7-Epitaxol Induces Apoptosis and Autophagy in Head and Neck Squamous Cell Carcinoma through Inhibition of the ERK Pathway
Abstract
:1. Introduction
2. Materials and Methods
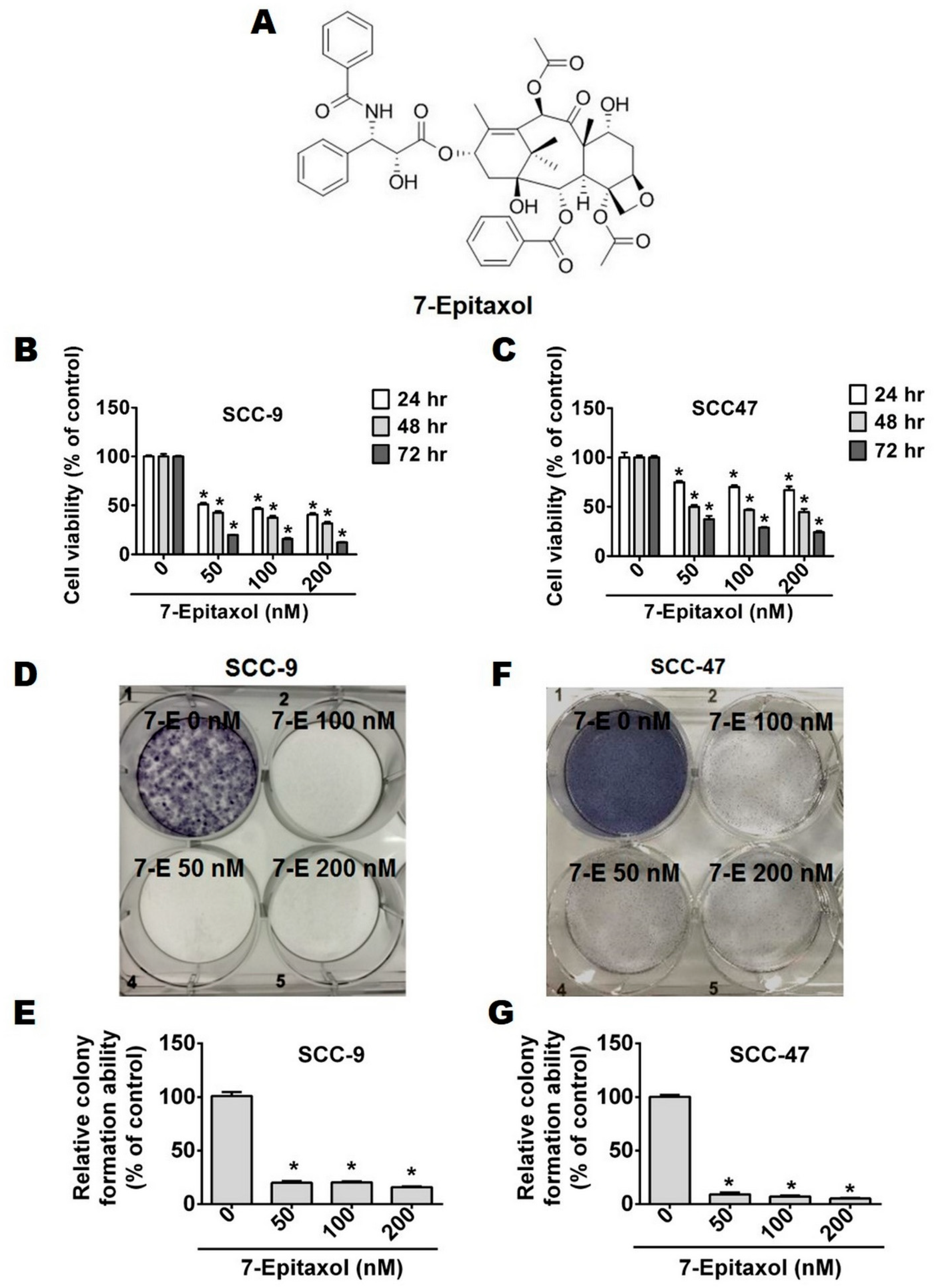
2.1. Chemical
2.2. Cell Culture
2.3. Cell Cytotoxicity
2.4. Colony Formation Assay
2.5. Cell Cycle Analysis
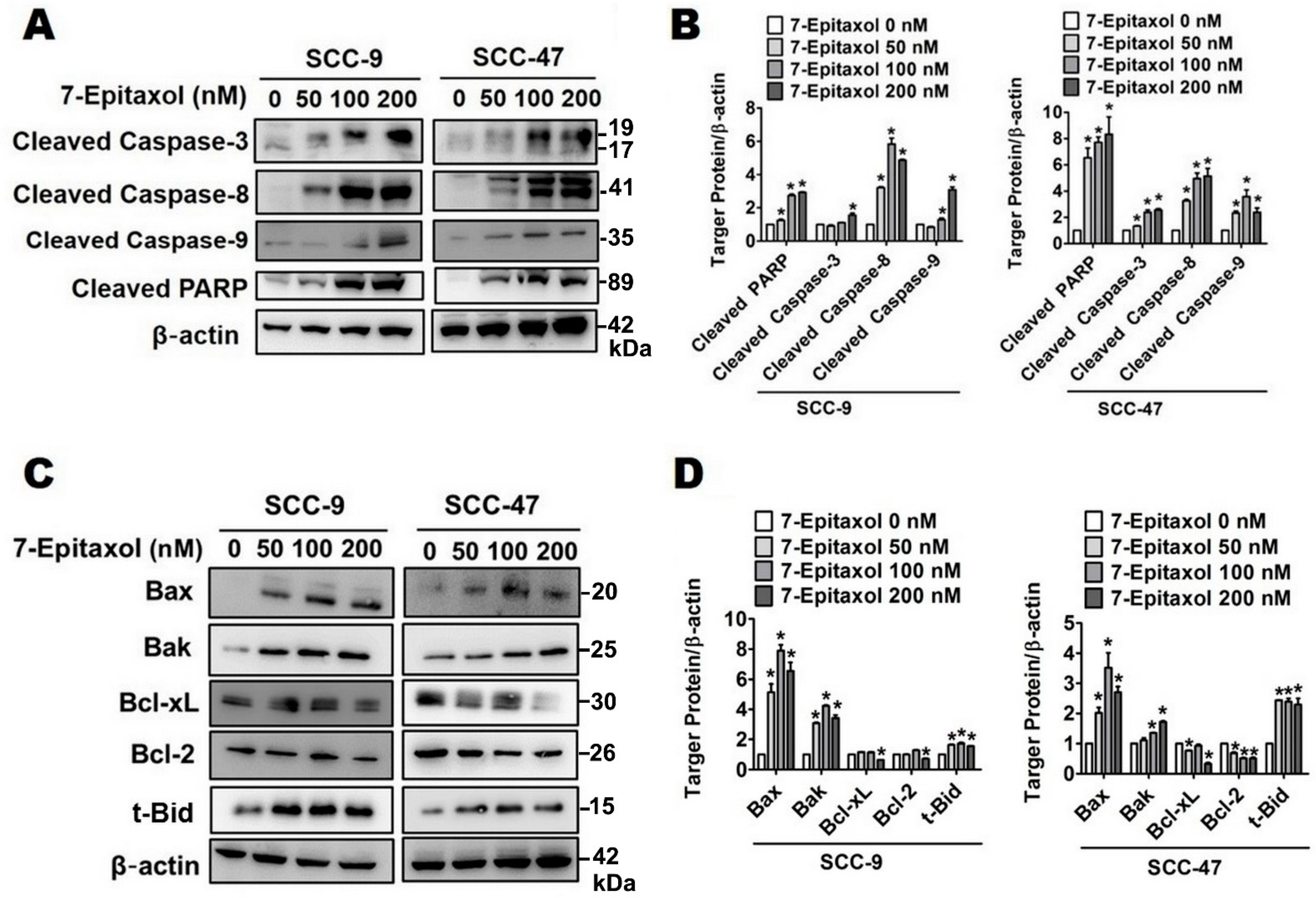
2.6. Western Blot Analysis
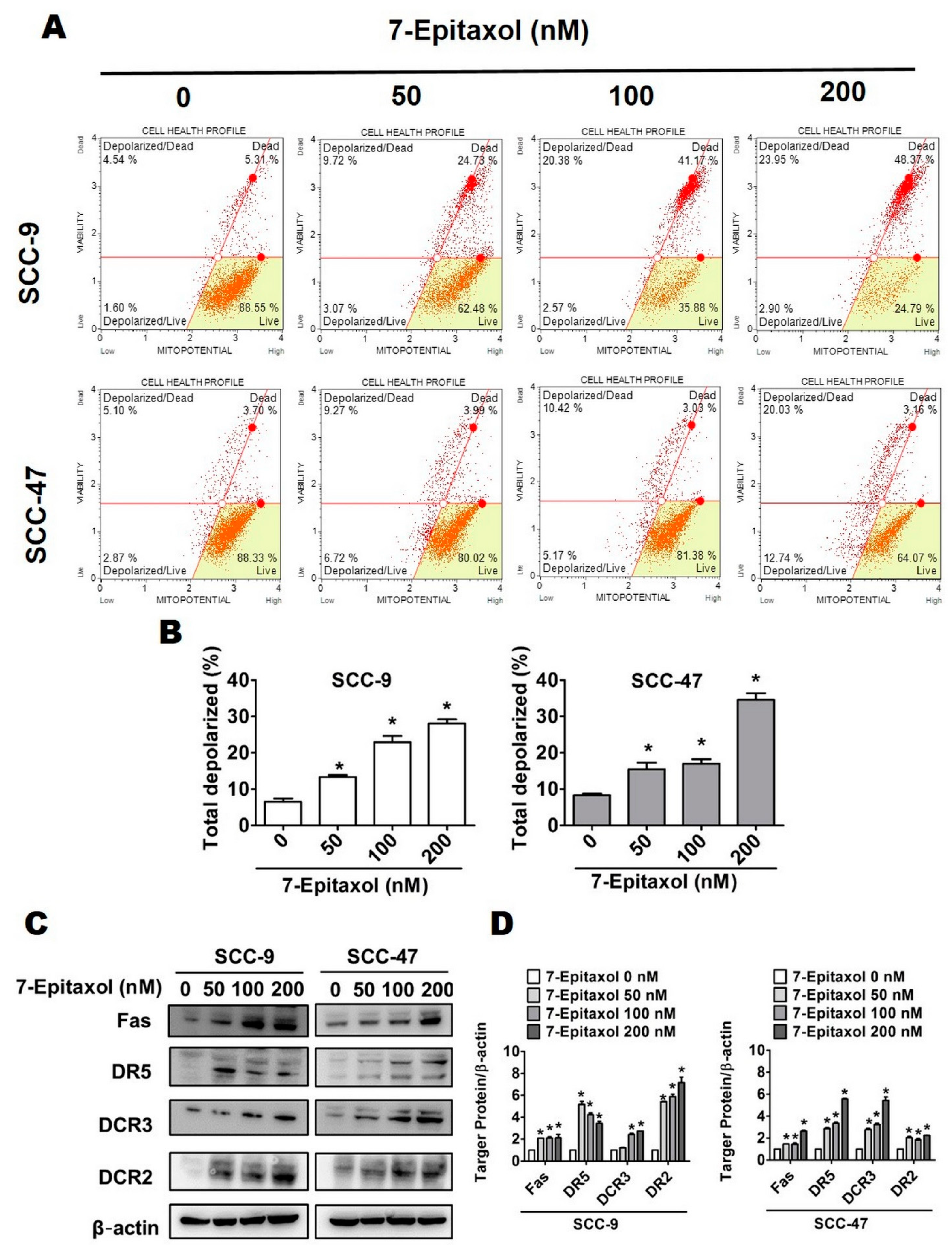
2.7. Annexin V/PI Double Staining Assay
2.8. DAPI Staining
2.9. Mitochondrial Membrane Potential Measurement
2.10. Detection of Autophagy
2.11. Statistical Analysis
3. Results
3.1. Cytotoxic Effects of 7-Epitaxol on HNCSS Cell Lines
3.2. Effect of 7-Epitaxol on Cell Cycle Progression and Apoptosis of HNCSS Cells
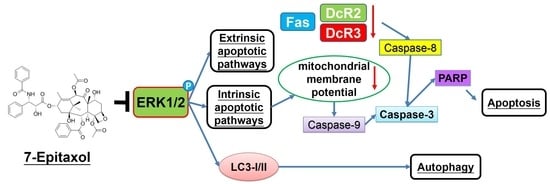
3.3. Effect of 7-Epitaxol on Apoptotic Signaling Pathways
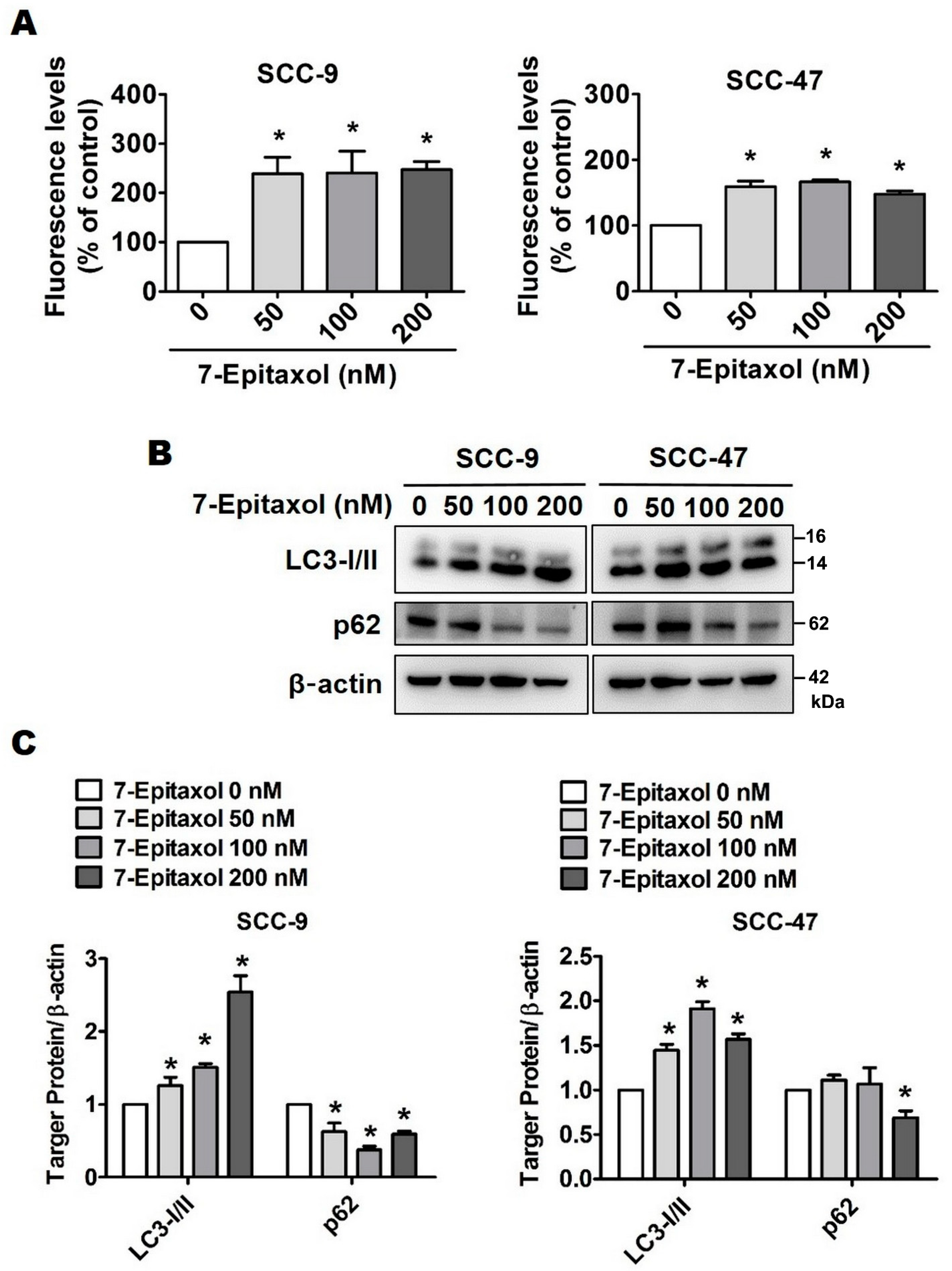
3.4. Effect of 7-Epitaxol on Autophagy Signaling Pathway
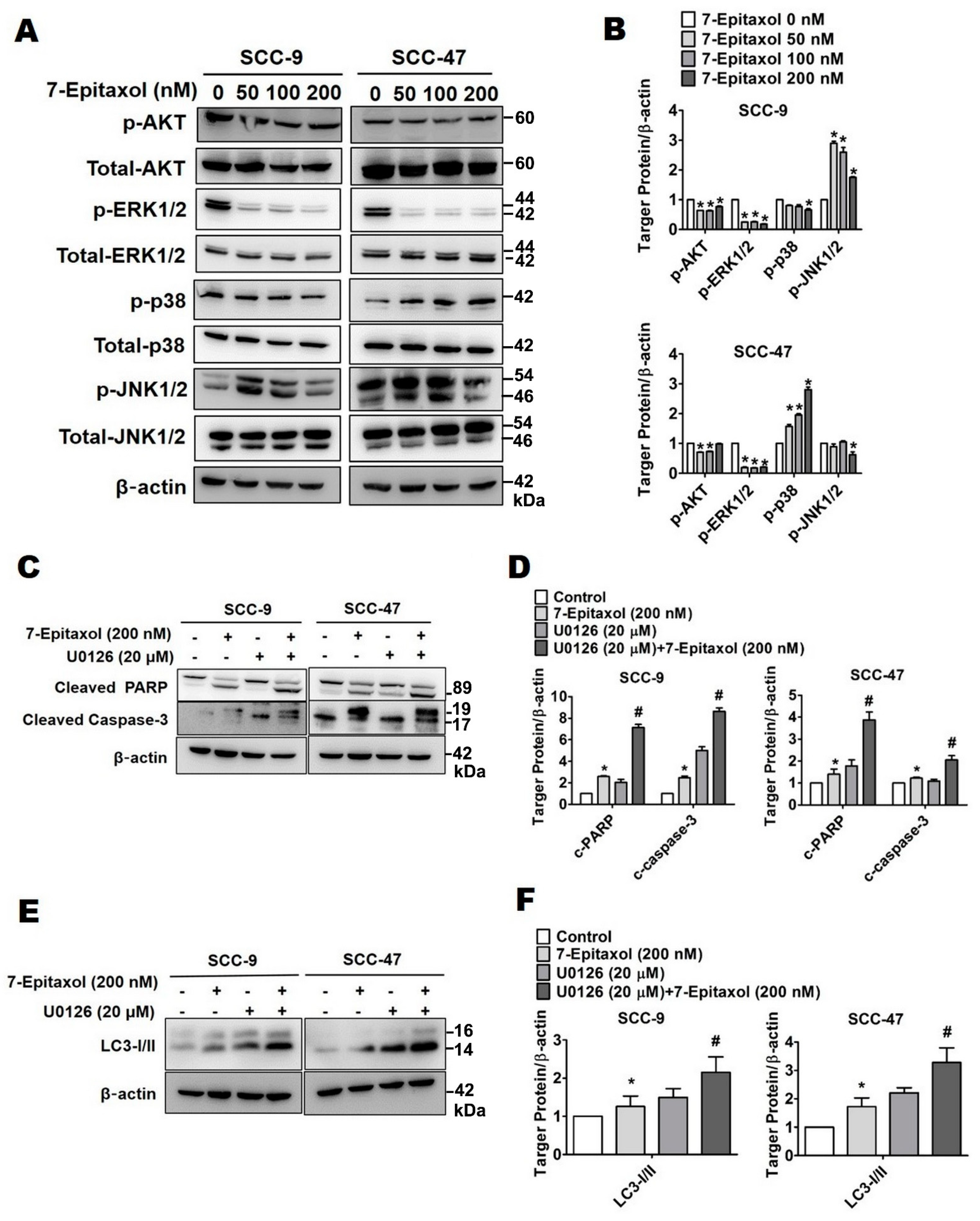
3.5. Effect of 7-Epitaxol on AKT and MAPK Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, K.P.; Shema, S.; Wu, S.; Gomez, S.L.; Chang, E.T.; Le, Q.T. Head and neck cancer-specific survival based on socioeconomic status in asians and pacific islanders. Cancer 2011, 117, 1935–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCarthy, D.; O’Sullivan, E. Public awareness of mouth, head and neck cancer. Ir. Med. J. 2010, 103, 317. [Google Scholar] [PubMed]
- Park, B.J.; Chiosea, S.I.; Grandis, J.R. Molecular changes in the multistage pathogenesis of head and neck cancer. Cancer Biomark. 2010, 9, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, P.; Eriksen, J.G.; Hamilton-Dutoit, S.; Tramm, T.; Alsner, J.; Overgaard, J.; Danish, H.; Danish Head and Neck Cancer Group (DAHANCA). Hpv-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother. Oncol. 2010, 94, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zeng, Q.; Kang, T.H.; Peng, S.; Roosinovich, E.; Pai, S.I.; Hung, C.F. Innovative DNA vaccine for human papillomavirus (hpv)-associated head and neck cancer. Gene Ther. 2011, 18, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Lee, J.H.; Wilke, W.W.; Quon, H.; Smith, G.; Maity, A.; Buatti, J.M.; Spitz, D.R. Radiation response in two hpv-infected head-and-neck cancer cell lines in comparison to a non-hpv-infected cell line and relationship to signaling through akt. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 928–933. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Jang, H.; Roh, J.L. A novel polyphenol conjugate sensitizes cisplatin-resistant head and neck cancer cells to cisplatin via nrf2 inhibition. Mol. Cancer Ther. 2016, 15, 2620–2629. [Google Scholar] [CrossRef]
- Kim, E.H.; Jang, H.; Shin, D.; Baek, S.H.; Roh, J.L. Targeting nrf2 with wogonin overcomes cisplatin resistance in head and neck cancer. Apoptosis 2016, 21, 1265–1278. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, L.L.; Xiang, R.L.; Yu, G.Y. Efficacy and safety of intro-arterial chemotherapy combined with radiotherapy on head and neck cancer: A systematic review and meta-analysis. J. Cancer 2019, 10, 6233–6243. [Google Scholar] [CrossRef]
- Schuette, A.; Lander, D.P.; Kallogjeri, D.; Collopy, C.; Goddu, S.; Wildes, T.M.; Daly, M.; Piccirillo, J.F. Predicting hearing loss after radiotherapy and cisplatin chemotherapy in patients with head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 106–112. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.; Ma, Y.; Yang, Y.; An, R. Role of hsamir105 during the pathogenesis of paclitaxel resistance and its clinical implication in ovarian cancer. Oncol. Rep. 2021, 45, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Badr El-Din, N.K.; Mahmoud, A.Z.; Hassan, T.A.; Ghoneum, M. Baker’s yeast sensitizes metastatic breast cancer cells to paclitaxel in vitro. Integr. Cancer Ther. 2018, 17, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-targeting magnetic plga nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef] [PubMed]
- Leiva, M.C.; Ortiz, R.; Contreras-Caceres, R.; Perazzoli, G.; Mayevych, I.; Lopez-Romero, J.M.; Sarabia, F.; Baeyens, J.M.; Melguizo, C.; Prados, J. Tripalmitin nanoparticle formulations significantly enhance paclitaxel antitumor activity against breast and lung cancer cells in vitro. Sci. Rep. 2017, 7, 13506. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.J.; Kim, Y.J.; Chang, H.; Kim, J.W.; Lee, J.O.; Lee, K.W.; Kim, J.H.; Bang, S.M.; Lee, J.S. Paclitaxel as third-line chemotherapy for small cell lung cancer failing both etoposide- and camptothecin-based chemotherapy. Medicine 2017, 96, e8176. [Google Scholar] [CrossRef]
- Bida, O.; Gidoni, M.; Ideses, D.; Efroni, S.; Ginsberg, D. A novel mitosis-associated lncrna, ma-linc1, is required for cell cycle progression and sensitizes cancer cells to paclitaxel. Oncotarget 2015, 6, 27880–27890. [Google Scholar] [CrossRef]
- Yasuhira, S.; Shibazaki, M.; Nishiya, M.; Maesawa, C. Paclitaxel-induced aberrant mitosis and mitotic slippage efficiently lead to proliferative death irrespective of canonical apoptosis and p53. Cell Cycle 2016, 15, 3268–3277. [Google Scholar] [CrossRef] [Green Version]
- Royer, I.; Alvinerie, P.; Armand, J.P.; Ho, L.K.; Wright, M.; Monsarrat, B. Paclitaxel metabolites in human plasma and urine: Identification of 6 alpha-hydroxytaxol, 7-epitaxol and taxol hydrolysis products using liquid chromatography/atmospheric-pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1995, 9, 495–502. [Google Scholar] [CrossRef]
- Ringel, I.; Horwitz, S.B. Taxol is converted to 7-epitaxol, a biologically active isomer, in cell culture medium. J. Pharmacol. Exp. Ther. 1987, 242, 692–698. [Google Scholar]
- Liu, Y.; Ma, X.; Zhou, M.; Hao, X.; Zhu, X. An effective method to produce 7-epitaxol from taxol in HCO3. Bioorg. Med. Chem. Lett. 2020, 30, 127285. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chen, J.C.; Yang, W.E.; Chien, S.Y.; Chen, M.K.; Lo, Y.S.; Hsi, Y.T.; Chuang, Y.C.; Lin, C.C.; Yang, S.F. Dehydroandrographolide inhibits oral cancer cell migration and invasion through nf-κb-, ap-1-, and sp-1-modulated matrix metalloproteinase-2 inhibition. Biochem. Pharmacol. 2017, 130, 10–20. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Lin, C.W.; Su, S.C.; Reiter, R.J.; Chen, A.W.; Chen, M.K.; Yang, S.F. Effects of mir-34b/mir-892a upregulation and inhibition of abcb1/abcb4 on melatonin-induced apoptosis in vcr-resistant oral cancer cells. Mol. Ther. Nucleic Acids 2020, 19, 877–889. [Google Scholar] [CrossRef]
- Chen, J.C.; Hsieh, M.C.; Lin, S.H.; Lin, C.C.; Hsi, Y.T.; Lo, Y.S.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Coronarin d induces reactive oxygen species-mediated cell death in human nasopharyngeal cancer cells through inhibition of p38 mapk and activation of jnk. Oncotarget 2017, 8, 108006–108019. [Google Scholar] [CrossRef]
- Du, W.; Hao, X.; Yuan, Z.; Wang, Y.; Zhang, X.; Liu, J. Shikonin potentiates paclitaxel antitumor efficacy in esophageal cancer cells via the apoptotic pathway. Oncol. Lett. 2019, 18, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbuti, A.M.; Chen, Z.S. Paclitaxel through the ages of anticancer therapy: Exploring its role in chemoresistance and radiation therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Mao, J.W.; Tan, X.L. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar] [PubMed]
- Zhang, X.; Xu, Y.; Zhang, W.; Fu, X.; Hao, Z.; He, M.; Trefilov, D.; Ning, X.; Ge, H.; Chen, Y. Controllable subtractive nanoimprint lithography for precisely fabricating paclitaxel-loaded plga nanocylinders to enhance anticancer efficacy. ACS Appl. Mater. Interfaces 2020, 12, 14797–14805. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Lo, H.W.; Lin, J. Combined bazedoxifene and paclitaxel treatments inhibit cell viability, cell migration, colony formation, and tumor growth and induce apoptosis in breast cancer. Cancer Lett. 2019, 448, 11–19. [Google Scholar] [CrossRef]
- Untch, M.; Untch, A.; Sevin, B.U.; Angioli, R.; Perras, J.P.; Koechli, O.; Averette, H.E. Comparison of paclitaxel and docetaxel (taxotere) in gynecologic and breast cancer cell lines with the atp-cell viability assay. Anticancer Drugs 1994, 5, 24–30. [Google Scholar] [CrossRef]
- Kuittinen, T.; Rovio, P.; Staff, S.; Luukkaala, T.; Kallioniemi, A.; Grenman, S.; Laurila, M.; Maenpaa, J. Paclitaxel, carboplatin and 1,25-d3 inhibit proliferation of endometrial cancer cells in vitro. Anticancer Res. 2017, 37, 6575–6581. [Google Scholar]
- Tan, K.T.; Li, S.; Li, Y.R.; Cheng, S.L.; Lin, S.H.; Tung, Y.T. Synergistic anticancer effect of a combination of paclitaxel and 5-demethylnobiletin against lung cancer cell line in vitro and in vivo. Appl. Biochem. Biotechnol. 2019, 187, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Kundranda, M.N.; Niu, J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Dev. Ther. 2015, 9, 3767–3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Lee, J.B.; Deshpande, K.; Garbuzenko, O.B.; Minko, T.; Kagan, L. Prevention of paclitaxel-induced neuropathy by formulation approach. J. Control. Release 2019, 303, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Levit, S.L.; Gade, N.R.; Roper, T.D.; Yang, H.; Tang, C. Self-assembly of ph-labile polymer nanoparticles for paclitaxel prodrug delivery: Formulation, characterization, and evaluation. Int. J. Mol. Sci. 2020, 21, 9292. [Google Scholar] [CrossRef]
- De Clercq, K.; Xie, F.; De Wever, O.; Descamps, B.; Hoorens, A.; Vermeulen, A.; Ceelen, W.; Vervaet, C. Preclinical evaluation of local prolonged release of paclitaxel from gelatin microspheres for the prevention of recurrence of peritoneal carcinomatosis in advanced ovarian cancer. Sci. Rep. 2019, 9, 14881. [Google Scholar] [CrossRef]
- Rimoldi, J.M.; Kingston, D.G.; Chaudhary, A.G.; Samaranayake, G.; Grover, S.; Hamel, E. Modified taxols, 9. Synthesis and biological evaluation of 7-substituted photoaffinity analogues of taxol. J. Nat. Prod. 1993, 56, 1313–1330. [Google Scholar] [CrossRef]
- Mariotti, M.; Colognato, R.; Rimoldi, M.; Rizzetto, M.; Sisto, F.; Cocce, V.; Bonomi, A.; Parati, E.; Alessandri, G.; Bagnati, R.; et al. Mesenchymal stromal cells uptake and release paclitaxel without reducing its anticancer activity. Anticancer Agents Med. Chem. 2015, 15, 400–405. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, R.; Wang, S.; Dong, Z. Paclitaxel: New uses for an old drug. Drug Des. Dev. Ther. 2014, 8, 279–284. [Google Scholar]
- Yuan, J.; Yan, R.; Kramer, A.; Eckerdt, F.; Roller, M.; Kaufmann, M.; Strebhardt, K. Cyclin b1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene 2004, 23, 5843–5852. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.H.; An, H.J.; Kang, S.; Hong, S.; Choi, Y.P.; Kim, Y.T.; Choi, Y.D.; Cho, N.H. Loss of cyclin b1 followed by downregulation of cyclin a/cdk2, apoptosis and antiproliferation in hela cell line. Int. J. Cancer 2005, 116, 520–525. [Google Scholar] [CrossRef]
- Foland, T.B.; Dentler, W.L.; Suprenant, K.A.; Gupta, M.L., Jr.; Himes, R.H. Paclitaxel-induced microtubule stabilization causes mitotic block and apoptotic-like cell death in a paclitaxel-sensitive strain of saccharomyces cerevisiae. Yeast 2005, 22, 971–978. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, B.; Chang, H.; Xiao, M.; Wu, Y.; Liu, Y. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ros and the akt/mapk signaling pathway in canine mammary gland tumor cells. Mol. Med. Rep. 2018, 17, 8289–8299. [Google Scholar] [CrossRef] [Green Version]
- Calaf, G.M.; Ponce-Cusi, R.; Carrion, F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018, 40, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, P.; Zhang, J.; Wu, H.; Sui, S.; Zhang, J.; Wang, Q.; Qiao, K.; Yang, W.; Xu, H.; et al. Ai-lncrna egot enhancing autophagy sensitizes paclitaxel cytotoxicity via upregulation of itpr1 expression by rna-rna and rna-protein interactions in human cancer. Mol. Cancer 2019, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M. Potential role of p62 in tumor development. Autophagy 2011, 7, 1088–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic regulation of p62 is critical for cancer therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef] [Green Version]
- Nihira, K.; Miki, Y.; Ono, K.; Suzuki, T.; Sasano, H. An inhibition of p62/sqstm1 caused autophagic cell death of several human carcinoma cells. Cancer Sci. 2014, 105, 568–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, J.S.; Bi, W.C.; Chan, G.K.L.; Jin, Y.; Wong, C.W.; Zhou, Z.Y.; Wang, H.Y.; Yao, P.; Dong, T.T.X.; Tsim, K.W.K. Ginkgetin induces autophagic cell death through p62/sqstm1-mediated autolysosome formation and redox setting in non-small cell lung cancer. Oncotarget 2017, 8, 93131–93148. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting mapk signaling in cancer: Mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [Green Version]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The akt pathway in oncology therapy and beyond (review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar]
- Wang, P.; Song, D.; Wan, D.; Li, L.; Mei, W.; Li, X.; Han, L.; Zhu, X.; Yang, L.; Cai, Y.; et al. Ginsenoside panaxatriol reverses tnbc paclitaxel resistance by inhibiting the irak1/nf-kappab and erk pathways. PeerJ 2020, 8, e9281. [Google Scholar] [CrossRef]
- Sanchez-Carranza, J.N.; Diaz, J.F.; Redondo-Horcajo, M.; Barasoain, I.; Alvarez, L.; Lastres, P.; Romero-Estrada, A.; Aller, P.; Gonzalez-Maya, L. Gallic acid sensitizes paclitaxel-resistant human ovarian carcinoma cells through an increase in reactive oxygen species and subsequent downregulation of erk activation. Oncol. Rep. 2018, 39, 3007–3014. [Google Scholar]
- Feng, S.L.; Tian, Y.; Huo, S.; Qu, B.; Liu, R.M.; Xu, P.; Li, Y.Z.; Xie, Y. Nobiletin potentiates paclitaxel anticancer efficacy in a549/t xenograft model: Pharmacokinetic and pharmacological study. Phytomedicine 2020, 67, 153141. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.B.; Hsieh, M.-J.; Mahalakshmi, B.; Chuang, Y.-C.; Lin, C.-C.; Lo, Y.-S.; Ho, H.-Y.; Lin, J.-T. 7-Epitaxol Induces Apoptosis and Autophagy in Head and Neck Squamous Cell Carcinoma through Inhibition of the ERK Pathway. Cells 2021, 10, 2633. https://doi.org/10.3390/cells10102633
Kumar VB, Hsieh M-J, Mahalakshmi B, Chuang Y-C, Lin C-C, Lo Y-S, Ho H-Y, Lin J-T. 7-Epitaxol Induces Apoptosis and Autophagy in Head and Neck Squamous Cell Carcinoma through Inhibition of the ERK Pathway. Cells. 2021; 10(10):2633. https://doi.org/10.3390/cells10102633
Chicago/Turabian StyleKumar, V. Bharath, Ming-Ju Hsieh, B. Mahalakshmi, Yi-Ching Chuang, Chia-Chieh Lin, Yu-Sheng Lo, Hsin-Yu Ho, and Jen-Tsun Lin. 2021. "7-Epitaxol Induces Apoptosis and Autophagy in Head and Neck Squamous Cell Carcinoma through Inhibition of the ERK Pathway" Cells 10, no. 10: 2633. https://doi.org/10.3390/cells10102633
APA StyleKumar, V. B., Hsieh, M.-J., Mahalakshmi, B., Chuang, Y.-C., Lin, C.-C., Lo, Y.-S., Ho, H.-Y., & Lin, J.-T. (2021). 7-Epitaxol Induces Apoptosis and Autophagy in Head and Neck Squamous Cell Carcinoma through Inhibition of the ERK Pathway. Cells, 10(10), 2633. https://doi.org/10.3390/cells10102633