Interneuron Dysfunction and Inhibitory Deficits in Autism and Fragile X Syndrome
Abstract
:1. Introduction
2. Excitatory–Inhibitory (E–I) Imbalance
3. Autism and Fragile X Syndrome
4. Inhibitory System Alteration
5. ASD and FXS Are Interneuronopathy
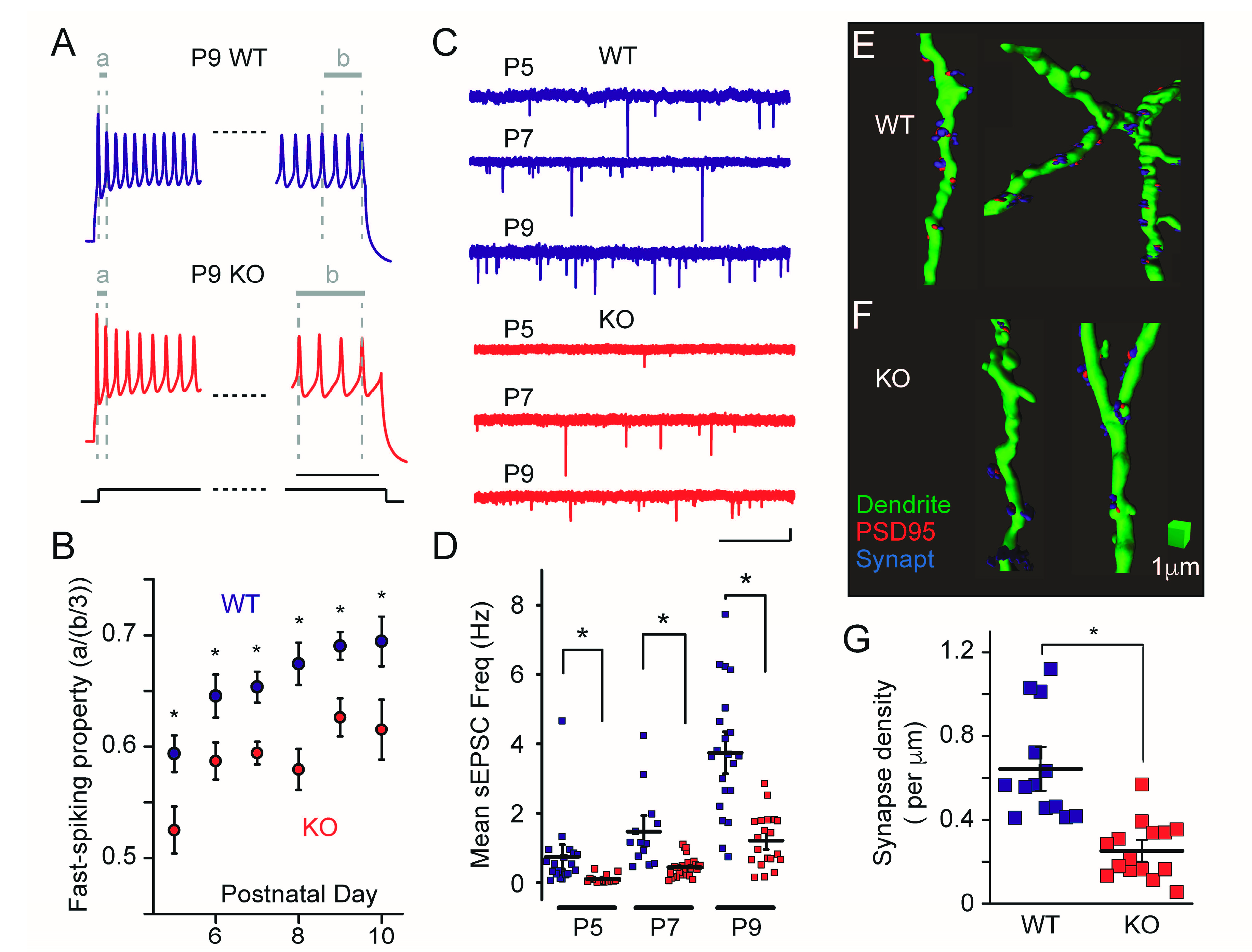
6. Developmental Alteration
7. Translational Perspective
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kofke, W.A.; Tempelhoff, R.; Dasheiff, R.M. Anesthetic implications of epilepsy, status epilepticus, and epilepsy surgery. J. Neurosurg. Anesthesiol. 1997, 9, 349–372. [Google Scholar] [CrossRef]
- Eichler, S.A.; Meier, J.C. E–I balance and human diseases—From molecules to networking. Front. Mol. Neurosci. 2008, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Tatti, R.; Haley, M.S.; Swanson, O.K.; Tselha, T.; Maffei, A. Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biol. Psychiatry 2017, 81, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological bases of autism-epilepsy comorbidity: A focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2018, 47, 534–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohal, V.S.; Rubenstein, J.L.R. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Talantova, M.; McKercher, S.R.; Lipton, S.A. Novel Therapeutic Approach for Excitatory/Inhibitory Imbalance in Neurodevelopmental and Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, J.; Kim, E. Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders. Biol. Psychiatry 2017, 81, 838–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Harris, J.C. The origin and natural history of autism spectrum disorders. Nat. Neurosci. 2016, 19, 1390–1391. [Google Scholar] [CrossRef]
- Mottron, L.; Bzdok, D. Autism spectrum heterogeneity: Fact or artifact? Mol. Psychiatry 2020, 25, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R.; Kyzar, E.J.; Scattoni, M.L.; Norton, W.H.; Pittman, J.; Gaikwad, S.; Nguyen, M.; Poudel, M.K.; Ullmann, J.F.; Diamond, D.M.; et al. Genetic and environmental modulation of neurodevelopmental disorders: Translational insights from labs to beds. Brain Res. Bull. 2016, 125, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Homberg, J.R.; Kyzar, E.J.; Nguyen, M.; Norton, W.H.; Pittman, J.; Poudel, M.K.; Gaikwad, S.; Nakamura, S.; Koshiba, M.; Yamanouchi, H.; et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci. Biobehav. Rev. 2016, 65, 292–312. [Google Scholar] [CrossRef] [Green Version]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.; Rivero-Arias, O.; Angelov, A.; Kim, E.; Fotheringham, I.; Leal, J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. Am. J. Med. Genet A 2014, 164, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.; Mailick, M.; Berry-Kravis, E.; Bolen, J. The Future of Fragile X Syndrome: CDC Stakeholder Meeting Summary. Pediatrics 2017, 139, S147–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry-Kravis, E.; Knox, A.; Hervey, C. Targeted treatments for fragile X syndrome. J. Neurodev. Disord. 2011, 3, 193–210. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.; Berry-Kravis, E.M.; Bassell, G.J. Therapeutic strategies in fragile X syndrome: Dysregulated mGluR signaling and beyond. Neuropsychopharmacology 2012, 37, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Penagarikano, O.; Mulle, J.G.; Warren, S.T. The pathophysiology of fragile x syndrome. Annu. Rev. Genomics Hum. Genet. 2007, 8, 109–129. [Google Scholar] [CrossRef] [Green Version]
- Contractor, A.; Klyachko, V.A.; Portera-Cailliau, C. Altered Neuronal and Circuit Excitability in Fragile X Syndrome. Neuron 2015, 87, 699–715. [Google Scholar] [CrossRef] [Green Version]
- Devys, D.; Lutz, Y.; Rouyer, N.; Bellocq, J.P.; Mandel, J.L. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat. Genet. 1993, 4, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Zalfa, F.; Giorgi, M.; Primerano, B.; Moro, A.; Di Penta, A.; Reis, S.; Oostra, B.; Bagni, C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 2003, 112, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Dutch-Belgian Fragile X Consorthium; Bakker, C.E.; Verheij, C.; Willemsen, R.; van der Helm, R.; Oerlemans, F.; Vermey, M.; Bygrave, A.; Hoogeveen, A.; Oostra, B.A.; et al. Fmr1 knockout mice: A model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 1994, 78, 23–33. [Google Scholar]
- Zhang, Y.Q.; Bailey, A.M.; Matthies, H.J.; Renden, R.B.; Smith, M.A.; Speese, S.D.; Rubin, G.M.; Broadie, K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 2001, 107, 591–603. [Google Scholar] [CrossRef] [Green Version]
- Tucker, B.; Richards, R.I.; Lardelli, M. Contribution of mGluR and Fmr1 functional pathways to neurite morphogenesis, craniofacial development and fragile X syndrome. Hum. Mol. Genet. 2006, 15, 3446–3458. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.M.; Green, J.R.; Veeraragavan, S.; Yuva, L.; McCoy, A.; Wu, Y.; Warren, J.; Little, L.; Ji, D.; Cui, X.; et al. Fmr1 and Nlgn3 knockout rats: Novel tools for investigating autism spectrum disorders. Behav. Neurosci. 2014, 128, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Bontekoe, C.J.; Bakker, C.E.; Nieuwenhuizen, I.M.; van der Linde, H.; Lans, H.; de Lange, D.; Hirst, M.C.; Oostra, B.A. Instability of a (CGG)98 repeat in the Fmr1 promoter. Hum. Mol. Genet. 2001, 10, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, D.; Errijgers, V.; Kooy, R.F.; Willemsen, R.; Mientjes, E.; Oostra, B.A.; De Deyn, P.P. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS). Behav. Brain Res. 2005, 162, 233–239. [Google Scholar] [CrossRef]
- Brouwer, J.R.; Mientjes, E.J.; Bakker, C.E.; Nieuwenhuizen, I.M.; Severijnen, L.A.; Van der Linde, H.C.; Nelson, D.L.; Oostra, B.A.; Willemsen, R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp. Cell Res. 2007, 313, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascano, M., Jr.; Mukherjee, N.; Bandaru, P.; Miller, J.B.; Nusbaum, J.D.; Corcoran, D.L.; Langlois, C.; Munschauer, M.; Dewell, S.; Hafner, M.; et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 2012, 492, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Bhakar, A.L.; Dolen, G.; Bear, M.F. The pathophysiology of fragile X (and what it teaches us about synapses). Annu. Rev. Neurosci. 2012, 35, 417–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castren, M.L.; Castren, E. BDNF in fragile X syndrome. Neuropharmacology 2014, 76 Pt C, 729–736. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Krueger, D.D.; Bear, M.F. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu. Rev. Med. 2011, 62, 411–429. [Google Scholar] [CrossRef] [Green Version]
- Scharf, S.H.; Jaeschke, G.; Wettstein, J.G.; Lindemann, L. Metabotropic glutamate receptor 5 as drug target for Fragile X syndrome. Curr. Opin. Pharmacol. 2015, 20, 124–134. [Google Scholar] [CrossRef]
- Van der Aa, N.; Kooy, R.F. GABAergic abnormalities in the fragile X syndrome. Eur. J. Paediatr. Neurol. 2020, 24, 100–104. [Google Scholar] [CrossRef]
- Kidd, S.A.; Lachiewicz, A.; Barbouth, D.; Blitz, R.K.; Delahunty, C.; McBrien, D.; Visootsak, J.; Berry-Kravis, E. Fragile X syndrome: A review of associated medical problems. Pediatrics 2014, 134, 995–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cellot, G.; Cherubini, E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front. Pediatr. 2014, 2, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braat, S.; Kooy, R.F. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- D’Hulst, C.; Heulens, I.; Van der Aa, N.; Goffin, K.; Koole, M.; Porke, K.; Van De Velde, M.; Rooms, L.; Van Paesschen, W.; Van Esch, H.; et al. Positron Emission Tomography (PET) Quantification of GABAA Receptors in the Brain of Fragile X Patients. PLoS ONE 2015, 10, e0131486. [Google Scholar] [CrossRef] [Green Version]
- Morin-Parent, F.; Champigny, C.; Lacroix, A.; Corbin, F.; Lepage, J.F. Hyperexcitability and impaired intracortical inhibition in patients with fragile-X syndrome. Transl. Psychiatry 2019, 9, 312. [Google Scholar] [CrossRef]
- Chen, L.; Toth, M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 2001, 103, 1043–1050. [Google Scholar] [CrossRef]
- Gonzalez, D.; Tomasek, M.; Hays, S.; Sridhar, V.; Ammanuel, S.; Chang, C.W.; Pawlowski, K.; Huber, K.M.; Gibson, J.R. Audiogenic Seizures in the Fmr1 Knock-Out Mouse Are Induced by Fmr1 Deletion in Subcortical, VGlut2-Expressing Excitatory Neurons and Require Deletion in the Inferior Colliculus. J. Neurosci. 2019, 39, 9852–9863. [Google Scholar] [CrossRef]
- Rotschafer, S.; Razak, K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Res. 2013, 1506, 12–24. [Google Scholar] [CrossRef]
- Heulens, I.; D’Hulst, C.; Van Dam, D.; De Deyn, P.P.; Kooy, R.F. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav. Brain Res. 2012, 229, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Song, S.; Li, F.; Yuan, F. Reduction of alpha1GABAA receptor mediated by tyrosine kinase C (PKC) phosphorylation in a mouse model of fragile X syndrome. Int. J. Clin. Exp. Med. 2015, 8, 13219–13226. [Google Scholar] [PubMed]
- Pacey, L.K.; Heximer, S.P.; Hampson, D.R. Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol. Pharmacol. 2009, 76, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Pacey, L.K.; Tharmalingam, S.; Hampson, D.R. Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome. J. Pharmacol. Exp. Ther. 2011, 338, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos-Serrano, J.L.; Corbin, J.G.; Burns, M.P. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev. Neurosci. 2011, 33, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Frankland, P.W.; Wang, Y.; Rosner, B.; Shimizu, T.; Balleine, B.W.; Dykens, E.M.; Ornitz, E.M.; Silva, A.J. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol. Psychiatry 2004, 9, 417–425. [Google Scholar] [CrossRef]
- de Vrij, F.M.; Levenga, J.; van der Linde, H.C.; Koekkoek, S.K.; De Zeeuw, C.I.; Nelson, D.L.; Oostra, B.A.; Willemsen, R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol. Dis. 2008, 31, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Kokash, J.; Alderson, E.M.; Reinhard, S.M.; Crawford, C.A.; Binder, D.K.; Ethell, I.M.; Razak, K.A. Genetic reduction of MMP-9 in the Fmr1 KO mouse partially rescues prepulse inhibition of acoustic startle response. Brain Res. 2019, 1719, 24–29. [Google Scholar] [CrossRef]
- Puts, N.A.; Edden, R.A.; Evans, C.J.; McGlone, F.; McGonigle, D.J. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci. 2011, 31, 16556–16560. [Google Scholar] [CrossRef]
- Puts, N.A.J.; Wodka, E.L.; Harris, A.D.; Crocetti, D.; Tommerdahl, M.; Mostofsky, S.H.; Edden, R.A.E. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism. Res. 2017, 10, 608–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapey-Triomphe, L.A.; Lamberton, F.; Sonie, S.; Mattout, J.; Schmitz, C. Tactile hypersensitivity and GABA concentration in the sensorimotor cortex of adults with autism. Autism. Res. 2019, 12, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Wood, E.T.; Cummings, K.K.; Jung, J.; Patterson, G.; Okada, N.; Guo, J.; O’Neill, J.; Dapretto, M.; Bookheimer, S.Y.; Green, S.A. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl. Psychiatry 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; Cantu, D.A.; Mantri, S.S.; Zeiger, W.A.; Goel, A.; Portera-Cailliau, C. Tactile Defensiveness and Impaired Adaptation of Neuronal Activity in the Fmr1 Knock-Out Mouse Model of Autism. J. Neurosci. 2017, 37, 6475–6487. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Dobyns, W.B. X-linked lissencephaly with abnormal genitalia as a tangential migration disorder causing intractable epilepsy: Proposal for a new term, “interneuronopathy”. J. Child Neurol. 2005, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, D.; Toutain, A.; Laquerriere, A.; Marret, S.; Saugier-Veber, P.; Barthez, M.A.; Radi, S.; Biran-Mucignat, V.; Rodriguez, D.; Gelot, A. X-linked lissencephaly with absent corpus callosum and ambiguous genitalia (XLAG): Clinical, magnetic resonance imaging, and neuropathological findings. Ann. Neurol. 2002, 51, 340–349. [Google Scholar] [CrossRef]
- Mullen, S.A.; Scheffer, I.E. Translational research in epilepsy genetics: Sodium channels in man to interneuronopathy in mouse. Arch. Neurol. 2009, 66, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A. Dravet Syndrome: A Sodium Channel Interneuronopathy. Curr. Opin. Physiol. 2018, 2, 42–50. [Google Scholar] [CrossRef]
- Kato, M. A new paradigm for West syndrome based on molecular and cell biology. Epilepsy Res. 2006, 70 (Suppl. 1), S87–S95. [Google Scholar] [CrossRef]
- Ruggieri, M.; Pavone, P.; Scapagnini, G.; Romeo, L.; Lombardo, I.; Li Volti, G.; Corsello, G.; Pavone, L. The aristaless (Arx) gene: One gene for many “interneuronopathies”. Front. Biosci. (Elite Ed) 2010, 2, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [Green Version]
- Fishell, G.; Kepecs, A. Interneuron Types as Attractors and Controllers. Annu. Rev. Neurosci. 2020, 43, 1–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, L.; Mi, D.; Llorca, A.; Marin, O. Development and Functional Diversification of Cortical Interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Paul, A. The diversity of GABAergic neurons and neural communication elements. Nat. Rev. Neurosci. 2019, 20, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Gouwens, N.W.; Sorensen, S.A.; Baftizadeh, F.; Budzillo, A.; Lee, B.R.; Jarsky, T.; Alfiler, L.; Baker, K.; Barkan, E.; Berry, K.; et al. Integrated Morphoelectric and Transcriptomic Classification of Cortical GABAergic Cells. Cell 2020, 183, 935–953.e919. [Google Scholar] [CrossRef]
- Melzer, S.; Monyer, H. Diversity and function of corticopetal and corticofugal GABAergic projection neurons. Nat. Rev. Neurosci. 2020, 21, 499–515. [Google Scholar] [CrossRef]
- Beaulieu, C. Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res. 1993, 609, 284–292. [Google Scholar] [CrossRef]
- Jones, E.G. GABAergic neurons and their role in cortical plasticity in primates. Cereb. Cortex 1993, 3, 361–372. [Google Scholar] [CrossRef]
- Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Filice, F.; Janickova, L.; Henzi, T.; Bilella, A.; Schwaller, B. The Parvalbumin Hypothesis of Autism Spectrum Disorder. Front. Cell. Neurosci. 2020, 14, 577525. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.; Zhang, C.; Sun, Q.Q. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci. Lett. 2007, 412, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.H.; Afroz, S.; Reinhard, S.M.; Palacios, A.R.; Tapia, K.; Binder, D.K.; Razak, K.A.; Ethell, I.M. Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Interneurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb. Cortex 2018, 28, 3951–3964. [Google Scholar] [CrossRef] [Green Version]
- Benali, A.; Trippe, J.; Weiler, E.; Mix, A.; Petrasch-Parwez, E.; Girzalsky, W.; Eysel, U.T.; Erdmann, R.; Funke, K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J. Neurosci. 2011, 31, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Musial, T.F.; Marshall, J.J.; Zhu, Y.; Remmers, C.L.; Xu, J.; Nicholson, D.A.; Contractor, A. Delayed Maturation of Fast-Spiking Interneurons Is Rectified by Activation of the TrkB Receptor in the Mouse Model of Fragile X Syndrome. J. Neurosci. 2017, 37, 11298–11310. [Google Scholar] [CrossRef] [Green Version]
- Olmos-Serrano, J.L.; Paluszkiewicz, S.M.; Martin, B.S.; Kaufmann, W.E.; Corbin, J.G.; Huntsman, M.M. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J. Neurosci. 2010, 30, 9929–9938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoine, M.W.; Langberg, T.; Schnepel, P.; Feldman, D.E. Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron 2019, 101, 648–661.e644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berzhanskaya, J.; Phillips, M.A.; Shen, J.; Colonnese, M.T. Sensory hypo-excitability in a rat model of fetal development in Fragile X Syndrome. Sci. Rep. 2016, 6, 30769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, L.M.; Okray, Z.; Linneweber, G.A.; Hassan, B.A.; Yaksi, E. Reduced Lateral Inhibition Impairs Olfactory Computations and Behaviors in a Drosophila Model of Fragile X Syndrome. Curr. Biol. 2017, 27, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Cantu, D.A.; Guilfoyle, J.; Chaudhari, G.R.; Newadkar, A.; Todisco, B.; de Alba, D.; Kourdougli, N.; Schmitt, L.M.; Pedapati, E.; et al. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat. Neurosci. 2018, 21, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Arsenault, J.; Bah, A.; Krzeminski, M.; Fekete, A.; Chao, O.Y.; Pacey, L.K.; Wang, A.; Forman-Kay, J.; Hampson, D.R.; et al. Identification of a molecular locus for normalizing dysregulated GABA release from interneurons in the Fragile X brain. Mol. Psychiatry 2020, 25, 2017–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, P.T.; Hull, C.; Chu, Y.; Greene-Colozzi, E.; Sadowski, A.R.; Leech, J.M.; Steinberg, J.; Crawley, J.N.; Regehr, W.G.; Sahin, M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 2012, 488, 647–651. [Google Scholar] [CrossRef] [Green Version]
- Cupolillo, D.; Hoxha, E.; Faralli, A.; De Luca, A.; Rossi, F.; Tempia, F.; Carulli, D. Autistic-Like Traits and Cerebellar Dysfunction in Purkinje Cell PTEN Knock-Out Mice. Neuropsychopharmacology 2016, 41, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- Peter, S.; Ten Brinke, M.M.; Stedehouder, J.; Reinelt, C.M.; Wu, B.; Zhou, H.; Zhou, K.; Boele, H.J.; Kushner, S.A.; Lee, M.G.; et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 2016, 7, 12627. [Google Scholar] [CrossRef]
- Chao, O.Y.; Marron Fernandez de Velasco, E.; Pathak, S.S.; Maitra, S.; Zhang, H.; Duvick, L.; Wickman, K.; Orr, H.T.; Hirai, H.; Yang, Y.M. Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism. Neuropsychopharmacology 2020, 45, 1159–1170. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Nimchinsky, E.A.; Oberlander, A.M.; Svoboda, K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 2001, 21, 5139–5146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Martin, A.; Crespo, M.; Portera-Cailliau, C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 2010, 30, 7793–7803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Martin, A.; Crespo, M.; Portera-Cailliau, C. Glutamate induces the elongation of early dendritic protrusions via mGluRs in wild type mice, but not in fragile X mice. PLoS ONE 2012, 7, e32446. [Google Scholar] [CrossRef]
- Till, S.M.; Wijetunge, L.S.; Seidel, V.G.; Harlow, E.; Wright, A.K.; Bagni, C.; Contractor, A.; Gillingwater, T.H.; Kind, P.C. Altered maturation of the primary somatosensory cortex in a mouse model of fragile X syndrome. Hum. Mol. Genet. 2012, 21, 2143–2156. [Google Scholar] [CrossRef] [Green Version]
- Wijetunge, L.S.; Angibaud, J.; Frick, A.; Kind, P.C.; Nagerl, U.V. Stimulated emission depletion (STED) microscopy reveals nanoscale defects in the developmental trajectory of dendritic spine morphogenesis in a mouse model of fragile X syndrome. J. Neurosci. 2014, 34, 6405–6412. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, E.D.; Fiole, D.; Mantri, S.S.; Huang, C.; Portera-Cailliau, C. Dendritic Spines in Early Postnatal Fragile X Mice Are Insensitive to Novel Sensory Experience. J. Neurosci. 2019, 39, 412–419. [Google Scholar] [CrossRef]
- Harlow, E.G.; Till, S.M.; Russell, T.A.; Wijetunge, L.S.; Kind, P.; Contractor, A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 2010, 65, 385–398. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Arroyo, E.D.; Smukowski, S.N.; Xu, J.; Piochon, C.; Savas, J.N.; Portera-Cailliau, C.; Contractor, A. Critical period inhibition of NKCC1 rectifies synapse plasticity in the somatosensory cortex and restores adult tactile response maps in fragile X mice. Mol. Psychiatry 2019, 24, 1732–1747. [Google Scholar] [CrossRef] [Green Version]
- Bureau, I.; Shepherd, G.M.; Svoboda, K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J. Neurosci. 2008, 28, 5178–5188. [Google Scholar] [CrossRef] [Green Version]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef]
- Domanski, A.P.F.; Booker, S.A.; Wyllie, D.J.A.; Isaac, J.T.R.; Kind, P.C. Cellular and synaptic phenotypes lead to disrupted information processing in Fmr1-KO mouse layer 4 barrel cortex. Nat. Commun. 2019, 10, 4814. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.R.; Bartley, A.F.; Hays, S.A.; Huber, K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 2008, 100, 2615–2626. [Google Scholar] [CrossRef]
- Patel, A.B.; Hays, S.A.; Bureau, I.; Huber, K.M.; Gibson, J.R. A target cell-specific role for presynaptic Fmr1 in regulating glutamate release onto neocortical fast-spiking inhibitory neurons. J. Neurosci. 2013, 33, 2593–2604. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.D.; Pinto, D.J.; Simons, D.J. Processing in layer 4 of the neocortical circuit: New insights from visual and somatosensory cortex. Curr. Opin. Neurobiol. 2001, 11, 488–497. [Google Scholar] [CrossRef]
- Feldmeyer, D.; Qi, G.; Emmenegger, V.; Staiger, J.F. Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex. Neuroscience 2018, 368, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.T.; Anstey, J.E.; Golshani, P.; Portera-Cailliau, C. Circuit level defects in the developing neocortex of Fragile X mice. Nat. Neurosci. 2013, 16, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Arnett, M.T.; Herman, D.H.; McGee, A.W. Deficits in tactile learning in a mouse model of fragile X syndrome. PLoS ONE 2014, 9, e109116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bonnan, A.; Bony, G.; Ferezou, I.; Pietropaolo, S.; Ginger, M.; Sans, N.; Rossier, J.; Oostra, B.; LeMasson, G.; et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat. Neurosci. 2014, 17, 1701–1709. [Google Scholar] [CrossRef]
- Boland, M.J.; Nazor, K.L.; Tran, H.T.; Szucs, A.; Lynch, C.L.; Paredes, R.; Tassone, F.; Sanna, P.P.; Hagerman, R.J.; Loring, J.F. Molecular analyses of neurogenic defects in a human pluripotent stem cell model of fragile X syndrome. Brain 2017, 140, 582–598. [Google Scholar] [CrossRef]
- Vitrac, A.; Cloez-Tayarani, I. Induced pluripotent stem cells as a tool to study brain circuits in autism-related disorders. Stem Cell Res. Ther. 2018, 9, 226. [Google Scholar] [CrossRef]
- Telias, M.; Segal, M.; Ben-Yosef, D. Neural differentiation of Fragile X human Embryonic Stem Cells reveals abnormal patterns of development despite successful neurogenesis. Dev. Biol. 2013, 374, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Telias, M.; Kuznitsov-Yanovsky, L.; Segal, M.; Ben-Yosef, D. Functional Deficiencies in Fragile X Neurons Derived from Human Embryonic Stem Cells. J. Neurosci. 2015, 35, 15295–15306. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, H.; Sauvey, C.; Yao, L.; Zarnowska, E.D.; Zhang, S.C. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat. Protoc. 2013, 8, 1670–1679. [Google Scholar] [CrossRef] [Green Version]
- Maroof, A.M.; Keros, S.; Tyson, J.A.; Ying, S.W.; Ganat, Y.M.; Merkle, F.T.; Liu, B.; Goulburn, A.; Stanley, E.G.; Elefanty, A.G.; et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013, 12, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, C.R.; Chen, J.; Tang, Y.; Southwell, D.G.; Chalmers, N.; Vogt, D.; Arnold, C.M.; Chen, Y.J.; Stanley, E.G.; Elefanty, A.G.; et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 2013, 12, 573–586. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.G.; Yao, R.; Monnell, T.; Cho, J.H.; Vasudevan, A.; Koh, A.; Peeyush, K.T.; Moon, M.; Datta, D.; Bolshakov, V.Y.; et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells 2014, 32, 1789–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry-Kravis, E.M.; Hessl, D.; Rathmell, B.; Zarevics, P.; Cherubini, M.; Walton-Bowen, K.; Mu, Y.; Nguyen, D.V.; Gonzalez-Heydrich, J.; Wang, P.P.; et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: A randomized, controlled, phase 2 trial. Sci. Transl. Med. 2012, 4, 152ra127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry-Kravis, E.; Hagerman, R.; Visootsak, J.; Budimirovic, D.; Kaufmann, W.E.; Cherubini, M.; Zarevics, P.; Walton-Bowen, K.; Wang, P.; Bear, M.F.; et al. Arbaclofen in fragile X syndrome: Results of phase 3 trials. J. Neurodev. Disord. 2017, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligsay, A.; Van Dijck, A.; Nguyen, D.V.; Lozano, R.; Chen, Y.; Bickel, E.S.; Hessl, D.; Schneider, A.; Angkustsiri, K.; Tassone, F.; et al. A randomized double-blind, placebo-controlled trial of ganaxolone in children and adolescents with fragile X syndrome. J. Neurodev. Disord. 2017, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, L.K.; Zhang, Y. Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance. Physiol. Rev. 2017, 97, 1431–1468. [Google Scholar] [CrossRef] [Green Version]
- Rosato-Siri, M.D.; Zambello, E.; Mutinelli, C.; Garbati, N.; Benedetti, R.; Aldegheri, L.; Graziani, F.; Virginio, C.; Alvaro, G.; Large, C.H. A Novel Modulator of Kv3 Potassium Channels Regulates the Firing of Parvalbumin-Positive Cortical Interneurons. J. Pharmacol. Exp. Ther. 2015, 354, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Boddum, K.; Hougaard, C.; Xiao-Ying Lin, J.; von Schoubye, N.L.; Jensen, H.S.; Grunnet, M.; Jespersen, T. Kv3.1/Kv3.2 channel positive modulators enable faster activating kinetics and increase firing frequency in fast-spiking GABAergic interneurons. Neuropharmacology 2017, 118, 102–112. [Google Scholar] [CrossRef]
- Brown, M.R.; El-Hassar, L.; Zhang, Y.; Alvaro, G.; Large, C.H.; Kaczmarek, L.K. Physiological modulators of Kv3.1 channels adjust firing patterns of auditory brain stem neurons. J. Neurophysiol. 2016, 116, 106–121. [Google Scholar] [CrossRef] [Green Version]
- El-Hassar, L.; Song, L.; Tan, W.J.T.; Large, C.H.; Alvaro, G.; Santos-Sacchi, J.; Kaczmarek, L.K. Modulators of Kv3 Potassium Channels Rescue the Auditory Function of Fragile X Mice. J. Neurosci. 2019, 39, 4797–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cea-Del Rio, C.A.; Nunez-Parra, A.; Freedman, S.M.; Kushner, J.K.; Alexander, A.L.; Restrepo, D.; Huntsman, M.M. Disrupted inhibitory plasticity and homeostasis in Fragile X syndrome. Neurobiol. Dis. 2020, 142, 104959. [Google Scholar] [CrossRef]
- Svalina, M.N.; Guthman, E.M.; Cea-Del Rio, C.A.; Kushner, J.K.; Baca, S.M.; Restrepo, D.; Huntsman, M.M. Hyperexcitability and Loss of Feedforward Inhibition Contribute to Aberrant Plasticity in the Fmr1KO Amygdala. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S. Preclinical research: Make mouse studies work. Nature 2014, 507, 423–425. [Google Scholar] [CrossRef]
- Begley, C.G.; Ellis, L.M. Drug development: Raise standards for preclinical cancer research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Jannot, A.S.; Agoritsas, T.; Gayet-Ageron, A.; Perneger, T.V. Citation bias favoring statistically significant studies was present in medical research. J. Clin. Epidemiol. 2013, 66, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Fragile X disappointments upset autism ambitions. Nat. Rev. Drug Discov. 2015, 14, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Duyx, B.; Urlings, M.J.E.; Swaen, G.M.H.; Bouter, L.M.; Zeegers, M.P. Scientific citations favor positive results: A systematic review and meta-analysis. J. Clin. Epidemiol. 2017, 88, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlinaric, A.; Horvat, M.; Supak Smolcic, V. Dealing with the positive publication bias: Why you should really publish your negative results. Biochem. Med. (Zagreb) 2017, 27, 030201. [Google Scholar] [CrossRef] [Green Version]
- Murad, M.H.; Chu, H.; Lin, L.; Wang, Z. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ Evid. Based Med. 2018, 23, 84–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, C.D.; Forstmann, B.; Pruszynski, J.A. Science in flux: Registered reports and beyond at the European Journal of Neuroscience. Eur. J. Neurosci 2019, 49, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Chambers, C. What’s next for Registered Reports? Nature 2019, 573, 187–189. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, T. Interneuron Dysfunction and Inhibitory Deficits in Autism and Fragile X Syndrome. Cells 2021, 10, 2610. https://doi.org/10.3390/cells10102610
Nomura T. Interneuron Dysfunction and Inhibitory Deficits in Autism and Fragile X Syndrome. Cells. 2021; 10(10):2610. https://doi.org/10.3390/cells10102610
Chicago/Turabian StyleNomura, Toshihiro. 2021. "Interneuron Dysfunction and Inhibitory Deficits in Autism and Fragile X Syndrome" Cells 10, no. 10: 2610. https://doi.org/10.3390/cells10102610
APA StyleNomura, T. (2021). Interneuron Dysfunction and Inhibitory Deficits in Autism and Fragile X Syndrome. Cells, 10(10), 2610. https://doi.org/10.3390/cells10102610





