The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms
Abstract
:1. Introduction
2. Large Scale Clinical Trial Outcomes
3. The Pathophysiology of Atherosclerosis
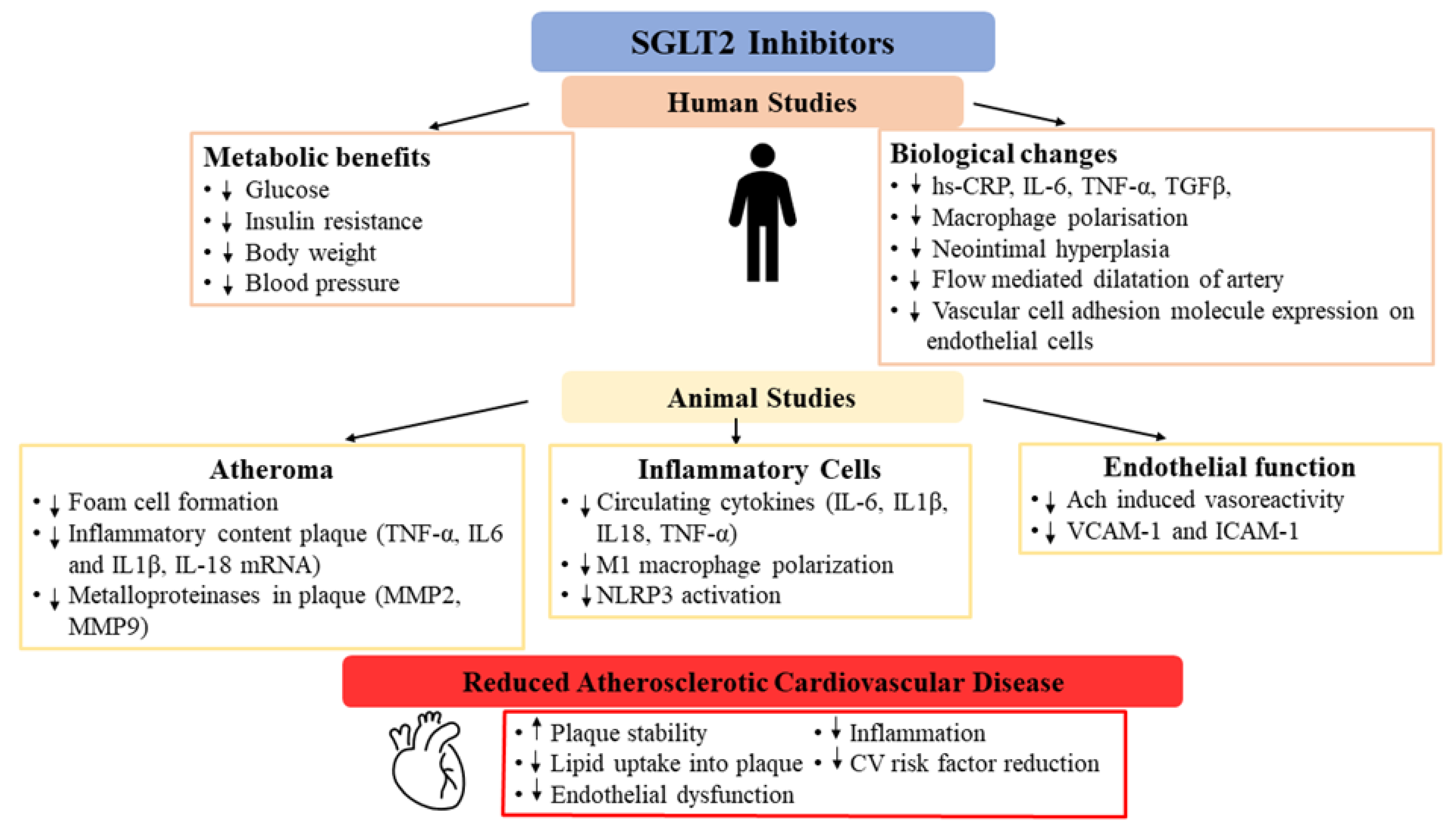
4. Effects of SGLT2 Inhibitors on Atherosclerosis Pathways
4.1. Glycaemia
4.2. Lipid Metabolism
4.3. Plaque Volume and Characteristics
5. Effects of SGLT2 Inhibitors on Inflammation
6. Effects of SGLT2 Inhibitors on Endothelial Function
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Trimarco, B.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnott, C.; Li, Q.; Kang, A.; Neuen, B.L.; Bompoint, S.; Lam, C.S.P.; Rodgers, A.; Mahaffey, K.W.; Cannon, C.P.; Perkovic, V.; et al. Sodium-Glucose Cotransporter 2 Inhibition for the Prevention of Cardiovascular Events in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Am. Hear. Assoc. 2020, 9, e014908. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.; Young, T.; Heerspink, H.J.L.; Neal, B.; Perkovic, V.; Billot, L.; Mahaffey, K.W.; Charytan, D.M.; Wheeler, D.C.; Arnott, C.; et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019, 7, 845–854. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.; McMurray, J.J.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- McMurray, J.J.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. NEJM 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Salah, H.M.; Al’Aref, S.J.; Khan, M.S.; Al-Hawwas, M.; Vallurupalli, S.; Mehta, J.L.; Mounsey, J.P.; Greene, S.J.; McGuire, D.K.; Lopes, R.D.; et al. Effects of sodium-glucose cotransporter 1 and 2 inhibitors on cardiovascular and kidney outcomes in type 2 diabetes: A meta-analysis update. Am. Hear. J. 2021, 233, 86–91. [Google Scholar] [CrossRef]
- Li, J.; Woodward, M.; Perkovic, V.; Figtree, G.A.; Heerspink, H.J.; Mahaffey, K.W.; de Zeeuw, D.; Vercruyss, F.; Shaw, W.; Matthews, D.R.; et al. Mediators of the Effects of Canagliflozin on Heart Failure in Patients With Type 2 Di-abetes. JACC Heart Fail. 2020, 8, 57–66. [Google Scholar] [CrossRef]
- Cannon, C.P.; McGuire, D.K.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Charbonnel, B.; Shih, W.J.; Gallo, S.; Masiukiewicz, U.; et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am. Hear. J. 2018, 206, 11–23. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef] [PubMed]
- St. Clair RWP. Pathogenesis of Atherosclerosis. Cardiol. Rev. 1997, 5, 14–24. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Zhan, F.; He, M.; Liu, Z.; Song, X. Anti-inflammatory effects of sodium-glucose co-transporter 2 inhibitors on athero-sclerosis. Vasc. Pharmacol. 2020, 133-134, 106779. [Google Scholar] [CrossRef] [PubMed]
- Idzkowska, E.; Eljaszewicz, A.; Miklasz, P.; Musial, W.J.; Tycinska, A.M.; Moniuszko, M. The Role of Different Monocyte Subsets in the Pathogenesis of Atherosclerosis and Acute Coronary Syndromes. Scand. J. Immunol. 2015, 82, 163–173. [Google Scholar] [CrossRef]
- Baldrighi, M.; Mallat, Z.; Li, X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis 2018, 267, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.-Q. Innate and Adaptive Immunity in the Pathogenesis of Atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Durante, W.; Behnammanesh, G.; Peyton, K.J. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int. J. Mol. Sci. 2021, 22, 8786. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109 (Suppl. S1), III27–III32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gong, D.; Li, S.; Zhou, X. Meta-analysis of the effects of statin therapy on endothelial function in patients with diabetes mellitus. Atherosclerosis 2012, 223, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, R.; Koundinya, K.S.S.; Malati, T.; Kutala, V.K. Endothelial Dysfunction in Type 2 Diabetes Mellitus. Indian J. Clin. Biochem. 2016, 31, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Fukuhara-Takaki, K.; Sakai, M.; Sakamoto, Y.I.; Takeya, M.; Horiuchi, S. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: One mechanism for an increased rate of atherosclerosis in diabetes. J. Biol. Chem. 2005, 280, 3355–3364. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sawamura, T.; Renier, G. Glucose enhances human macrophage LOX-1 expression: Role for LOX-1 in glucose-induced macrophage foam cell formation. Circ. Res. 2004, 94, 892–901. [Google Scholar] [CrossRef]
- Terasaki, M.; Hiromura, M.; Mori, Y.; Kohashi, K.; Nagashima, M.; Kushima, H.; Watanabe, T.; Hirano, T. Amelioration of Hyperglycemia with a Sodium-Glucose Cotransporter 2 Inhibitor Prevents Macrophage-Driven Atherosclerosis through Macrophage Foam Cell Formation Suppression in Type 1 and Type 2 Diabetic Mice. PLoS ONE 2015, 10, e0143396. [Google Scholar] [CrossRef]
- Idris, I.; Donnelly, R. Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug. Diabetes Obes. Metab. 2009, 11, 79–88. [Google Scholar] [CrossRef]
- Sheahan, K.H.; Wahlberg, E.A.; Gilbert, M.P. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad. Med. J. 2019, 96, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considera-tions. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef] [PubMed]
- Elley, C.R.; Kenealy, T.; Robinson, E.; Drury, P.L. Glycated Haemoglobin and Cardiovascular Outcomes in People with Type 2 Diabetes: A Large Prospective Cohort Study. Diabet. Med. 2008, 25, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Pogue, J.; Mann, J.F.E.; Lonn, E.; Dagenais, G.R.; McQueen, M.; Yusuf, S. HOPE investigators The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: A prospective epidemiological analysis. Diabetol. 2005, 48, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Klein, T.; Leung, P.S. Effects of Combining Linagliptin Treatment with BI-38335, A Novel SGLT2 Inhibitor, on Pancreatic Islet Function and Inflammation in db/db Mice. Curr. Mol. Med. 2012, 12, 995–1004. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.-J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Beverly, J.K.; Budoff, M.J. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes 2020, 12, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Bornfeldt, K.E.; Tabas, I. Insulin Resistance, Hyperglycemia, and Atherosclerosis. Cell Metabol. 2011, 14, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Han, J.H.; Oh, T.J.; Lee, G.; Maeng, H.J.; Lee, D.H.; Kim, K.M.; Choi, S.H.; Jang, H.C.; Lee, H.S.; Park, K.S.; et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia 2017, 60, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Mudaliar, S.; Henry, R.R.; Boden, G.; Smith, S.; Chalamandaris, A.-G.; Duchesne, D.; Iqbal, N.; List, J. Changes in Insulin Sensitivity and Insulin Secretion with the Sodium Glucose Cotransporter 2 Inhibitor Dapagliflozin. Diabetes Technol. Ther. 2014, 16, 137–144. [Google Scholar] [CrossRef]
- Merovci, A.; Abdul-Ghani, M.; Mari, A.; Herrera, C.S.; Xiong, J.; Daniele, G.; Tripathy, D.; DeFronzo, R.A. Effect of Dapagliflozin With and Without Acipimox on Insulin Sensitivity and Insulin Secretion in T2DM Males. J. Clin. Endocrinol. Metab. 2016, 101, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Daniele, G.; Xiong, J.; Solis-Herrera, C.; Merovci, A.; Eldor, R.; Tripathy, D.; DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Dapagliflozin Enhances Fat Oxidation and Ketone Production in Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 2036–2041. [Google Scholar] [CrossRef] [Green Version]
- Latva-Rasku, A.; Honka, M.-J.; Kullberg, J.; Mononen, N.; Lehtimäki, T.; Saltevo, J.; Kirjavainen, A.K.; Saunavaara, V.; Iozzo, P.; Johansson, L.; et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care 2019, 42, 931–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sharea, A.; Murphy, A.J.; Huggins, L.A.; Hu, Y.; Goldberg, I.J.; Nagareddy, P.R. SGLT2 inhibition reduces atherosclerosis by en-hancing lipoprotein clearance in Ldlr−/− type 1 diabetic mice. Atherosclerosis 2018, 271, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, Y.; Kokubo, H.; Bumdelger, B.; Yoshizumi, M.; Yamamotoya, T.; Matsunaga, Y.; Ueda, K.; Inoue, Y.; Inoue, M.-K.; Fujishiro, M.; et al. The SGLT2 Inhibitor Luseogliflozin Rapidly Normalizes Aortic mRNA Levels of Inflammation-Related but Not Lipid-Metabolism-Related Genes and Suppresses Atherosclerosis in Diabetic ApoE KO Mice. Int. J. Mol. Sci. 2017, 18, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, S. Anti-inflammatory effects of empagliflozin in patients with type 2 diabetes and insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, L.; Yuan, L.; Li, D.; Zhang, Y.; Zheng, R.; Liu, C.; Feng, X.; Li, Q.; Ma, J. Sodium-glucose co-transporter-2 inhibitors suppress atrial natriuretic peptide secretion in patients with newly diagnosed Type 2 diabetes. Diabet. Med. 2016, 33, 1732–1736. [Google Scholar] [CrossRef]
- Storgaard, H.; Gluud, L.L.; Bennett, C.; Grøndahl, M.F.G.; Christensen, M.; Knop, F.K.; Vilsbøll, T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166125. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol. Res. 2020, 160, 105068. [Google Scholar] [CrossRef]
- Leng, W.; Ouyang, X.; Lei, X.; Wu, M.; Chen, L.; Wu, Q.; Deng, W.; Liang, Z. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE−/−Mice. Mediat. Inflamm. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nasiri-Ansari, Ν.; Dimitriadis, G.K.; Agrogiannis, G.; Perrea, D.; Kostakis, I.D.; Kaltsas, G.; Papavassiliou, A.G.; Randeva, H.S.; Kassi, E. Canagliflozin attenuates the progression of atherosclerosis and in-flammation process in APOE knockout mice. Cardiovasc. Diabetol. 2018, 17, 106. [Google Scholar] [CrossRef] [Green Version]
- Aso, Y.; Kato, K.; Sakurai, S.; Kishi, H.; Shimizu, M.; Jojima, T.; Iijima, T.; Maejima, Y.; Shimomura, K.; Usui, I. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int. J. Clin. Pract. 2019, 73, e13335. [Google Scholar] [CrossRef]
- Fadini, G.P.; Bonora, B.M.; Zatti, G.; Vitturi, N.; Iori, E.; Marescotti, M.C.; Albiero, M.; Avogaro, A. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: A randomized placebo-controlled trial. Cardiovasc. Diabetol. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, M.; Nakatani, Y.; Tanka, S.; Aoki, C.; Sagara, M.; Yanagi, K.; Suzuki, K.; Aso, Y. Efficacy of Additional Canagliflozin Administration to Type 2 Diabetes Patients Receiving Insulin Therapy: Examination of Diurnal Glycemic Patterns Using Continuous Glucose Monitoring (CGM). Diabetes Ther. 2017, 8, 821–827. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Pedicino, D.; Severino, A.; Ucci, S.; Bugli, F.; Flego, D.; Giglio, A.F.; Trotta, F.; Ruggio, A.; Lucci, C.; Iaconelli, A.; et al. Epicardial adipose tissue microbial colonization and inflammasome activation in acute coronary syndrome. Int. J. Cardiol. 2017, 236, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xing, S.; Gong, Z.; Xing, Q. NLRP3 Inflammasomes Show High Expression in Aorta of Patients with Atherosclerosis. Hear. Lung Circ. 2013, 22, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bajaj, M.; Yang, H.-C.; Perez-Polo, J.R.; Birnbaum, Y. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc. Drugs Ther. 2017, 31, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.J.; Matsumura, N.; Maayah, Z.H.; Ferdaoussi, M.; Takahara, S.; Darwesh, A.M.; Levasseur, J.L.; Jahng, J.W.S.; Vos, D.; Parajuli, N.; et al. Empagliflozin Blunts Worsening Cardiac Dysfunction Associated With Reduced NLRP3 (Nucleotide-Binding Domain-Like Receptor Protein 3) Inflammasome Activation in Heart Failure. Circ. Hear. Fail. 2020, 13, e006277. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Chinetti, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- Hess, D.A.; Terenzi, D.C.; Trac, J.Z.; Quan, A.; Mason, T.; Al-Omran, M.; Bhatt, D.L.; Dhingra, N.; Rotstein, O.D.; Leiter, L.A.; et al. SGLT2 Inhibition with Empagliflozin Increases Circulating Provascular Progenitor Cells in People with Type 2 Diabetes Mellitus. Cell Metab. 2019, 30, 609–613. [Google Scholar] [CrossRef]
- Fadini, G.P.; Avogaro, A. Cell-based methods for ex vivo evaluation of human endothelial biology. Cardiovasc. Res. 2010, 87, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Dimitriadis, G.K.; Nasiri-Ansari, N.; Agrogiannis, G.; Kostakis, I.D.; Randeva, M.S.; Nikiteas, N.; Patel, V.H.; Kaltsas, G.; Papavassiliou, A.G.; Randeva, H.S.; et al. Empagliflozin improves primary haemodynamic parameters and at-tenuates the development of atherosclerosis in high fat diet fed APOE knockout mice. Mol. Cell. Endocrinol. 2019, 494, 110487. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shin, S.E.; Seo, M.S.; An, J.R.; Choi, I.-W.; Jung, W.-K.; Firth, A.L.; Lee, D.-S.; Yim, M.-J.; Choi, G.; et al. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci. 2018, 197, 46–55. [Google Scholar] [CrossRef]
- Sukhanov, S.; Higashi, Y.; Yoshida, T.; Mummidi, S.; Aroor, A.R.; Russell, J.J.; Bender, S.B.; DeMarco, V.G.; Chandrasekar, B. The SGLT2 inhibitor Empagliflozin attenuates interleukin-17A-induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1β and IL-18 secretion. Cell. Signal. 2021, 77, 109825. [Google Scholar] [CrossRef] [PubMed]
- Adingupu, D.D.; Göpel, S.O.; Grönros, J.; Behrendt, M.; Sotak, M.; Miliotis, T.; Dahlqvist, U.; Gan, L.-M.; Jönsson-Rylander, A.-C. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob−/− mice. Cardiovasc. Diabetol. 2019, 18, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Daly, M.; Venu, V.K.P.; Saifeddine, M.; Mihara, K.; Kang, S.; Fedak, P.W.; Alston, L.A.; Hirota, S.A.; Ding, H.; Triggle, C.R.; et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vasc. Pharmacol. 2018, 109, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.; Spizzo, I.; Liu, H.; Hu, Y.; Simpson, R.W.; Widdop, R.E.; Dear, A.E. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diabetes Vasc. Dis. Res. 2017, 15, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaou, P.E.; Efentakis, P.; Abu Qourah, F.; Femminò, S.; Makridakis, M.; Kanaki, Z.; Varela, A.; Tsoumani, M.; Davos, C.H.; Dimitriou, C.A.; et al. Chronic Empagliflozin Treatment Reduces Myocardial Infarct Size in Nondiabetic Mice Through STAT-3-Mediated Protection on Microvascular Endothelial Cells and Reduction of Oxidative Stress. Antioxidants Redox Signal. 2021, 34, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Römer, G.; Kerindongo, R.; Hermanides, J.; Albrecht, M.; Hollmann, M.; Zuurbier, C.; Preckel, B.; Weber, N. Sodium Glucose Co-Transporter 2 Inhibitors Ameliorate Endothelium Barrier Dysfunction Induced by Cyclic Stretch through Inhibition of Reactive Oxygen Species. Int. J. Mol. Sci. 2021, 22, 6044. [Google Scholar] [CrossRef] [PubMed]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashikata, T.; Ikutomi, M.; Jimba, T.; Shindo, A.; Kakuda, N.; Katsushika, S.; Yokoyama, M.; Kishi, M.; Sato, T.; Matsushita, M.; et al. Empagliflozin attenuates neointimal hyperplasia after drug-eluting-stent implantation in patients with type 2 diabetes. Hear. Vessel. 2020, 35, 1378–1389. [Google Scholar] [CrossRef]
- Mori, Y.; Terasaki, M.; Hiromura, M.; Saito, T.; Kushima, H.; Koshibu, M.; Osaka, N.; Ohara, M.; Fukui, T.; Ohtaki, H.; et al. Luseogliflozin attenuates neointimal hyperplasia after wire injury in high-fat diet-fed mice via inhibition of perivascular adipose tissue remodeling. Cardiovasc. Diabetol. 2019, 18, 143–149. [Google Scholar] [CrossRef]
- Striepe, K.; Jumar, A.; Ott, C.; Karg, M.V.; Schneider, M.P.; Kannenkeril, D.; Schmieder, R.E. Effects of the Selective Sodium-Glucose Cotransporter 2 Inhibitor Empagliflozin on Vascular Function and Central Hemodynamics in Patients With Type 2 Diabetes Mellitus. Circulation 2017, 136, 1167–1169. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Har, R.; Fagan, N.; Johansen, O.E.; Woerle, H.J.; von Eynatten, M.; Broedl, U.C. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc. Diabetol. 2014, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
| Completed Trial | Intervention | Study Size (n) | CV Disease Proportion, n (%) | Primary Outcome | MACE, HR (95% CI) | MI 1, HR (95% CI) | Stroke 1, HR (95% CI) | CV Mortality, HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| EMPA-REG OUTCOME [2] | empagliflozin | 7020 | 6964 (99.2) 2 | MACE | 0.86 (0.74–0.99) | 0.87 (0.70–1.09) | 1.18 (0.89–1.56) | 0.62 (0.49–0.77) |
| CANVAS Program [1] | canagliflozin | 10142 | 6656 (65.6) 2 | MACE | 0.86 (0.75–0.97) | 0.89 (0.73–1.09) | 0.87 (0.69–1.09) | 0.87 (0.72–1.06) |
| DECLARE-TIMI 58 [3] | dapagliflozin | 17160 | 6974 (40.6) 2 | MACE | 0.93 (0.84–1.03) | 0.89 (0.77–1.01) | 1.01 (0.84–1.21) | 0.98 (0.82–1.17) |
| CREDENCE [4] | canagliflozin | 4401 | 2220 (55.4) 2 | Composite of ESKD, doubling of serum creatinine, renal, or CV death | 0.80 (0.67–0.95) | 0.86 (0.64–1.06) | 0.77 (0.55–1.08) | 0.78 (0.61–1.00) |
| DAPA-HF [10] | dapagliflozin | 4744 | 2674 (56.4) 3 | Worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or CV death | NA | NA | NA | 0.82 (0.69–0.98) |
| VERTIS-CV [8] | Ertugliflozin | 8246 | 8236 (99.9) 2 [14] | MACE | 0.97 (0.85–1.11) | 1.04 (0.86–1.26) | 1.06 (0.82–1.37) | 0.92 (0.77–1.11) |
| DAPA-CKD [9] | dapagliflozin | 4304 | 1610 (37.4) 4 | Composite of ≥ 50% sustained decline in eGFR, ESKD, renal, or CV death | NA | NA | NA | 0.81 (0.58–1.12) |
| EMPEROR-Reduced [11] | empagliflozin | 3730 | 1929 (51.7) 3 | CV death or hospitalization for worsening HF | NA | NA | NA | 0.92 (0.75–1.12) |
| SOLOIST-WHF [15] | sotagliflozin | 1222 | NA | CV death and hospitalizations and urgent visits for HF | NA | NA | NA | 0.84 (0.58–1.22) |
| SCORED [7] | sotagliflozin | 10,584 | 5144 (48.6) 5 | CV death, hospitalizations for HF, and urgent visits for HF | 0.84 (0.72–0.99) | 0.68 (0.52–0.89) | 0.66 (0.48–0.91) | 0.90 (0.73–1.12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraclough, J.Y.; Patel, S.; Yu, J.; Neal, B.; Arnott, C. The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms. Cells 2021, 10, 2699. https://doi.org/10.3390/cells10102699
Barraclough JY, Patel S, Yu J, Neal B, Arnott C. The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms. Cells. 2021; 10(10):2699. https://doi.org/10.3390/cells10102699
Chicago/Turabian StyleBarraclough, Jennifer Y., Sanjay Patel, Jie Yu, Bruce Neal, and Clare Arnott. 2021. "The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms" Cells 10, no. 10: 2699. https://doi.org/10.3390/cells10102699
APA StyleBarraclough, J. Y., Patel, S., Yu, J., Neal, B., & Arnott, C. (2021). The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms. Cells, 10(10), 2699. https://doi.org/10.3390/cells10102699








