Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction
Abstract
1. Introduction
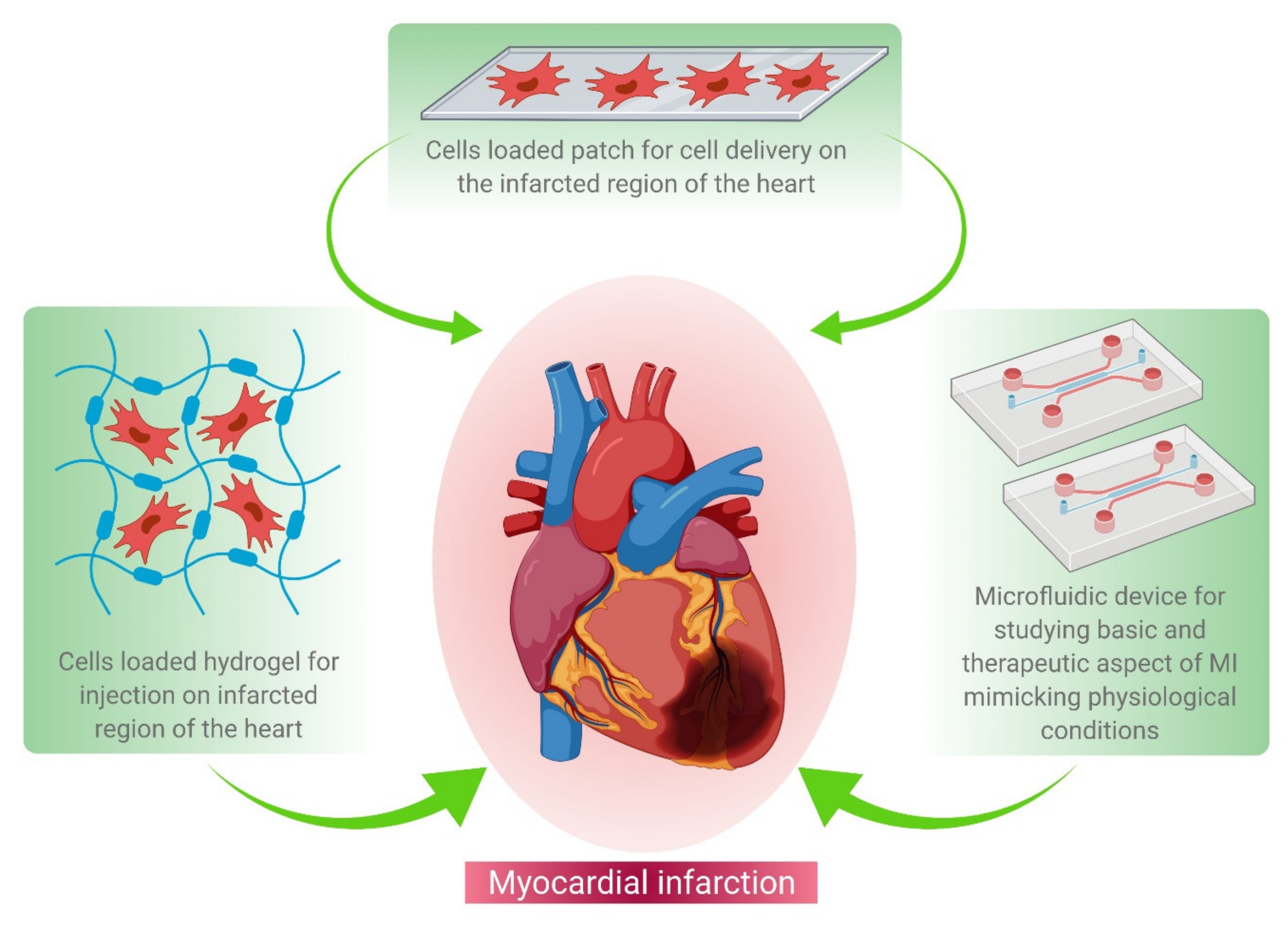
2. Regenerative Therapy
3. Cell Based Therapy
- Embryonic stem cells (ESCs)
- b.
- Induced pluripotent stem cells (iPSCs)
- c.
- Adult stem cells (ASCs)
- d.
- Cardiac stem cells (CSCs)
| Initial Cell Type | Target Cell Type | Composition of Delivery Vehicle | Mode of Delivery | Animal Models | Outcomes | Limitations | References |
|---|---|---|---|---|---|---|---|
| iPSCs | CMs | Polyethylene glycol hydrogel | Trans-epicardial | MI in nude rats | Increased infarct thickness and improved muscle content | No donor cell engraftment was observed | [47] |
| Mouse ESCs | CMs | PA-RGDS based gel | Trans-epicardial | Mice | Engraftment and integration of mESC-CMs into host myocardium improved cardiac function | No information available on cardiac remodelling | [12] |
| iPSCs | CMs | PBS solution | Trans-epicardial | Post-infarcted swine | Enhanced angiogenesis, reduced apoptosis, and blunted cardiac remodelling | No detailed information available on the engraftment of donor cell | [48] |
| MSCs | **** | Self-assembling peptide hydrogels (3-D Matrix, Ltd.) | Surface immobilization by spreading | Lewis rats | Augmented microvascular formation and reduced interstitial fibrosis | No detailed information available on the engraftment of donor cell and CMs differentiation from MSC | [49] |
| MSCs | **** | Si-HPMC | Trans-epicardial | Lewis rats | Short-term recovery of ventricular function and attenuated mid-term remodelling | No detailed information available on the engraftment of donor cell and CMs differentiation from MSC | [50] |
| c-Kit overexpressing CSCs | **** | PBS solution | Intracoronary | Fischer 344 rats | Preserved LV function and structure | Increased cell dose was found to be harmful. Cell tracing or engraftment were not available in detail | [50] |
| CSCs | **** | Matrigel and dimethylpolysiloxane mixture gel | Trans-epicardial | NOD-SCID mice | Improved long-term retention of CSCs, cardiac structure and function | Cell tracing or engraftment were not available | [51] |
- e.
- Skeletal myoblast cells (SMs)
- f.
- Umbilical cord blood cells (UCBC)
- g.
- Amniotic fluid stem Cells (AFSCs)
- h.
- Cells Aggregates
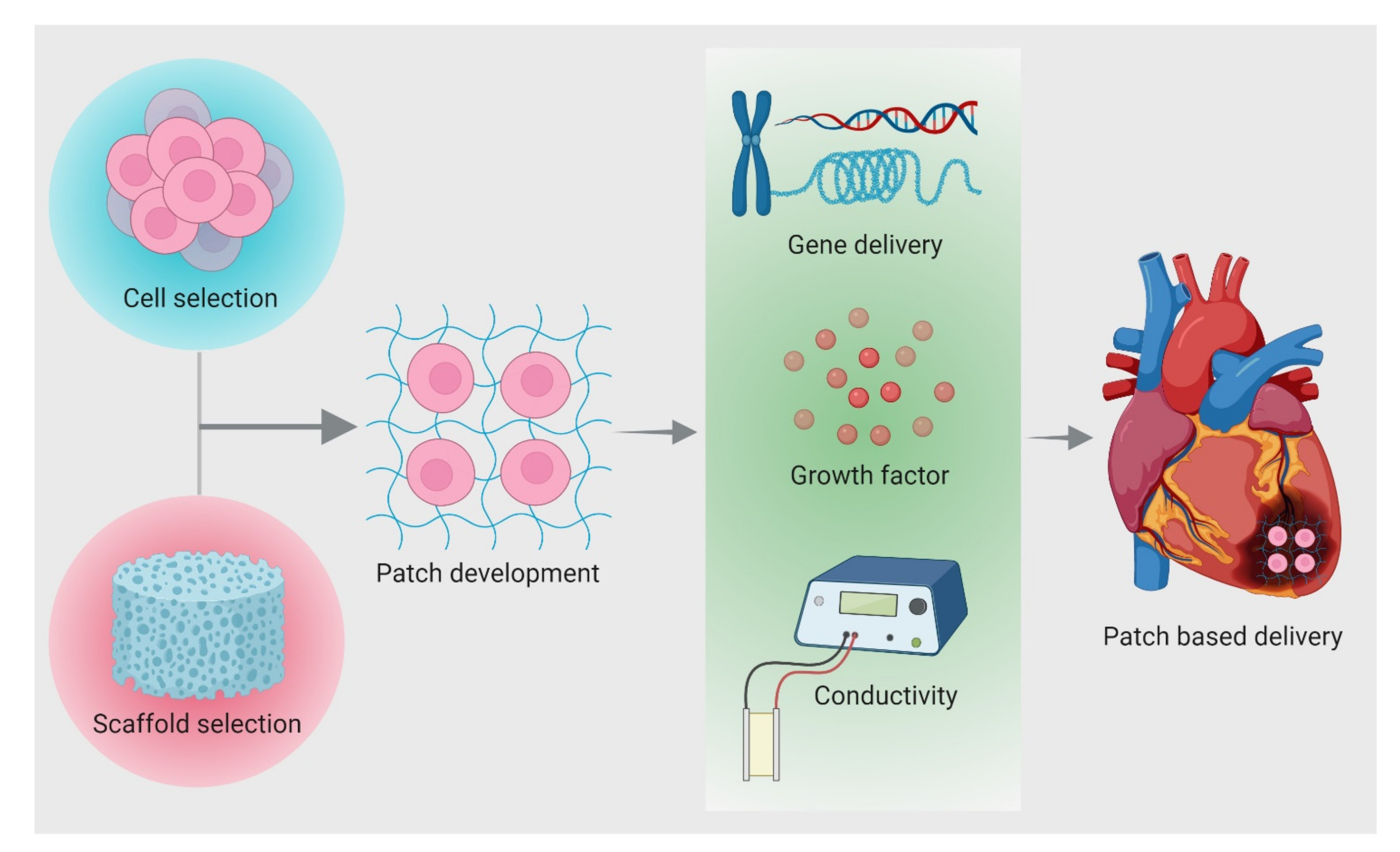
4. Patch Based Cell Therapy Development
4.1. Properties for Patch Design
- Chemical: Surface properties (e.g., surface energy, chemistry, charge, surface area)
- Electrical: Conductivity
- Physical: Mechanical competence (e.g., compressive and tensile strength), External geometry (e.g., macrostructure, microstructure, interconnectivity), porosity, and pore size
- Biological: Interface adherence, biocompatibility, biodegradation
4.1.1. Chemical Properties
4.1.2. Electrical Properties
4.1.3. Physical Properties
4.1.4. Biological Properties
4.2. Biomaterials Used for Cardiac Tissue Engineering
4.2.1. Natural Polymers
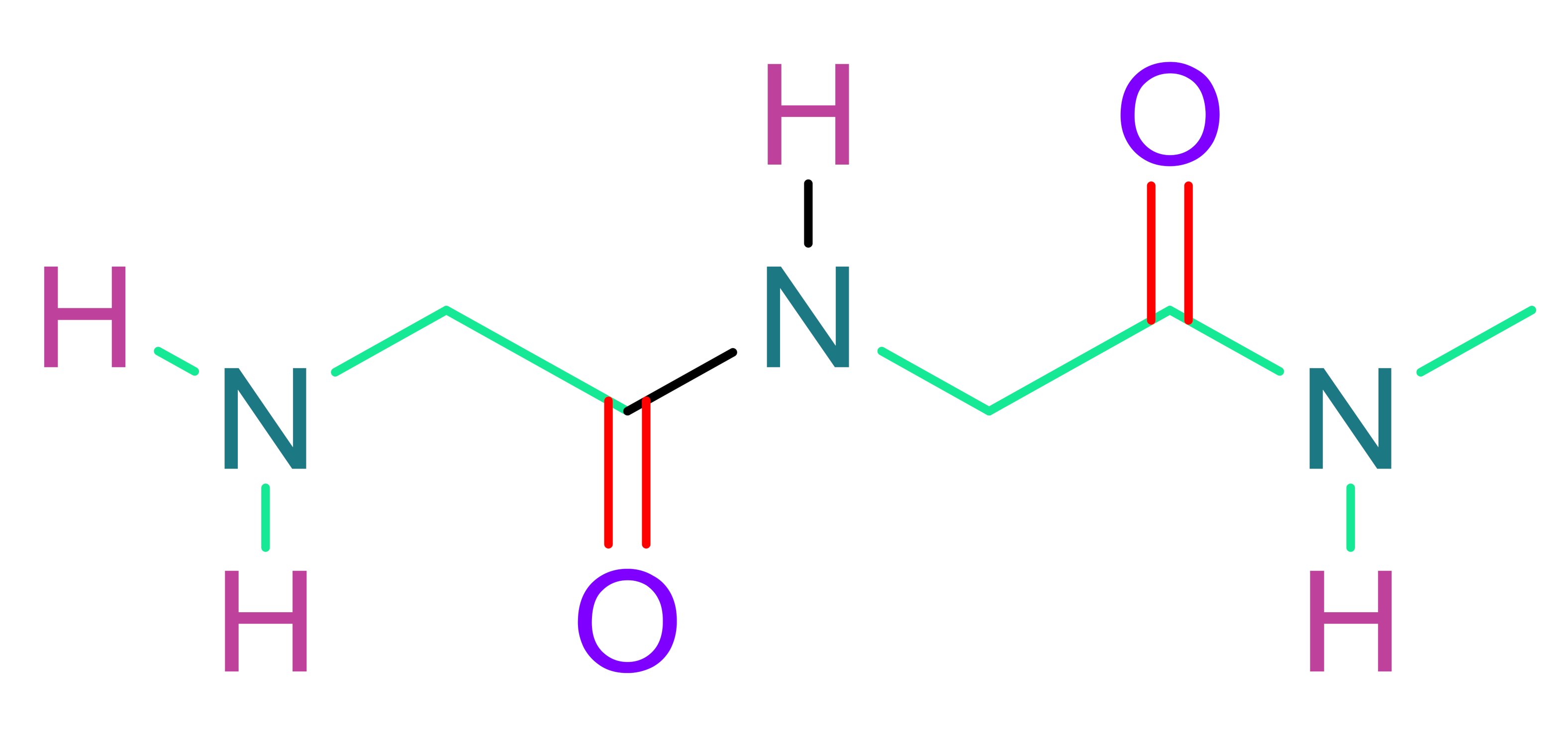
- (a)
- Fibrin
- (b)
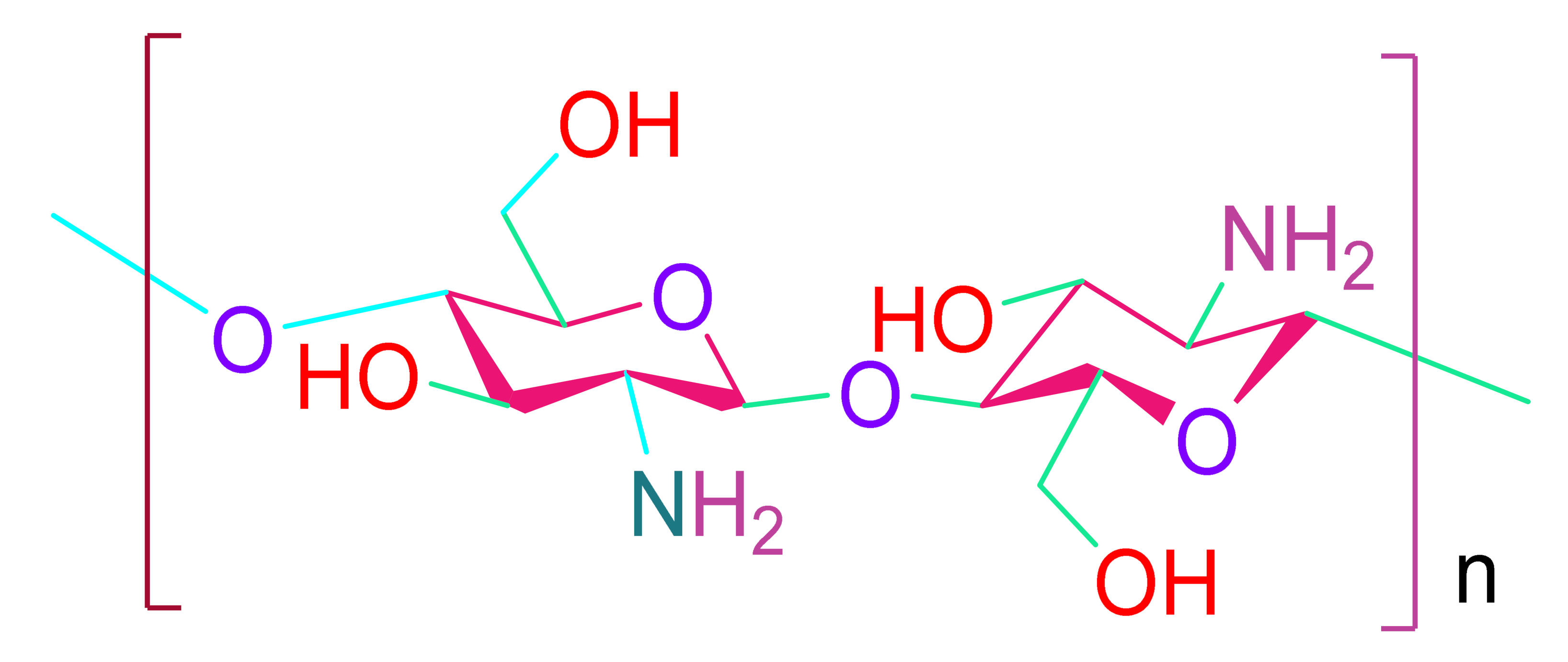
- Chitosan
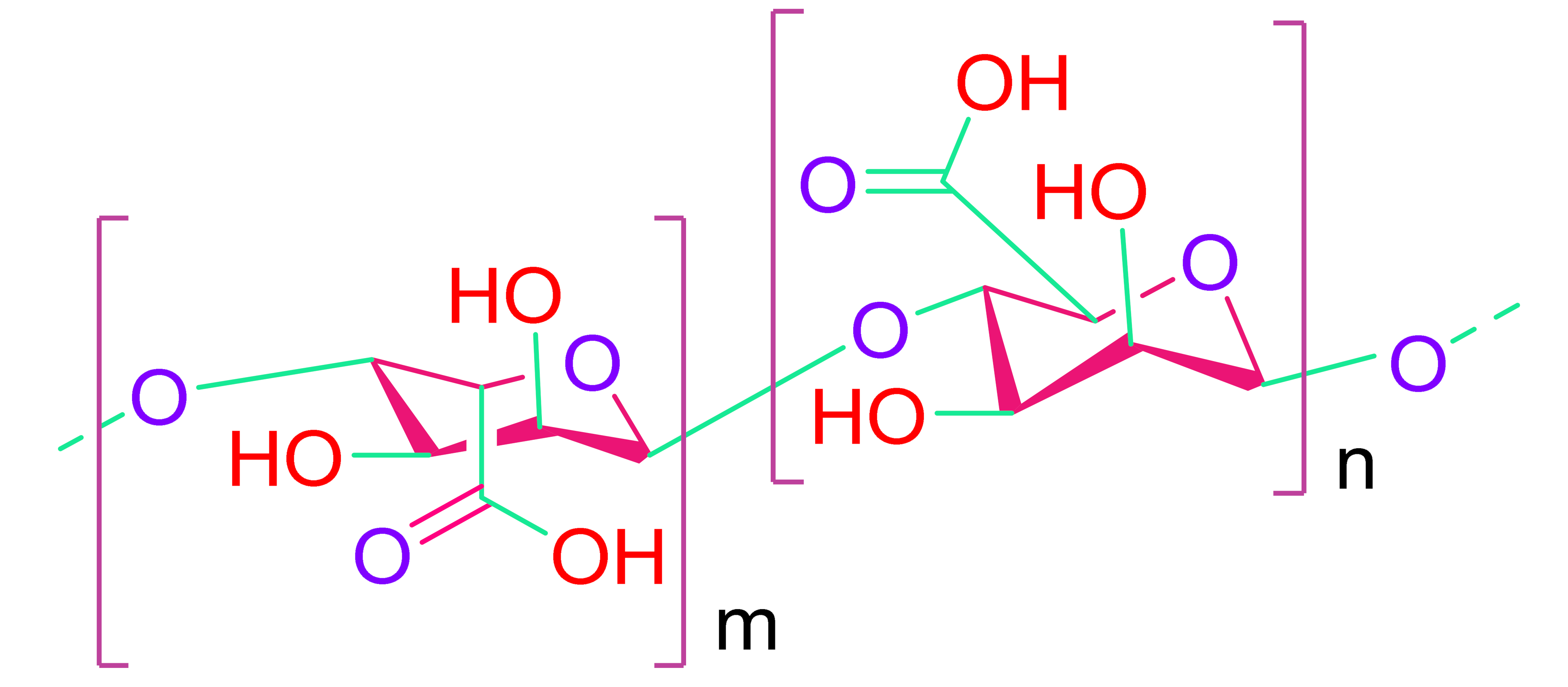
- (c)
- Alginate
- (d)
- Hyaluronic acid
- (e)
- Collagen
- (f)
- ECM
4.2.2. Synthetic Polymers
- (a)
- Poly(ethylene glycol)
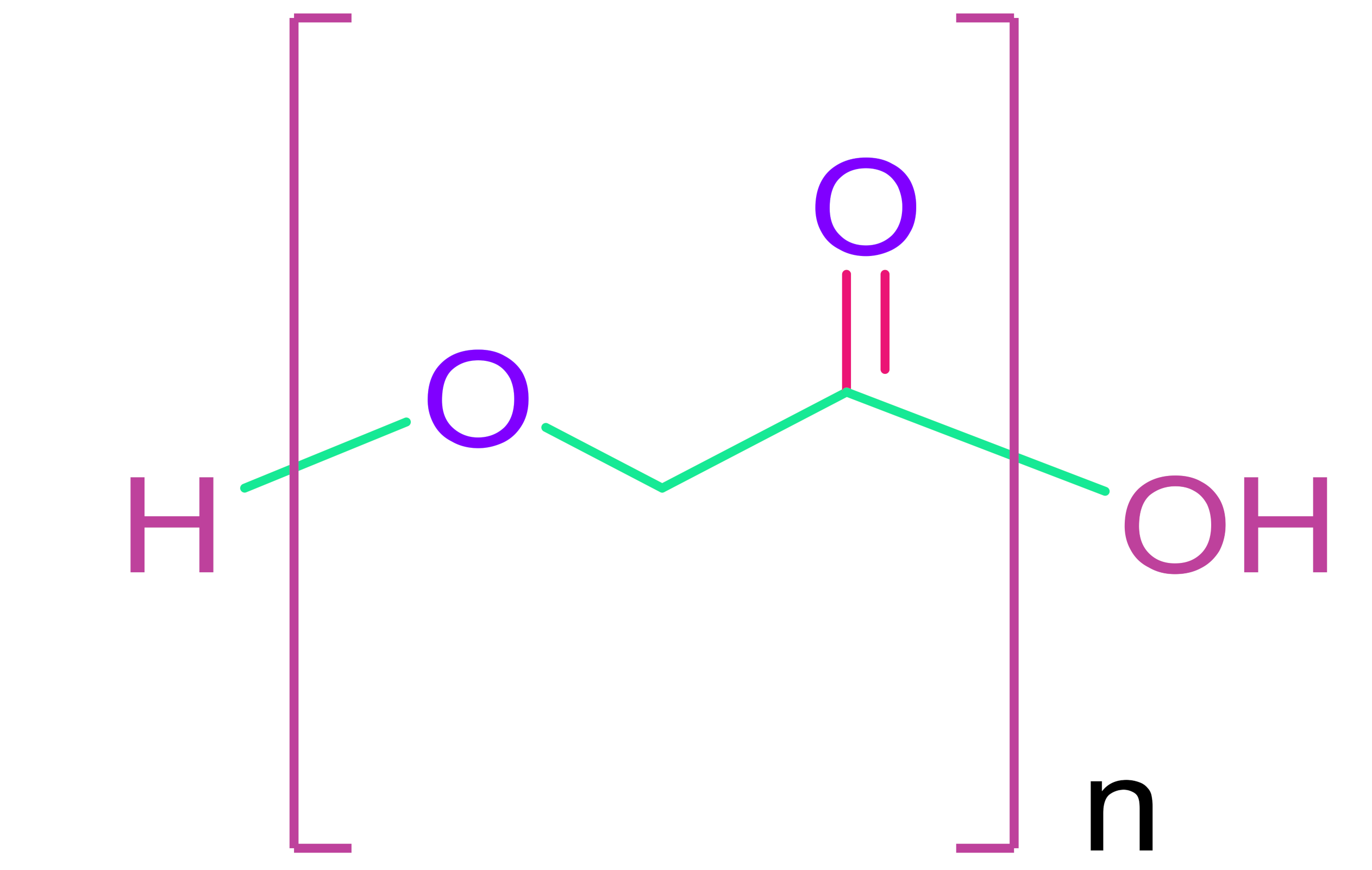
- (b)
- Poly(glycolic acid) & Poly(lactic acid)
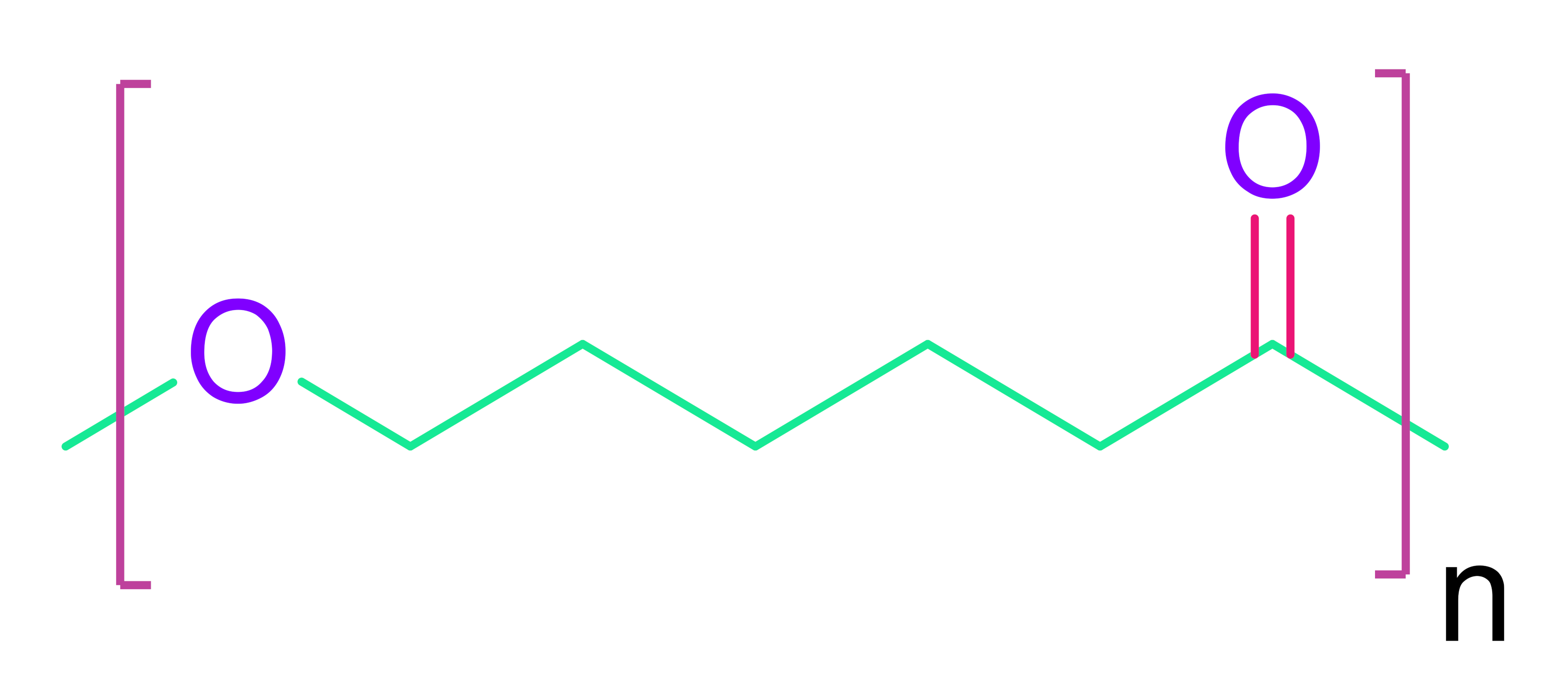
- (c)
- Poly(ε-caprolactone)
- (d)
- N-isopropylacrylamide (poly(N-isopropylacrylamide)) (PNIPAAm)
- (e)
- Hybrid gelatin methacryloyl (GelMA)
4.3. Delivery Strategies of Cells from Patch
- (a)
- Invasive method
- (b)
- Minimum Invasive Method
4.4. Advantages and Disadvantages of Patch
4.4.1. Advantages
4.4.2. Disadvantages
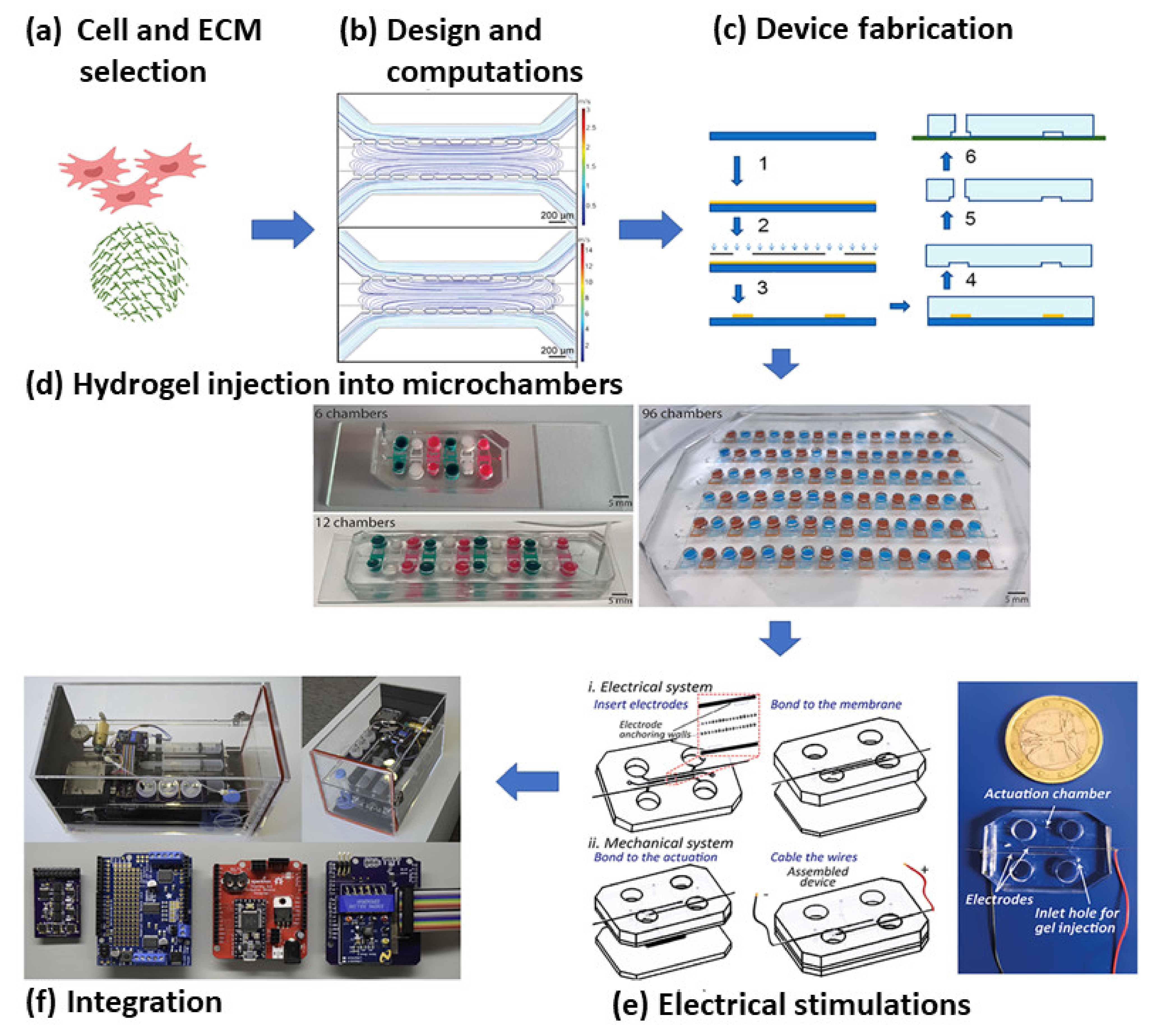
5. Microfluidics Based MI Research
5.1. Microfluidics for Cardiac Cell Biology
5.2. Application of Microfluidics in MI Research
5.3. Microfluidic 3D Culture Models
5.4. Implementation of Physical Forces
| Device Function | Cell Source | Techniques Used | Chemical or Physical Cues Studied | Scaffold Used | Fabrication Technique | Important Observations | References |
|---|---|---|---|---|---|---|---|
| Differentiation to CMs | ESCs | External motor for stretching the microfluidic device | Uniaxial cyclic mechanical stretch | 2D culture | Lithography | Reduction in cardiogenesis | [197] |
| hESCs | Micropatterned surface generation through direct micro contact printing | ------ | Micropatterned fibronectin hydrogel | ------ | Display of beating foci earlier than non-patterned substrates | [204] | |
| Drug toxicity testing | Human iPSC-CBs | Micro niches to trap CBs in microchannel, Perfusion based system | Veparamil, Quinidine, Doxorubicin | No external scaffold | Standard photo lithography | 3D environment showed different effect on beating frequency of cells | [193] |
| Human CMs | Micropillar based system to prohibit direct contact between 3D cell matrix from media flow, diffusion-based transport | Isoproterenol | Puramatrix hydrogel | PMMA micromilling | Cell viability appeared better in 3D culture | [192] | |
| Contractile stress measurement | Neonatal rat ventricular myocytes and human iPSC derived CMs | Electronic quantification of stress through Cantilever deflection measurement | Isoproterenol | 3D printed matrix of PDMS with polyamide electrical network | Multimaterial 3D printing | Positive chronotropic response to drug similar to engineered NRVM microtissues and ESC-derived CM tissue | [196] |
| Neonatal mouse CMs | Stress measurement by use of PIV technique to capture nanoparticle displacement coupled with finite element method. | Epinephrine | Sandwich of GelMA hydrogel and polyacrylamide hydrogels | 3D patterning | Increased frequency and amplitude of contraction cycles | [195] | |
| Generation of in vitro constructs for tissue engineering application | Neonatal rat CMs | Coaxial needle extrusion system | ------ | GelMA | 3D printing | Generated complex heterogenous structures with single bioink extruder | [194] |
| Hydraulic pressure and mechanical strain condition generation | H9c2 cells | Use of peristaltic pump coupled with pneumatically actuated valve to generate pathological heart conditions | ------ | ------ | PDMS molding | Organized F actin alignment similar to in vivo | [198] |
| Neonatal rat CM | Pneumatic deflection of thin PDMS membrane to generate stretch | Uniaxial cyclic stretch | Cell laden gel | Lithography | Superior cardiac differentiation with better electrical and mechanical coupling | [202] | |
| Effect of electrical field on proliferation and differentiation | Neonatal rat CM | ------ | Square monophasic electrical pulses | 2D cell culture on collagen coated matrix | Laser ablation of ITO coated glass slides to generate electrodes | Cell aligned in the direction perpendicular to the electric field | [207] |
| 3D environment mimicking shear protection from endothelial barrier | hiPSC derived CMs | ------ | Verapamil, Isoproterenol, Metoprolol, E-4031 | ------ | Two step photolithography process | IC50 and EC50 values were more consistent with the data on tissue-scale references | [191] |
5.5. Drug Discovery and Disease Modelling
5.6. Point of Care Devices and Disease Diagnosis
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics’ 2017 Update. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021. Circulation 2021, 143, 254–743. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Jeemon, P.; Roy, A. Cardiovascular Diseases in India: Current Epidemiology and Future Directions. Circulation 2016, 133, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Pfuntner, A.; Wier, L.M.; Stocks, C. Most Frequent Conditions in U.S. Hospitals, 2011: Statistical Brief#162; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. [Google Scholar]
- Frangogiannis, N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [PubMed]
- Tonsho, M.; Michel, S.; Ahmed, Z.; Alessandrini, A.; Madsen, J.C. Heart transplantation: Challenges facing the field. Cold Spring Harb. Perspect. Med. 2014, 4, a015636. [Google Scholar] [CrossRef]
- Feric, N.T.; Radisic, M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv. Drug Deliv. Rev. 2016, 96, 110–134. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in cardiac tissue engineering. Tissue Eng. Part B Rev. 2020, 16, 169–187. [Google Scholar] [CrossRef]
- Cahill, T.J.; Choudhury, R.P.; Riley, P.R. Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Nat. Rev. Drug Discov. 2017, 16, 699–717. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Ban, K.; Park, H.-J.; Kim, S.; Andukuri, A.; Cho, K.-W.; Hwang, J.W.; Cha, H.J.; Kim, S.Y.; Kim, W.-S.; Jun, H.-W.; et al. Cell Therapy with Embryonic Stem Cell-Derived Cardiomyocytes Encapsulated in Injectable Nanomatrix Gel Enhances Cell Engraftment and Promotes Cardiac Repair. ACS Nano 2014, 8, 10815–10825. [Google Scholar] [CrossRef]
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Günter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational cardiac stem cell therapy: Advancing from first-generation to next-generation cell types. Npj Regen. Med. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chetty, S.S.; Praneetha, S.; Vadivel Murugan, A.; Varthana, K.; Verma, R.S. Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells Labeled with Mn2+ and Gd3+ Co-Doped CuInS2-ZnS Nanocrystals for Multimodality Imaging in a Tumor Mice Model. ACS Appl. Mater. Interfaces 2020, 12, 3415–3429. [Google Scholar] [CrossRef]
- Behfar, A.; Perez-Terzic, C.; Faustino, R.S.; Arrell, D.K.; Hodgson, D.M.; Yamada, S.; Puceat, M.; Niederländer, N.; Alekseev, A.E.; Zingman, L.V.; et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J. Exp. Med. 2007, 19, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shapiro, L.; Flynn, A. The clinical application of mesenchymal stem cells and cardiac stem cells as a therapy for cardiovascular disease. Pharmacology 2015, 151, 8–15. [Google Scholar] [CrossRef]
- Ji, L.L.; Long, X.F.; Tian, H.; Liu, Y.F. Effect of transplantation of bone marrow stem cells on myocardial infarction size in a rabbit model. World J. Emerg. Med. 2013, 4, 304–310. [Google Scholar] [CrossRef][Green Version]
- Roura, S.; Gálvez-Montón, C.; Mirabel, C.; Vives, J.; Bayes-Genis, A. Mesenchymal stem cells for cardiac repair: Are the actors ready for the clinical scenario? Stem Cell Res. Ther. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Tang, J.N.; Cores, J.; Huang, K.; Cui, X.L.; Luo, L.; Zhang, J.Y.; Li, T.S.; Qian, L.; Cheng, K. Concise Review: Is Cardiac Cell Therapy Dead? Embarrassing Trial Outcomes and New Directions for the Future. Stem Cells Transl. Med. 2018, 7, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. Preclinical Studies of Stem Cell Therapy for Heart Disease. Circ. Res. 2018, 122, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, L.; Zeng, C.; Wang, W.E. Functionally improved mesenchymal stem cells to better treat myocardial infarction. Stem Cells Int. 2018, 2018, 7045245. [Google Scholar] [CrossRef]
- Verma, R.S. Recent Advances in Induced Pluripotent Stem Cell (iPSC) based Therapeutics. J. Stem Cell Res. Ther. 2017, 16, 115–130. [Google Scholar] [CrossRef][Green Version]
- Xu, J.-Y.; Cai, W.-Y.; Tian, M.; Liu, D.; Huang, R.-C. Stem cell transplantation dose in patients with acute myocardial infarction: A meta-analysis. Chronic Dis. Transl. Med. 2016, 2, 92–101. [Google Scholar] [CrossRef]
- Hartman, M.E.; Librande, J.R.; Medvedev, I.O.; Ahmad, R.N.; Moussavi-Harami, F.; Gupta, P.P.; Chien, W.M.; Chin, M.T. An optimized and simplified system of mouse embryonic stem cell cardiac differentiation for the assessment of differentiation modifiers. PLoS ONE 2014, 9, e93033. [Google Scholar] [CrossRef]
- Hodgson, D.M.; Behfar, A.; Zingman, L.V.; Kane, G.C.; Alekseev, A.E.; Pucéat, M.; Terzic, A. Cellular Plasticity in the Cardiovascular System Stable benefit of embryonic stem cell therapy in myocardial infarction. Am. J. Physiol. 2009, 55905, 471–479. [Google Scholar]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Dementyeva, E.V.; Medvedev, S.P.; Kovalenko, V.R.; Vyatkin, Y.V.; Kretov, E.I.; Slotvitsky, M.M.; Shtokalo, D.N.; Pokushalov, E.A.; Zakian, S.M. Applying Patient-Specific Induced Pluripotent Stem Cells to Create a Model of Hypertrophic Cardiomyopathy. Biochem. Biokhimiia 2019, 84, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mauritz, C.; Martens, A.; Rojas, S.V.; Schnick, T.; Rathert, C.; Schecker, N.; Menke, S.; Glage, S.; Zweigerdt, R.; Haverich, A.; et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur. Heart J. 2011, 32, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Pushp, P.; Nogueira, D.E.S.; Rodrigues, C.A.V.; Ferreira, F.C.; Cabral, J.M.S.; Gupta, M.K. A Concise Review on Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Personalized Regenerative Medicine. Stem Cell Rev. Rep. 2020, 748–776. [Google Scholar]
- Maza, I.; Caspi, I.; Zviran, A.; Chomsky, E.; Rais, Y.; Viukov, S.; Geula, S.; Buenrostro, J.D.; Weinberger, L.; Krupalnik, V.; et al. Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat. Biotechnol. 2015, 33, 769–774. [Google Scholar] [CrossRef]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res. Ther. 2017, 8, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, R.; Darbandi-Azar, A.; Sadeghpour, A.; Majidzadeh, A.-K.; Eini, L.; Jafarbeik-Iravani, N.; Hoseinpour, P.; Vajhi, A.; Bakhshaiesh, T.O.; Masoudkabir, F.; et al. Mesenchymal stem cells Pretreatment with stromal-derived factor-1 alpha augments cardiac function and angiogenesis in infarcted myocardium. Am. J. Med. Sci. 2021, 361, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, Y.; Chen, W.; Li, J.; Wang, X.; Chen, Y.; Yu, Y.; Shen, Z.; Zhang, Y. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J. Mater. Chem. B 2020, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Kumaraswamy, P.; Krishnan, U.M.; Sethuraman, S. Engineering a growth factor embedded nanofiber matrix niche to promote vascularization for functional cardiac regeneration. Biomaterials 2016, 97, 176–195. [Google Scholar] [CrossRef]
- Duelen, R.; Sampaolesi, M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. EBioMedicine 2017, 16, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Tracy, E.P.; Gettler, B.C.; Zakhari, J.S.; Schwartz, R.J.; Williams, S.K.; Birla, R.K. 3D Bioprinting the Cardiac Purkinje System Using Human Adipogenic Mesenchymal Stem Cell Derived Purkinje Cells. Cardiovasc. Eng. Technol. 2020, 11, 587–604. [Google Scholar] [CrossRef]
- Dawn, B.; Stein, A.B.; Urbanek, K.; Rota, M.; Whang, B.; Rastaldo, R.; Torella, D.; Tang, X.L.; Rezazadeh, A.; Kajstura, J.; et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc. Natl. Acad. Sci. USA 2005, 102, 3766–3771. [Google Scholar] [CrossRef]
- Li, N.; Huang, R.; Zhang, X.; Xin, Y.; Li, J.; Huang, Y.; Cui, W.; Stoltz, J.F.; Zhou, Y.; Kong, Q. Stem cells cardiac patch from decellularized umbilical artery improved heart function after myocardium infarction. Bio-Med Mater. Eng. 2017, 28, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mauretti, A.; Spaans, S.; Bax, N.A.M.; Sahlgren, C.; Bouten, C.V.C. Cardiac Progenitor Cells and the Interplay with Their Microenvironment. Stem Cells Int. 2017, 2017, 7471582. [Google Scholar] [CrossRef]
- Su, T.; Huang, K.; Daniele, M.A.; Hensley, M.T.; Young, A.T.; Tang, J.; Allen, T.A.; Vandergriff, A.C.; Erb, P.D.; Ligler, F.S.; et al. Cardiac Stem Cell Patch Integrated with Microengineered Blood Vessels Promotes Cardiomyocyte Proliferation and Neovascularization after Acute Myocardial Infarction. ACS Appl. Mater. Interfaces 2018, 10, 33088–33096. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.J.; Berman, D.; Czer, L.S.C.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-derived aUtologous stem CElls to reverse ventricular dysfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J. Cardiol. 2015, 7, 243. [Google Scholar] [CrossRef]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, X.; Shen, Y. Qian, L.; Kong, X.; Chen, M.; Cao, K.; Zhang, F. Transplantation of iPSc Restores Cardiac Function by Promoting Angiogenesis and Ameliorating Cardiac Remodeling in a Post-infarcted Swine Model. Cell Biochem. Biophys. 2015, 71, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, Y.; Kaneko, M.; Yamahara, K.; Koulouroudias, M.; Sato, N.; Uppal, R.; Yamazaki, K.; Saito, S.; Suzuki, K. Self-assembling peptide hydrogel enables instant epicardial coating of the heart with mesenchymal stromal cells for the treatment of heart failure. Biomaterials 2018, 154, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Lamirault, G.; Toquet, C.; Lhommet, P.; Rederstorff, E.; Sourice, S.; Biteau, K.; Hulin, P.; Forest, V.; Weiss, P.; et al. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PLoS ONE 2012, 7, e51991. [Google Scholar] [CrossRef]
- Mayfield, A.E.; Tilokee, E.L.; Latham, N.; McNeill, B.; Lam, B.-K.; Ruel, M.; Suuronen, E.J.; Courtman, D.W.; Stewart, D.J.; Davis, D.R. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials 2014, 35, 133–142. [Google Scholar] [CrossRef]
- Durrani, S.; Konoplyannikov, M.; Ashraf, M.; Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010, 5, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Murtuza, B.; Beauchamp, J.R.; Smolenski, R.T.; Varela-Carver, A.; Fukushima, S.; Coppen, S.R.; Partridge, T.A.; Yacoub, M.H. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004, 18, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Menasché, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.-T.; Marolleau, J.-P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Rijen, H.V.M.; van Forest, V.; Evain, S.; Leblond, A.-L.; Mérot, J.; Charpentier, F.; Bakker, J.M.T.; de Lemarchand, P. Cardiac cell therapy: Overexpression of connexin43 in skeletal myoblasts and prevention of ventricular arrhythmias. J. Cell. Mol. Med. 2009, 13, 3703–3712. [Google Scholar] [CrossRef]
- Hirata, Y.; Sata, M.; Motomura, N.; Takanashi, M.; Suematsu, Y.; Ono, M.; Takamoto, S. Human umbilical cord blood cells improve cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2005, 327, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, K.; Gupta, P.K.; Ramasamy, D.; Kumar, P.; Mahadevan, S.; Verma, R.S. DNA methylation microarray uncovers a permissive methylome for cardiomyocyte differentiation in human mesenchymal stem cells. Genomics 2020, 112, 1384–1395. [Google Scholar] [CrossRef]
- Govarthanan, K.; Vidyasekar, P.; Gupta, P.K.; Lenka, N.; Verma, R.S. Glycogen synthase kinase 3β inhibitor-CHIR99021 augments the differentiation potential of mesenchymal stem cells. Cytotherapy 2020, 22, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Lee, W.-Y.; Yu, C.-L.; Hwang, S.-M.; Chung, M.-F.; Hsu, L.-W.; Chang, Y.; Lin, W.-W.; Tsai, M.-S.; Wei, H.-J.; et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 2010, 31, 6444–6453. [Google Scholar] [CrossRef]
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, Z.; Wang, H.; Reese, B.E.; Gushchina, L.V.; Jiang, M.; Agarwal, P.; Xu, j.; Zhang, M.; Shen, R.; et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Bauer, M.; Kang, L.; Qiu, Y.; Wu, J.; Peng, M.; Chen, H.H.; Camci-Unal, G.; Bayomy, A.F.; Sosnovik, D.E.; Khademhosseini, A.; et al. Adult Cardiac Progenitor Cell Aggregates Exhibit Survival Benefit Both In Vitro and In Vivo. PLoS ONE 2012, 7, e50491. [Google Scholar]
- Monsanto, M.M.; Wang, B.J.; Ehrenberg, Z.R.; Echeagaray, O.; White, K.S.; Alvarez, R.; Fisher, K.; Sengphanith, S.; Muliono, A.; Gude, N.A.; et al. Enhancing myocardial repair with CardioClusters. Nat. Commun. 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable Scaffold with Built-in Vasculature for Organ-on-a-Chip Engineering and Direct Surgical Anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Shahabipour, F.; Banach, M.; Johnston, T.P.; Pirro, M.; Sahebkar, A. Novel approaches toward the generation of bioscaffolds as a potential therapy in cardiovascular tissue engineering. Int. J. Cardiol. 2017, 228, 319–326. [Google Scholar] [CrossRef]
- Fleischer, S.; Feiner, R.; Dvir, T. Cutting-edge platforms in cardiac tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 23–29. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-on-a-chip for in vitro disease models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Navaei, A.; Moore, N.; Sullivan, R.T.; Truong, D.; Migrino, R.Q.; Nikkhah, M. Electrically conductive hydrogel-based micro-topographies for the development of organized cardiac tissues. RSC Adv. 2017, 7, 3302–3312. [Google Scholar] [CrossRef]
- Monteiro, L.M.; Vasques-Nóvoa, F.; Ferreira, L.; Pinto-Do-ó, P.; Nascimento, D.S. Restoring heart function and electrical integrity: Closing the circuit. Npj Regen. Med. 2017, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Matsumura, Y.; Tang, Y.; Roy, S.; Hoff, R.; Wang, B.; Wagner, W.R. Sustained viral gene delivery from a micro-fibrous, elastomeric cardiac patch to the ischemic rat heart. Biomaterials 2017, 133, 132–143. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Cieślar-Pobuda, A.; Ejneby, M.S.; Sammalisto, L.; Gelmi, A.; de Muinck, E.; Brask, J.; Łos, M.J.; Rafat, M. Rational Design of a Conductive Collagen Heart Patch. Macromol. Biosci. 2017, 17, 1600446. [Google Scholar] [CrossRef]
- Fleischer, S.; Feiner, R.; Dvir, T. Cardiac tissue engineering: From matrix design to the engineering of bionic hearts. Regen. Med. 2017, 12, 275–284. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- KC, P.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef]
- Kuraitis, D.; Giordano, C.; Ruel, M.; Musarò, A.; Suuronen, E.J. Exploiting extracellular matrix-stem cell interactions: A review of natural materials for therapeutic muscle regeneration. Biomaterials 2012, 33, 428–443. [Google Scholar] [CrossRef]
- Li, H.; Bao, M.; Nie, Y. Extracellular matrix–based biomaterials for cardiac regeneration and repair. Heart Fail. Rev. 2020, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular matrix and heart development. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, Z.; Konttinen, Y.T.; Kaivosoja, E.; Stegajev, V.; Wagner, H.D.; Levón, J.; Tiainen, V.M. Extracellular matrix and tissue regeneration. In Regenerative Medicine 2016—from Protocol to Patient: 1. Biology of Tissue Regeneration; Springer: Cham, Switzerland, 2016; pp. 1–55. [Google Scholar]
- Pattar, S.S.; Fatehi Hassanabad, A.; Fedak, P.W.M. Acellular Extracellular Matrix Bioscaffolds for Cardiac Repair and Regeneration. Front. Cell Dev. Biol. 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Batalov, I. Engineering 2D Cardiac Tissues Using Biomimetic Protein Micropatterns Based on the Extracellular Matrix in the Embryonic Heart. ProQuest Diss. Ph.D. Thesis, Carnegie Mellon University, Gloucester, VA, USA, 2017; p. 183. [Google Scholar]
- Ciuffreda, M.C.; Malpasso, G.; Chokoza, C.; Bezuidenhout, D.; Goetsch, K.P.; Mura, M.; Pisano, F.; Davies, N.H.; Gnecchi, M. Synthetic extracellular matrix mimic hydrogel improves efficacy of mesenchymal stromal cell therapy for ischemic cardiomyopathy. Acta Biomater. 2018, 70, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Hsieh, P.C.H.; Grodzinsky, A.J.; Lee, R.T. Custom design of the cardiac microenvironment with biomaterials. Circ. Res. 2005, 97, 8–15. [Google Scholar] [CrossRef]
- Seif-Naraghi, S.B.; Singelyn, J.M.; Salvatore, M.A.; Osborn, K.G.; Wang, J.J.; Sampat, U.; Kwan, O.L.; Strachan, G.M.; Wong, J.; Schup-Magoffin, P.J.; et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 2013, 5, 173ra25. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Krishnan, U.M.; Sethuraman, S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014, 32, 449–461. [Google Scholar] [CrossRef]
- Silvestri, A.; Boffito, M.; Sartori, S.; Ciardelli, G. Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol. Biosci. 2013, 13, 984–1019. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Ruvinov, E.; Dvir, T.; Leor, J.; Cohen, S. Myocardial repair: From salvage to tissue reconstruction. Expert Rev. Cardiovasc. Ther. 2018, 6, 669–686. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Asti, A.; Gioglio, L. Natural and Synthetic Biodegradable Polymers: Different Scaffolds for Cell Expansion and Tissue Formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Hinderer, S.; Brauchle, E.; Schenke-Layland, K. Generation and Assessment of Functional Biomaterial Scaffolds for Applications in Cardiovascular Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2015, 4, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Chiu, L.L.Y.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Joch, C. The safety of fibrin sealants. Cardiovasc. Surg. 2003, 11, 23–28. [Google Scholar] [CrossRef]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ichihara, Y.; Sato, N.; Umeda, N.; Fields, L.; Fukumitsu, M.; Tago, Y.; Ito, T.; Kainuma, S.; Podaru, M.; et al. On-site fabrication of Bi-layered adhesive mesenchymal stromal cell-dressings for the treatment of heart failure. Biomaterials 2019, 209, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Radosevich, M.; Goubran, H.A.; Burnouf, T. Fibrin sealant: Scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997, 72, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; Polo-Corrales, L.; Ramirez-Vick, J.E.; Freytes, D.O. Tissue Engineering Strategies for Myocardial Regeneration: Acellular Versus Cellular Scaffolds? Tissue Eng. Part B Rev. 2016, 22, 438–458. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Hoeeg, C.; Dolatshahi-Pirouz, A.; Follin, B. Injectable hydrogels for improving cardiac cell therapy—in vivo evidence and translational challenges. Gels 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- Tormos, C.J.; Madihally, S.V. Chitosan for cardiac tissue engineering and regeneration. In Chitosan Based Biomaterials; Woodhead Publishing: Shaston, UK, 2017; Volume 2. [Google Scholar]
- Chen, J.; Zhan, Y.; Wang, Y.; Han, D.; Tao, B.; Luo, Z.; Ma, S.; Wang, Q.; Li, X.; Fan, L.; et al. Chitosan/silk fibroin modified nanofibrous patches with mesenchymal stem cells prevent heart remodeling post-myocardial infarction in rats. Acta Biomater. 2018, 80, 154–168. [Google Scholar] [CrossRef]
- Hasan, A.; Khattab, A.; Islam, M.A.; Hweij, K.A.; Zeitouny, J.; Waters, R.; Sayegh, M.; Hossain, M.M.; Paul, A. Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction. Adv. Sci. 2015, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J.S.; Cristian, G.; Dragomir, D.; Pusineri, E.; Piredda, M.; Bettari, L.; Dowling, R.; Volterrani, M.; Kirwan, B.-A.; et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial). Eur. Heart J. 2015, 36, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Dahlmann, J.; Krause, A.; Möller, L.; Kensah, G.; Möwes, M.; Diekmann, A.; Martin, U.; Kirschning, A.; Gruh, I.; Dräger, G. Biomaterials Fully de fi ned in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials 2013, 34, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Christman, K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2013, 10, 59–72. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Q.; Tu, J.; Zhu, J. Synthesis and characterization of low molecular weight hyaluronic acid-based cationic micelles for efficient siRNA delivery. Carbohydr. Polym. 2009, 77, 95–104. [Google Scholar] [CrossRef]
- Chi, N.H.; Yang, M.C.; Chung, T.W.; Chou, N.K.; Wang, S.S. Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydr. Polym. 2013, 92, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Kodavaty, J.; Deshpande, A.P. Regimes of microstructural evolution as observed from rheology and surface morphology of crosslinked poly(vinyl alcohol) and hyaluronic acid blends during gelation. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Wu, W.; Peng, S.; Song, Z.; Lin, S. Collagen biomaterial for the treatment of myocardial infarction: An update on cardiac tissue engineering and myocardial regeneration. Drug Deliv. Transl. Res. 2019, 9, 920–934. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for cardiac tissue engineering. NPG Asia Mater. 2014, 6, 99. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, J.; Huang, Z.; Qu, L.; Lin, N.; Liang, C.; Dai, R.; Tang, L.; Tian, F. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. Int. J. Nanomed. 2017, 12, 3109–3120. [Google Scholar] [CrossRef]
- Sarig, U.; Sarig, H.; de-Berardinis, E.; Chaw, S.-Y.; Nguyen, E.B.V.; Ramanujam, V.S.; Thang, V.D.; Al-Haddawi, M.; Liao, S.; Seliktar, D.; et al. Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomater. 2016, 44, 209–220. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huang, C. Composition and Mechanism of Three-Dimensional Hydrogel System in Regulating Stem Cell Fate. Tissue Engine. Part B Reviews. 2020, 26, 498–518. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Jeffords, M.E.; Wu, J.; Shah, M.; Hong, Y.; Zhang, G. Tailoring material properties of cardiac matrix hydrogels to induce endothelial differentiation of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 11053–11061. [Google Scholar] [CrossRef]
- Efraim, Y.; Sarig, H.; Cohen Anavy, N.; Sarig, U.; de Berardinis, E.; Chaw, S.-Y.; Krishnamoorthi, M.; Kalifa, J.; Bogireddi, H.; Duc, T.V.; et al. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater. 2017, 50, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Tian, L.; Li, Y.; Zhang, J.; Wei, Y.; Jin, Z.; Liu, Z.; Liu, H. Combining ECM Hydrogels of Cardiac Bioactivity with Stem Cells of High Cardiomyogenic Potential for Myocardial Repair. Stem Cells Int. 2019, 2019, 6708435. [Google Scholar] [CrossRef]
- Mewhort, H.E.M.; Turnbull, J.D.; Meijndert, H.C.; Ngu, J.M.C.; Fedak, P.W.M. Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J. Thorac. Cardiovasc. Surg. 2014, 147, 1650–1659. [Google Scholar] [CrossRef]
- Sreejit, P.; Verma, R.S. Natural ECM as Biomaterial for Scaffold Based Cardiac Regeneration Using Adult Bone Marrow Derived Stem Cells. Stem Cell Rev. Rep. 2013, 9, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Heras-Bautista, C.O.; Mikhael, N.; Lam, J.; Shinde, V.; Katsen-Globa, A.; Dieluweit, S.; Molcanyi, M.; Uvarov, V.; Jütten, P.; Sahito, R.G.A.; et al. Cardiomyocytes facing fibrotic conditions re-express extracellular matrix transcripts. Acta Biomater. 2019, 89, 180–192. [Google Scholar] [CrossRef]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-based localized nonviral gene delivery in regenerative medicine approaches—An overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef]
- Pascual-Gil, S.; Garbayo, E.; Díaz-Herráez, P.; Prosper, F.; Blanco-Prieto, M.J. Heart regeneration after myocardial infarction using synthetic biomaterials. J. Control. Release 2015, 203, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Jiang, X.; Li, Z.; Wen, Y.; Chen, D.; Li, X.; Zhang, X.; Zhuo, R.; Chu, H. Physical properties of poly(N-isopropylacrylamide) hydrogel promote its effects on cardiac protection after myocardial infarction. J. Int. Med. Res. 2012, 40, 2167–2182. [Google Scholar] [CrossRef]
- Nelson, D.M.; Ma, Z.; Fujimoto, K.L.; Hashizume, R.; Wagner, W.R. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011, 7, 1–15. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Prat-Vidal, C.; Bayes-Genis, A. In vivo experience with natural scaffolds for myocardial infarction: The times they are a-changin’. Stem Cell Res. Ther. 2015, 6, 248. [Google Scholar] [CrossRef]
- Mukherjee, R.; Zavadzkas, J.A.; Saunders, S.M.; McLean, J.E.; Jeffords, L.B.; Beck, C.; Stroud, R.E.; Leone, A.M.; Koval, C.N.; Rivers, W.T.; et al. Targeted myocardial microinjections of a biocomposite material reduces infarct expansion in pigs. Ann. Thorac. Surg. 2008, 86, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Gil, S.; Simón-Yarza, T.; Garbayo, E.; Prósper, F.; Blanco-Prieto, M.J. Cytokine-loaded PLGA and PEG-PLGA microparticles showed similar heart regeneration in a rat myocardial infarction model. Int. J. Pharm. 2017, 523, 531–533. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, X.-J.; Tang, Q.-Z.; Li, X.-Y.; Lin, T.; Wu, D.-Q.; Zhang, X.-Z.; Okello, E. Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009, 5, 2939–2944. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Wang, H.; Chang, N.; Zhou, X.-H.; Zhu, X.; Wang, J.; Xiong, J.-W. PEG–PLA nanoparticles facilitate siRNA knockdown in adult zebrafish heart. Dev. Biol. 2015, 406, 196–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alcon, A.; Cagavi Bozkulak, E.; Qyang, Y. Regenerating functional heart tissue for myocardial repair. Cell. Mol. Life Sci. CMLS 2012, 69, 2635–2656. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Prabhakaran, M.P.; Mukherjee, S.; Ravichandran, R.; Dan, K.; Ramakrishna, S. Biomaterial strategies for alleviation of myocardial infarction. J. R. Soc. Interface 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Huang, C.-C.; Wei, H.-J.; Yeh, Y.-C.; Wang, J.-J.; Lin, W.-W.; Lee, T.-Y.; Hwang, S.-M.; Choi, S.-W.; Xia, Y.; Chang, Y.; et al. Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials 2012, 33, 4069–4077. [Google Scholar] [CrossRef]
- McDevitt, T.C.; Angello, J.C.; Whitney, M.L.; Reinecke, H.; Hauschka, S.D.; Murry, C.E.; Stayton, P.S. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J. Biomed. Mater. Res. 2002, 60, 472–479. [Google Scholar] [CrossRef]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly(lactic–co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Radisic, M.; Lim, J.O.; Chang, B.H.; Vunjak-Novakovic, G. A novel composite scaffold for cardiac tissue engineering. Vitr. Cell. Dev. Biol. Anim. 2005, 41, 188–196. [Google Scholar] [CrossRef]
- Ishii, O.; Shin, M.; Sueda, T.; Vacanti, J.P. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix–like topography. J. Thorac. Cardiovasc. Surg. 2005, 130, 1358–1363. [Google Scholar] [CrossRef]
- Piao, H.; Kwon, J.-S.; Piao, S.; Sohn, J.-H.; Lee, Y.-S.; Bae, J.-W.; Hwang, K.-K.; Kim, D.-W.; Jeon, O.; Kim, B.-S.; et al. Effects of cardiac patches engineered with bone marrow-derived mononuclear cells and PGCL scaffolds in a rat myocardial infarction model. Biomaterials 2007, 28, 641–649. [Google Scholar] [CrossRef]
- Kim, E.H.; Joo, M.K.; Bahk, K.H.; Park, M.H.; Chi, B.; Lee, Y.M.; Jeong, B. Reverse Thermal Gelation of PAF-PLX-PAF Block Copolymer Aqueous Solution. Biomacromolecules 2009, 10, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zeng, F.; Huang, X.-P.; Chung, J.; Konecny, C.-Y.F.; Weisel, R.D.; Li, R.-K. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials 2011, 32, 579–586. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-based biohybrid injectable hydrogel for cardiac tissue engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Fu, M.; Xu, Z.; Zhang, B.; Li, Z.; Li, H.; Zhou, X.; Liu, X.; Duan, Y.; Lin, P.-H.; et al. Sustained Release of a Peptide-Based Matrix Metalloproteinase-2 Inhibitor to Attenuate Adverse Cardiac Remodeling and Improve Cardiac Function Following Myocardial Infarction. Biomacromolecules 2017, 18, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Deep, N.; Mishra, P. Fabrication and characterization of thermally conductive PMMA/MWCNT nanocomposites. Mater. Today Proc. 2018, 5, 28328–28336. [Google Scholar] [CrossRef]
- Koerner, H.; Price, G.; Pearce, N.A.; Alexander, M.; Vaia, R.A. Remotely actuated polymer nanocomposites—Stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 2014, 3, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lü, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Li, Y.; Poon, C.T.; Li, M.; Lu, T.J.; Pingguan-Murphy, B.; Xu, F. Hydrogel Fibers: Chinese-Noodle-Inspired Muscle Myofiber Fabrication (Adv. Funct. Mater. 37/2015). Adv. Funct. Mater. 2015, 25, 6020. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black III, L.D.; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huo, D.; Li, Y.; Wu, Y.; Zeng, L.; Cheng, P.; Xing, M.; Zeng, W.; Zhu, C. A VEGF delivery system targeting MI improves angiogenesis and cardiac function based on the tropism of MSCs and layer-by-layer self-assembly. Biomaterials 2017, 127, 117–131. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.J.; Kim, S.W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Hou, D.; Youssef, E.A.-S.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112 (Suppl. S9), I-150–I-156. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, A.; Sen, D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res. Ther. 2016, 7, 82. [Google Scholar] [CrossRef]
- Wang, Q.L.; Wang, H.J.; Li, Z.H.; Wang, Y.L.; Wu, X.P.; Tan, Y.Z. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J. Cell. Mol. Med. 2017, 21, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Jamaiyar, A.; Wan, W.; Ohanyan, V.; Enrick, M.; Janota, D.; Cumpston, D.; Song, H.; Stevanov, K.; Kolz, C.L.; Hakobyan, T.; et al. Alignment of inducible vascular progenitor cells on a micro-bundle scaffold improves cardiac repair following myocardial infarction. Basic Res. Cardiol. 2017, 112, 41. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Vandergriff, A.; Wang, Z.; Hensley, M.T.; Cores, J.; Allen, T.A.; Dinh, P.U.; Zhang, J.; Caranasos, T.G.; Cheng, K. A Regenerative Cardiac Patch Formed by Spray Painting of Biomaterials onto the Heart. Tissue Eng. Part C Methods 2017, 23, 146–155. [Google Scholar] [CrossRef]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef]
- Peña, B.; Bosi, S.; Aguado, B.A.; Borin, D.; Farnsworth, N.L.; Dobrinskikh, E.; Rowland, T.J.; Martinelli, V.; Jeong, M.; Taylor, M.R.G.; et al. Injectable Carbon Nanotube-Functionalized Reverse Thermal Gel Promotes Cardiomyocytes Survival and Maturation. ACS Appl. Mater. Interfaces 2017, 9, 31645–31656. [Google Scholar] [CrossRef]
- Merimi, M.; Lewalle, P.; Meuleman, N.; Agha, D.M.; El-kehdy, H.; Bouhtit, F.; Ayoub, S.; Burny, A.; Fahmi, H.; Lagneaux, L.; et al. Mesenchymal stem/stromal cell therapeutic features: The Bridge between the Bench and the Clinic. J. Clin. Med. 2021, 10, 905. [Google Scholar] [CrossRef]
- Inamdar, N.K.; Borenstein, J.T. Microfluidic cell culture models for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 681–689. [Google Scholar] [CrossRef]
- Ni, M.; Tong, W.H.; Choudhury, D.; Rahim, N.A.A.; Iliescu, C.; Yu, H. Cell culture on MEMS platforms: A review. Int. J. Mol. Sci. 2009, 10, 5411–5441. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Kobuszewska, A.; Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy, M.; Dybko, A.; Renaud, P.; Brzozka, Z. Heart-on-a-Chip: An Investigation of the Influence of Static and Perfusion Conditions on Cardiac (H9C2) Cell Proliferation, Morphology, and Alignment. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Dong, Q.; Li, B.; Obaid, S.; Miccile, C.; Yin, R.T.; Talapatra, T.; Lin, Z.; Li, S.; Li, Z.; et al. Multiparametric slice culture platform for the investigation of human cardiac tissue physiology. Prog. Biophys. Mol. Biol. 2019, 144, 139–150. [Google Scholar] [CrossRef]
- Visone, R.; Talò, G.; Occhetta, P.; Cruz-moreira, D.; Lopa, S.; Pappalardo, O.A.; Redaelli, A.; Moretti, M.; Rasponi, M. A microscale biomimetic platform for generation and electro-mechanical stimulation of 3D cardiac microtissues. APL Bioeng. 2018, 2, 046102. [Google Scholar] [CrossRef] [PubMed]
- Visone, R.; Ugolini, G.S.; Vinarsky, V.; Penati, M.; Redaelli, A.; Forte, G.; Rasponi, M. A Simple Vacuum-Based Microfluidic Technique to Establish High-Throughput Organs-On-Chip and 3D Cell Cultures at the Microscale. Adv. Mater. Technol. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Ong, S.-G.; Huber, B.C.; Hee Lee, W.; Kodo, K.; Ebert, A.D.; Ma, Y.; Nguyen, P.K.; Diecke, S.; Chen, W.-Y.; Wu, J.C. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes After Acute Myocardial. Infarction Circ. 2015, 132, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.A.; Gower, R.M.; Stanhope, K.L.; Havel, P.J.; Simon, S.I.; Armstrong, E.J. On-chip phenotypic analysis of inflammatory monocytes in atherogenesis and myocardial infarction. Proc. Natl. Acad. Sci. USA 2013, 110, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Kanda, P.; Benavente-Babace, A.; Parent, S.; Connor, M.; Soucy, N.; Steeves, A.; Lu, A.; Cober, N.D.; Courtman, D.; Variola, F.; et al. Deterministic paracrine repair of injured myocardium using microfluidic-based cocooning of heart explant-derived cells. Biomaterials 2020, 247, 120010. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.L.; Aw, W.Y.; Hickey, A.J.; Polacheck, W.J. Microfluidic and Organ-on-a-Chip Approaches to Investigate Cellular and Microenvironmental Contributions to Cardiovascular Function and Pathology. Front. Bioeng. Biotechnol. 2021, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.J.; Li, J.; Rickel, J.M.R.; Klibanov, A.L.; Zuo, Z.; Hossack, J.A. Efficacy of Sonothrombolysis Using Microbubbles Produced by a Catheter-Based Microfluidic Device in a Rat Model of Ischemic Stroke. Ann. Biomed. Eng. 2019, 47, 1012–1022. [Google Scholar] [CrossRef]
- Ng, E.X.; Wang, M.; Neo, S.H.; Tee, C.A.; Chen5, C.-H.; Van Vliet, K.J. Dissolvable gelatin-based microcarriers generated through droplet microfluidics for expansion and culture of mesenchymal stromal cells. Biotechnol. J. 2020, 16, 2000048. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Wang, E.Y.; Feric, N.T.; Lai, B.F.L.; Knee-Walden, E.J.; Backx, P.H.; Radisic, M. Engineering microenvironment for human cardiac tissue assembly in heart-on-a-chip platform. Matrix Biol. 2019, 85, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Loskill, P.; Deveshwar, N.; Spencer, C.I.; Judge, L.M.; Mandegar, M.A.; Fox, B.C.; Mohamed, T.M.A.A.; Ma, Z.; Mathur, A.; et al. Miniaturized iPS-Cell-Derived Cardiac Muscles for Physiologically Relevant Drug Response Analyses. Sci. Rep. 2016, 6, 24726. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Q.; Liu, H.; Yang, H.; Yun, J.X.; Eisenberg, C.; Borg, T.K.; Xu, M.; Gao, B.Z. Laser-patterned stem-cell bridges in a cardiac muscle model for on-chip electrical conductivity analyses. Lab Chip 2012, 12, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, J.; Loskill, P.; Huebsch, N.; Koo, S.; Svedlund, F.L.; Marks, N.C.; Hua, E.W.; Grigoropoulos, C.P.; Conklin, B.R.; et al. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 2015, 6, 7413. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human Cardiac Organoids for the Modelling of Myocardial Infarction and Drug Cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.G.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; et al. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tomecka, E.; Zukowski, K.; Jastrzebska, E.; Chudy Michałand Brzozka, Z.; Chudy, M.; Brzozka, Z. Microsystem with micropillar array for three- (gel-embaded) and two-dimensional cardiac cell culture. Sens. Actuators B Chem. 2018, 254, 973–983. [Google Scholar] [CrossRef]
- Bergström, G.; Christoffersson, J.; Schwanke, K.; Zweigerdt, R.; Mandenius, C.F. Stem cell derived in vivo-like human cardiac bodies in a microfluidic device for toxicity testing by beating frequency imaging. Lab Chip 2015, 15, 3242–3249. [Google Scholar] [CrossRef]
- Colosi, C.; Costantini, M.; Barbetta, A.; Dentini, M. Microfluidic bioprinting of heterogeneous 3d tissue constructs. Methods Mol. Biol. 2017, 1612, 369–380. [Google Scholar]
- Aung, A.; Bhullar, I.S.; Theprungsirikul, J.; Davey, S.K.; Lim, H.L.; Chiu, Y.-J.J.; Ma, X.; Dewan, S.; Lo, Y.-H.H.; McCulloch, A.; et al. 3D cardiac μtissues within a microfluidic device with real-time contractile stress readout. Lab Chip 2016, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.R.; Chung, S.; Kamm, R.D. Differentiation of embryonic stem cells into cardiomyocytes in a compliant microfluidic system. Ann. Biomed. Eng. 2011, 39, 1840–1847. [Google Scholar] [CrossRef]
- Giridharan, G.A.; Nguyen, M.D.; Estrada, R.; Parichehreh, V.; Hamid, T.; Ismahil, M.A.; Prabhu, S.D.; Sethu, P. Microfluidic Cardiac Cell Culture Model. Anal. Chem. 2010, 82, 7581–7587. [Google Scholar] [CrossRef]
- Sebastião, M.J.; Gomes-Alves, P.; Reis, I.; Sanchez, B.; Palacios, I.; Serra, M.; Alves, P.M. Bioreactor-based 3D human myocardial ischemia/reperfusion in vitro model: A novel tool to unveil key paracrine factors upon acute myocardial infarction. Transl. Res. 2020, 215, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Vunjak-Novakovic, G. Human Tissue-Engineered Model of Myocardial Ischemia-Reperfusion Injury. Tissue Eng. Part A 2019, 25, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.N.; Epshtein, M.; Korin, N. Endothelial cell activation in an embolic ischemia-reperfusion injury microfluidic model. Micromachines 2019, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Marsano, A.; Conficconi, C.; Lemme, M.; Occhetta, P.; Gaudiello, E.; Votta, E.; Cerino, G.; Redaelli, A.; Rasponi, M. Beating heart on a chip: A novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 2016, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, G.S.; Rasponi, M.; Pavesi, A.; Santoro, R.; Kamm, R.; Fiore, G.B.; Pesce, M.; Soncini, M. On-chip assessment of human primary cardiac fibroblasts proliferative responses to uniaxial cyclic mechanical strain. Biotechnol. Bioeng. 2016, 113, 859–869. [Google Scholar] [CrossRef]
- Castaño, A.G.; Hortigüela, V.; Lagunas, A.; Cortina, C.; Montserrat, N.; Samitier, J.; Martínez, E. Protein patterning on hydrogels by direct microcontact printing: Application to cardiac differentiation. RSC Adv. 2014, 4, 29120. [Google Scholar] [CrossRef]
- Sakamiya, M.; Fang, Y.; Mo, X.; Shen, J.; Zhang, T. A heart-on-a-chip platform for online monitoring of contractile behavior via digital image processing and piezoelectric sensing technique. Med. Eng. Phys. 2020, 75, 36–44. [Google Scholar] [CrossRef]
- Liu, J.; Miller, K.; Ma, X.; Dewan, S.; Lawrence, N.; Whang, G.; Chung, P.; McCulloch, A.D.; Chen, S. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials 2020, 256, 120204. [Google Scholar] [CrossRef]
- Tandon, N.; Marsano, A.; Maidhof, R.; Numata, K.; Montouri-Sorrentino, C.; Cannizzaro, C.; Voldman, J.; Vunjak-Novakovic, G. Surface-patterned electrode bioreactor for electrical stimulation. Lab Chip 2010, 10, 692. [Google Scholar] [CrossRef]
- Alassaf, A.; Tansik, G.; Mayo, V.; Wubker, L.; Carbonero, D.; Agarwal, A. Engineering anisotropic cardiac monolayers on microelectrode arrays for non-invasive analyses of electrophysiological properties. Analyst 2020, 145, 139–149. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Shao, C.; Sun, L.; Sun, L.; Zhao, Y. Graphene Hybrid Anisotropic Structural Color Film for Cardiomyocytes’ Monitoring. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Fang, J.; Koh, J.; Fang, Q.; Qiu, H.; Archang, M.M.; Hasani-Sadrabadi, M.M.; Miwa, H.; Zhong, X.; Sievers, R.; Gao, D.W.; et al. Injectable Drug-Releasing Microporous Annealed Particle Scaffolds for Treating Myocardial Infarction. Adv. Funct. Mater. 2020, 30, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Sun, L.; Zhao, C.; Qiu, Z.; Zhao, Y.; Jin, W. Protein microcapsules integrated hierarchical scaffolds for local treatment of acute myocardial infarction model. Appl. Mater. Today 2021, 22, 100901. [Google Scholar] [CrossRef]
- Dinh, N.D.; Kukumberg, M.; Nguyen, A.T.; Keramati, H.; Guo, S.; Phan, D.T.; Ja’afar, N.B.; Birgersson, E.; Leo, H.L.; Huang, R.Y.J.; et al. Functional reservoir microcapsules generated: Via microfluidic fabrication for long-term cardiovascular therapeutics. Lab Chip 2020, 20, 2756–2764. [Google Scholar] [CrossRef]
- Kamei, K.; Kato, Y.; Hirai, Y.; Ito, S.; Satoh, J.; Oka, A.; Tsuchiya, T.; Chen, Y.; Tabata, O. Integrated heart/cancer on a chip to reproduce the side effects of anti-cancer drugs in vitro. RSC Adv. 2017, 7, 36777–36786. [Google Scholar] [CrossRef]
- Liu, H.; Bolonduro, O.A.; Hu, N.; Ju, J.; Rao, A.A.; Duffy, B.M.; Huang, Z.; Black, L.D.; Timko, B.P. Heart-on-a-Chip Model with Integrated Extra- And Intracellular Bioelectronics for Monitoring Cardiac Electrophysiology under Acute Hypoxia. Nano Lett. 2020, 20, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Gao, Y.Y.; Zhang, L.; Shi, S.; Gao, Y.Y. A Novel High-Sensitivity Cardiac Multibiomarker Detection System Based on Microfluidic Chip and GMR Sensors. IEEE Trans. Magn. 2015, 51, 18–21. [Google Scholar] [CrossRef]
- Murata, K.; Glaser, L.; Nardiello, M.; Ramanathan, L.V.; Carlow, D.C. Data from the analytical performance of the Abaxis Piccolo Xpress point of care analyzer in whole blood, serum, and plasma. Data Brief 2018, 16, 81–89. [Google Scholar] [CrossRef]
- Li, F.; Guo, L.; Hu, Y.; Li, Z.; Liu, J.; He, J.; Cui, H. Multiplexed chemiluminescence determination of three acute myocardial infarction biomarkers based on microfluidic paper-based immunodevice dual amplified by multifunctionalized gold nanoparticles. Talanta 2020, 207, 120346. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Y.; Thevarajah, T.M.; Goh, B.T.; Khor, S.M. Paper microfluidic device for early diagnosis and prognosis of acute myocardial infarction via quantitative multiplex cardiac biomarker detection. Biosens. Bioelectron. 2019, 128, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-chip platforms for detection of cardiovascular disease and cancer biomarkers. Sensors 2017, 17, 2934. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Salazar, J.R.; Cruz, K.R.; Vásques, E.M.M.; de Oliveira, O.N. Microfluidic point-of-care devices: New trends and future prospects for ehealth diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 1–14. [Google Scholar] [CrossRef]


| Natural Polymers | |
|---|---|
| Chitosan | Hyaluronic acid |
 |  |
| Alginate | Fibrin |
 |  |
| Synthetic Polymers | |
| Poly(glycolic acid) | Poly(ε-caprolactone) |
 |  |
| Poly(N-isopropylacrylamide) | Poly(ethylene glycol) |
 | |
 | Poly(lactic acid) |
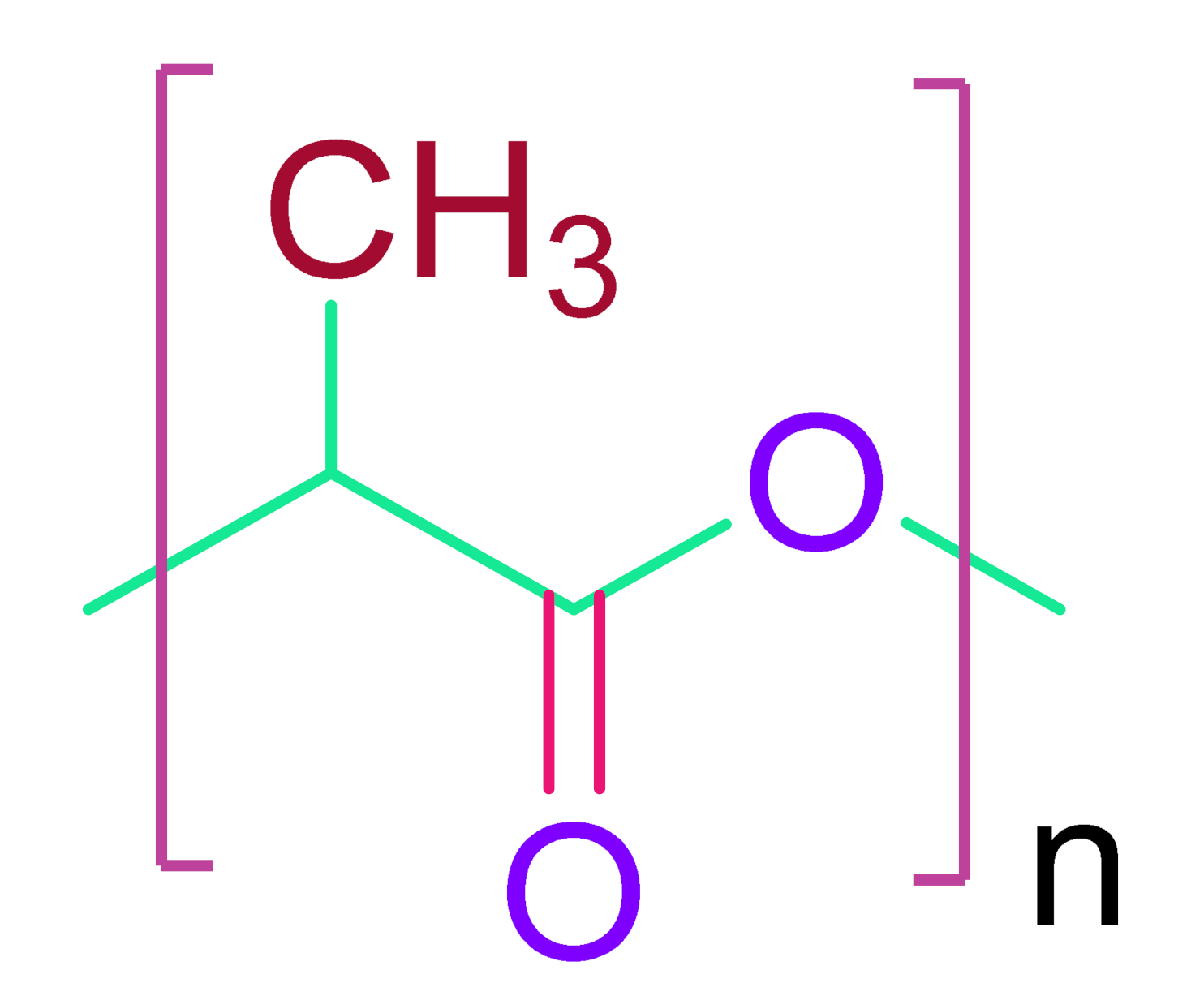 | |
Gelatin methacryloyl | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, V.; Dash, S.K.; Govarthanan, K.; Gahtori, R.; Negi, N.; Barani, M.; Tomar, R.; Chakraborty, S.; Mathapati, S.; Bishi, D.K.; et al. Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction. Cells 2021, 10, 2538. https://doi.org/10.3390/cells10102538
Sharma V, Dash SK, Govarthanan K, Gahtori R, Negi N, Barani M, Tomar R, Chakraborty S, Mathapati S, Bishi DK, et al. Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction. Cells. 2021; 10(10):2538. https://doi.org/10.3390/cells10102538
Chicago/Turabian StyleSharma, Vineeta, Sanat Kumar Dash, Kavitha Govarthanan, Rekha Gahtori, Nidhi Negi, Mahmood Barani, Richa Tomar, Sudip Chakraborty, Santosh Mathapati, Dillip Kumar Bishi, and et al. 2021. "Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction" Cells 10, no. 10: 2538. https://doi.org/10.3390/cells10102538
APA StyleSharma, V., Dash, S. K., Govarthanan, K., Gahtori, R., Negi, N., Barani, M., Tomar, R., Chakraborty, S., Mathapati, S., Bishi, D. K., Negi, P., Dua, K., Singh, S. K., Gundamaraju, R., Dey, A., Ruokolainen, J., Thakur, V. K., Kesari, K. K., Jha, N. K., ... Ojha, S. (2021). Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction. Cells, 10(10), 2538. https://doi.org/10.3390/cells10102538















