwarki, V.; Srivastava, A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999, 5, 71–77. [Google Scholar] [CrossRef]
- Pillay, S.; Meyer, N.L.; Puschnik, A.S.; Davulcu, O.; Diep, J.; Ishikawa, Y.; Jae, L.T.; Wosen, J.E.; Nagamine, C.M.; Chapman, M.S.; et al. An essential receptor for adeno-associated virus infection. Nature 2016, 530, 108–112. [Google Scholar] [CrossRef]
- Chen, S.; Kapturczak, M.; Loiler, S.A.; Zolotukhin, S.; Glushakova, O.Y.; Madsen, K.M.; Samulski, R.J.; Hauswirth, W.W.; Campbell-Thompson, M.; Berns, K.I.; et al. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum. Gene Ther. 2005, 16, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Hirsch, M.L.; Barker, J.C.; Connelly, J.P.; Steininger, R.J., 3rd; Porteus, M.H. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1–9) and one engineered adeno-associated virus serotype. Virol. J. 2013, 10, 74. [Google Scholar] [CrossRef]
- Dishart, K.L.; Denby, L.; George, S.J.; Nicklin, S.A.; Yendluri, S.; Tuerk, M.J.; Kelley, M.P.; Donahue, B.A.; Newby, A.C.; Harding, T.; et al. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: Implications for gene therapy. J. Mol. Cell Cardiol. 2003, 35, 739–748. [Google Scholar] [CrossRef]
- Li, J.; Wen, A.M.; Potla, R.; Benshirim, E.; Seebarran, A.; Benz, M.A.; Henry, O.Y.F.; Matthews, B.D.; Prantil-Baun, R.; Gilpin, S.E.; et al. AAV-mediated gene therapy targeting TRPV4 mechanotransduction for inhibition of pulmonary vascular leakage. APL Bioeng. 2019, 3, 046103. [Google Scholar] [CrossRef] [PubMed]
- Denby, L.; Nicklin, S.A.; Baker, A.H. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005, 12, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Xi, L.; Boye, S.L.; Han, S.; Chen, Z.J.; Hauswirth, W.W.; Lewin, A.S.; Boulton, M.E.; Law, B.K.; Jiang, W.G.; et al. Development of an anti-angiogenic therapeutic model combining scAAV2-delivered siRNAs and noninvasive photoacoustic imaging of tumor vasculature development. Cancer Lett. 2013, 332, 120–129. [Google Scholar] [CrossRef]
- Eslami, M.H.; Gangadharan, S.P.; Sui, X.; Rhynhart, K.K.; Snyder, R.O.; Conte, M.S. Gene delivery to in situ veins: Differential effects of adenovirus and adeno-associated viral vectors. J. Vasc. Surg. 2000, 31, 1149–1159. [Google Scholar] [CrossRef][Green Version]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef]
- Nicklin, S.A.; Buening, H.; Dishart, K.L.; de Alwis, M.; Girod, A.; Hacker, U.; Thrasher, A.J.; Ali, R.R.; Hallek, M.; Baker, A.H. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol. Ther. 2001, 4, 174–181. [Google Scholar] [CrossRef]
- Muller, O.J.; Kaul, F.; Weitzman, M.D.; Pasqualini, R.; Arap, W.; Kleinschmidt, J.A.; Trepel, M. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 2003, 21, 1040–1046. [Google Scholar] [CrossRef]
- Perabo, L.; Buning, H.; Kofler, D.M.; Ried, M.U.; Girod, A.; Wendtner, C.M.; Enssle, J.; Hallek, M. In vitro selection of viral vectors with modified tropism: The adeno-associated virus display. Mol. Ther. 2003, 8, 151–157. [Google Scholar] [CrossRef]
- Zhang, L.; Rossi, A.; Lange, L.; Meumann, N.; Koitzsch, U.; Christie, K.; Nesbit, M.A.; Moore, C.B.T.; Hacker, U.T.; Morgan, M.; et al. Capsid Engineering Overcomes Barriers Toward Adeno-Associated Virus Vector-Mediated Transduction of Endothelial Cells. Hum. Gene 2019, 30, 1284–1296. [Google Scholar] [CrossRef]
- Tan, C.; Lu, N.N.; Wang, C.K.; Chen, D.Y.; Sun, N.H.; Lyu, H.; Korbelin, J.; Shi, W.X.; Fukunaga, K.; Lu, Y.M.; et al. Endothelium-Derived Semaphorin 3G Regulates Hippocampal Synaptic Structure and Plasticity via Neuropilin-2/PlexinA4. Neuron 2019, 101, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.M.; Wang, Y.; Ma, Q.; Sagare, A.P.; Montagne, A.; Huuskonen, M.T.; Rege, S.V.; Kisler, K.; Dai, Z.; Korbelin, J.; et al. Endothelial LRP1 protects against neurodegeneration by blocking cyclophilin A. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, M.M.; Ahn, S.J.; Lane, D.; Faraco, G.; Garcia-Bonilla, L.; Racchumi, G.; Poon, C.; Schaeffer, S.; Segarra, S.G.; Korbelin, J.; et al. Endothelium-Macrophage Crosstalk Mediates Blood-Brain Barrier Dysfunction in Hypertension. Hypertension 2020, 76, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Sun, N.H.; Chen, X.; Gong, J.J.; Yuan, S.T.; Hu, Z.Z.; Lu, N.N.; Korbelin, J.; Fukunaga, K.; Liu, Q.H.; et al. Endothelium-derived semaphorin 3G attenuates ischemic retinopathy by coordinating beta-catenin-dependent vascular remodeling. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, S.; Huang, S.; Yoo, J.Y.; Korbelin, J.; Lee, T.J.; Kaur, B.; Dash, P.K.; Chen, P.R.; Kim, E. Selective Endothelial Hyperactivation of Oncogenic KRAS Induces Brain Arteriovenous Malformations in Mice. Ann. Neurol. 2021, 89, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.A.; Snellings, D.A.; Su, Y.S.; Hong, C.C.; Castro, M.; Tang, A.T.; Detter, M.R.; Hobson, N.; Girard, R.; Romanos, S.; et al. PIK3CA and CCM mutations fuel cavernomas through a cancer-like mechanism. Nature 2021, 594, 271–276. [Google Scholar] [CrossRef]
- Alvarez-Vergara, M.I.; Rosales-Nieves, A.E.; March-Diaz, R.; Rodriguez-Perinan, G.; Lara-Urena, N.; Ortega-de San Luis, C.; Sanchez-Garcia, M.A.; Martin-Bornez, M.; Gomez-Galvez, P.; Vicente-Munuera, P.; et al. Non-productive angiogenesis disassembles Ass plaque-associated blood vessels. Nat. Commun. 2021, 12, 3098. [Google Scholar] [CrossRef]
- Mehina, E.M.F.; Taylor, S.; Boghozian, R.; White, E.; Choi, S.E.; Cheema, M.S.; Korbelin, J.; Brown, C.E. Invasion of phagocytic Galectin 3 expressing macrophages in the diabetic brain disrupts vascular repair. Sci. Adv. 2021, 7, eabg2712. [Google Scholar] [CrossRef]
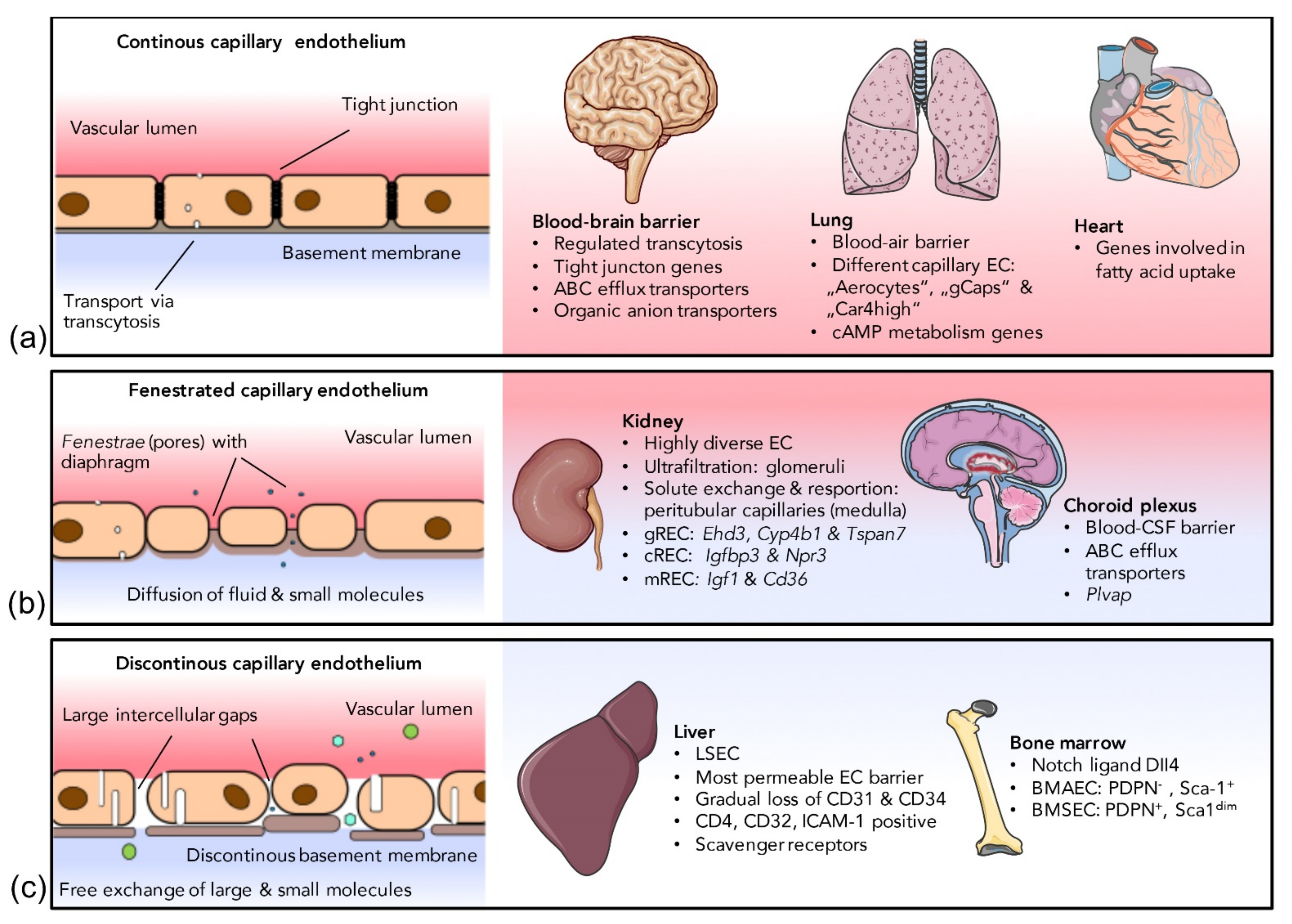
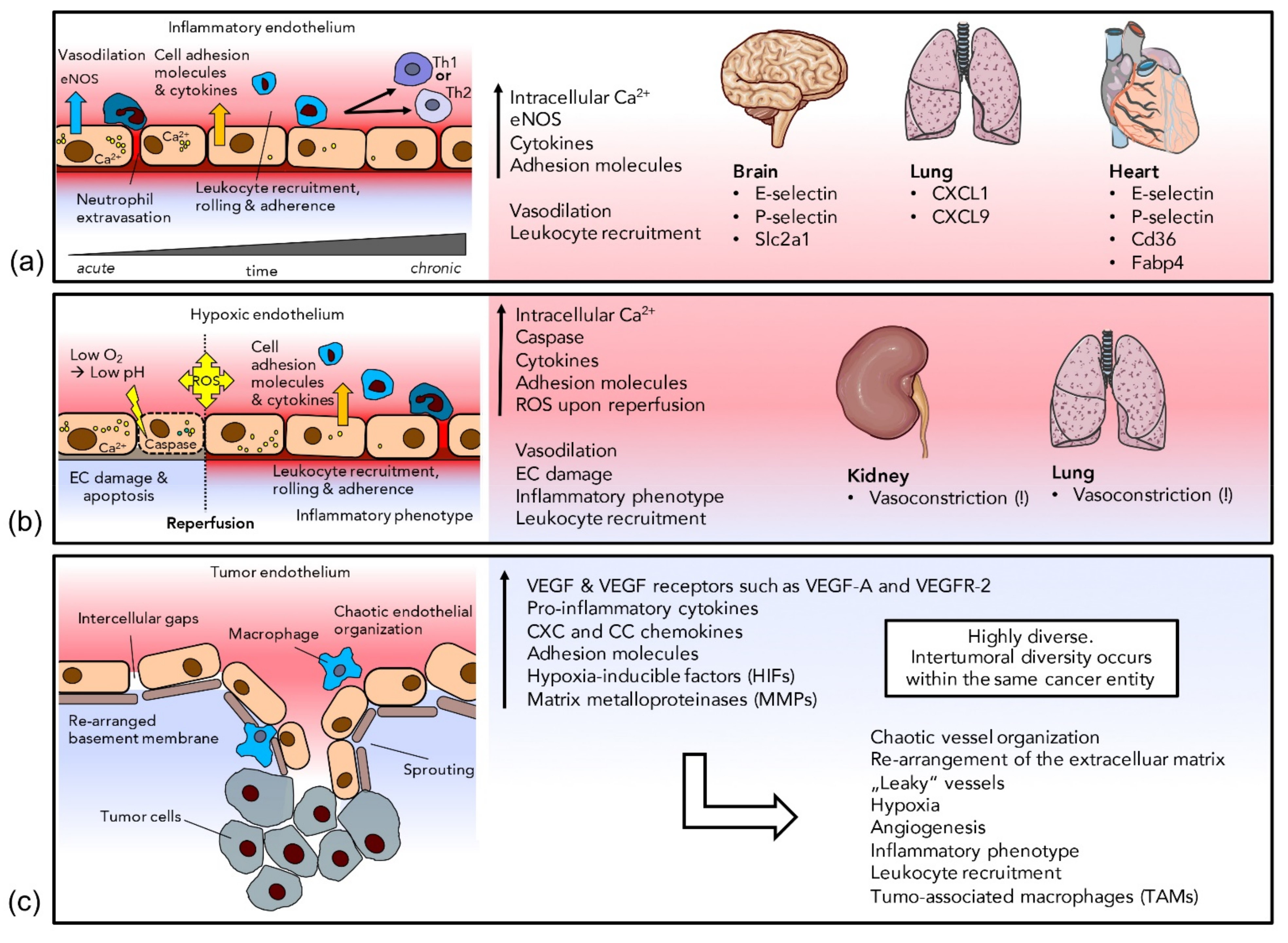
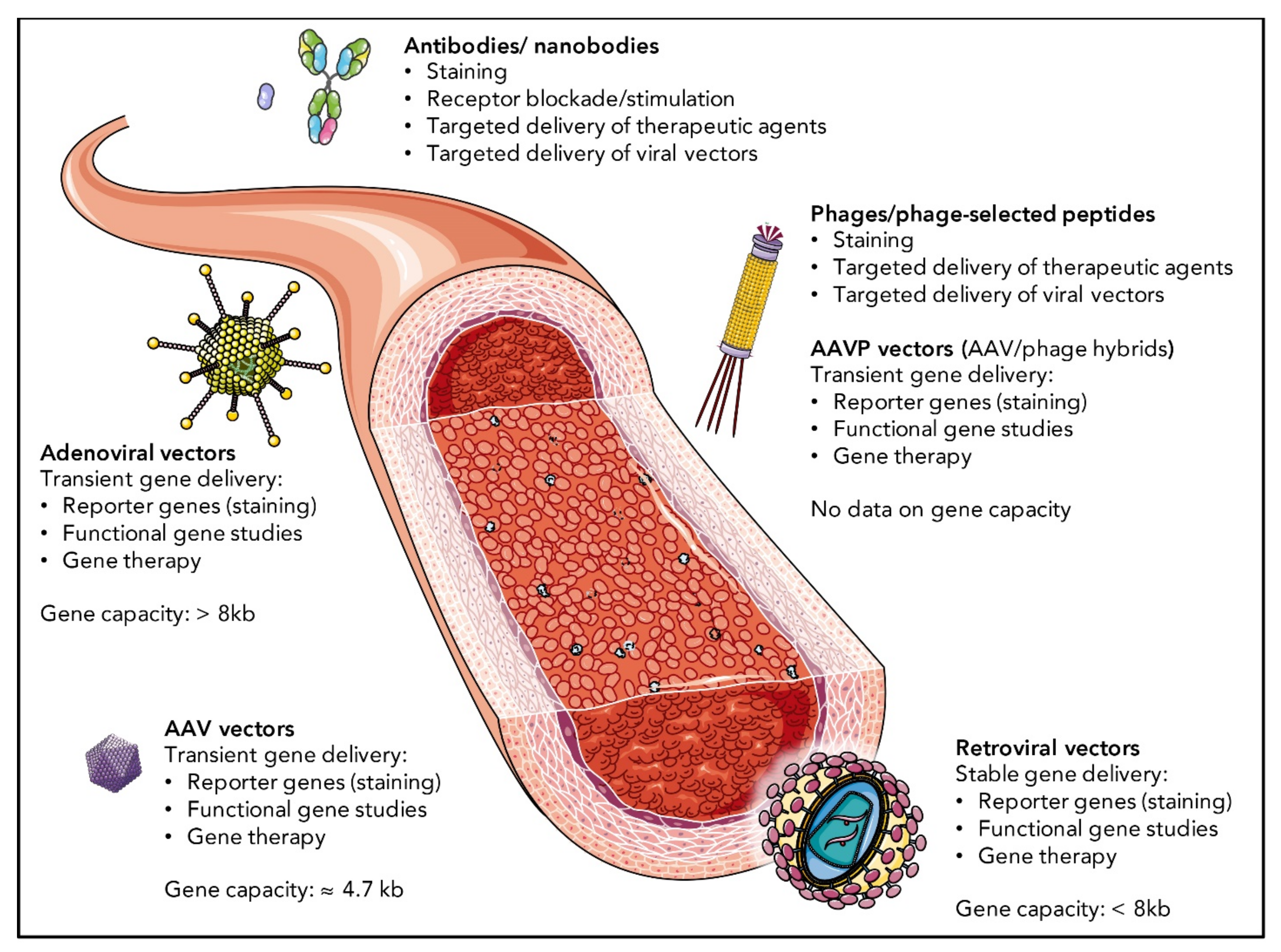
| EC Population | Species | Platform | Targeting Moiety | Application | Ref. |
|---|---|---|---|---|---|
| Cardiac ECs | Mouse | Bacteriophage | CRPPR peptide | - | [192] |
| Cerebral ECs | Mouse | Bacteriophage | CAGALCY peptide | - | [193] |
| AAV2 | NRGTEWD „BR1“ peptide (R588 insertion) | Nemo gene therapy of incontinentia pigmentii | [194,195] | ||
| Hex A/B gene therapy of Sandhoff disease | [196] | ||||
| Phage-selected DSPAHPS (“PPS”) peptide (I587 insertion) | - | [197] | |||
| Rat | Bacteriophage | QPEHSST peptide | - | [198] | |
| Cerebral ECs (ischemic) | Mouse | Antibody | PECAM-1 paratope | Urokinase-type plasminogen activator treatment of ischemic stroke | [199] |
| Cerebral EC junctions | Mouse | Bacteriophage | Peptides harboring the “FRW” morif | [200] | |
| Cerebral ECs (MPSVII mucopolysaccharidosis) | Mouse | AAV2 | Phage-selected WPFYGTP (“PFG”) peptide (I587 insertion) | β-glucuronidase gene therapy | [197] |
| Dermal ECs | Human | Bacteriophage | CHGGVGSGC peptide | - | [201] |
| Dermal ECs (inflamed) | Mouse | Ad vector | E-selectin paratope of antibody conjugated via PEG | - | [202] |
| High endothelial venule ECs (lymph nodes) | Mouse | Nanoparticle | PNAd paratope of MECA-79 monoclonal antibody | Improvement of heart allograft survival | [203] |
| Sheep | Antibody | Amelioration of asthma | [204] | ||
| Inflamed ECs | Mouse, human | Nanobody | VCAM-1 paratope | Imaging of atherosclerotic lesions by SPECT in mice | [205] |
| Mouse | Antibody | PLVAP paratope | Treatment of endotoxin-mediated inflammation in mice with SOD-coupled antibody | [206] | |
| Nanobody | VCAM-1 paratope | Imaging of atherosclerotic lesions by PET/MRI in mice | [207] | ||
| Imaging of atherosclerotic lesions by ultrasound in mice | [208] | ||||
| Ischemic muscle ECs | Mouse | Ad vector | Targeted gene expression by PPE1-3x promoter | HIF-1α gene therapy | [209] |
| Neovascular ECs | Mouse | Ad5 vector | Phage-selected “NGR” peptide motif | - | [210] |
| Pulmonary ECs | Mouse | Bacteriophage | CGFECVRQCPERC (“GFE-1”) peptide targeting membrane dipeptidase | - | [211,212] |
| AAV2 | AAV-selected peptide ESGHGYF (588 insertion) | - | [213] | ||
| Rat | Bacteriophage | VNTANST peptide | - | [198] | |
| Ad vector | ACE paratope of bi-specific antibody | BMPRII gene therapy in two rat models | [214,215] | ||
| eNOS gene therapy of stroke-prone hypertension | [216] | ||||
| ACE paratope of bi-specific antibody and targeted gene expression by flt-1 promoter | - | [217] | |||
| Antibody | ACE-paratope | Catalse treatment of lungs before transplantation | [218,219] | ||
| Fibrotic pulmonary ECs | Mouse | Antibody | PLVAP paratope | Treatment of pulmonary fibrosis with prostaglandin-coupled antibody | [220] |
| Ischemic pulmonary ECs | Mouse | Antibody | PECAM-1 paratope | Catalse treatment of acute lung injury | [221,222] |
| Thrombomodulin treatment of acute lung injury | [223] | ||||
| Urokinase-type plasminogen activator treatment of pulmonary embolism | [224,225] | ||||
| Rat | Antibody | PECAM-1 paratope | Catalse treatment of lungs before transplantation | [226] | |
| Pig | Antibody | PECAM-1 paratope | Catalse treatment of lungs before transplantation | [227] | |
| Prostate ECs | Human | Bacteriophage | IL-11Rα-binding CGRRAGGSC peptide | Treatment of metastatic prostate cancer in patients | [201,228] |
| Renal ECs | Mouse | Bacteriophage | CLPVASC peptide | - | [229] |
| Mouse/rat | Bacteriophage | PKNGSDP peptide | - | [230] | |
| DSHKDLK peptide | - | [230] | |||
| Rat | Ad19p pseudotyped Ad5 vector | Phage-selected peptides HTTHREP and HITSLLS | - | [231] | |
| Tumor ECs | Mouse | Bacteriophage | α integrin-binding peptide harboring the RGD motif | - | [232,233] |
| α integrin/Neuropilin1-binding peptide harboring iRGD motif | Imaging with iRGD-coated iron oxide nanoworms and tumor treatment with iRGD-coated abraxane | [234] | |||
| Lymphocyte infiltration in a xenograft mouse model of gastric cancer | [235] | ||||
| Improved efficacy of the anti-cancer membrane-active peptide HPRP-A1 | [236] | ||||
| AAVP vector | α integrin-binding peptide harboring the RGD motif | Transgene deliver to tumor EC | [237,238] | ||
| Ad5 vector | - | [239] | |||
| Oncolytic gene therapy | [240] | ||||
| VSC-pseudotyped lentivirus | Targeted gene expression by Tie2 promoter | - | [241] | ||
| Vena cava ECs | Mouse | AAV2 | Phage-selected peptides MSLTTPPAVARP and MTPFPTSNEANL (587 insertion) | - | [242] |
| White fat ECs | Mouse | Bacteriophage | CKGGRAKDC peptide | Ablation of adipose tissue in obese mice by apoptosis-inducing KLAKLAK peptide | [243] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. https://doi.org/10.3390/cells10102712
Hennigs JK, Matuszcak C, Trepel M, Körbelin J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells. 2021; 10(10):2712. https://doi.org/10.3390/cells10102712
Chicago/Turabian StyleHennigs, Jan K., Christiane Matuszcak, Martin Trepel, and Jakob Körbelin. 2021. "Vascular Endothelial Cells: Heterogeneity and Targeting Approaches" Cells 10, no. 10: 2712. https://doi.org/10.3390/cells10102712
APA StyleHennigs, J. K., Matuszcak, C., Trepel, M., & Körbelin, J. (2021). Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells, 10(10), 2712. https://doi.org/10.3390/cells10102712






