Putting Cells in Motion: Advantages of Endogenous Boosting of BDNF Production
Abstract
1. Introduction
2. BDNF: Gene Structure and Protein Localization
3. BDNF in Health and Disease
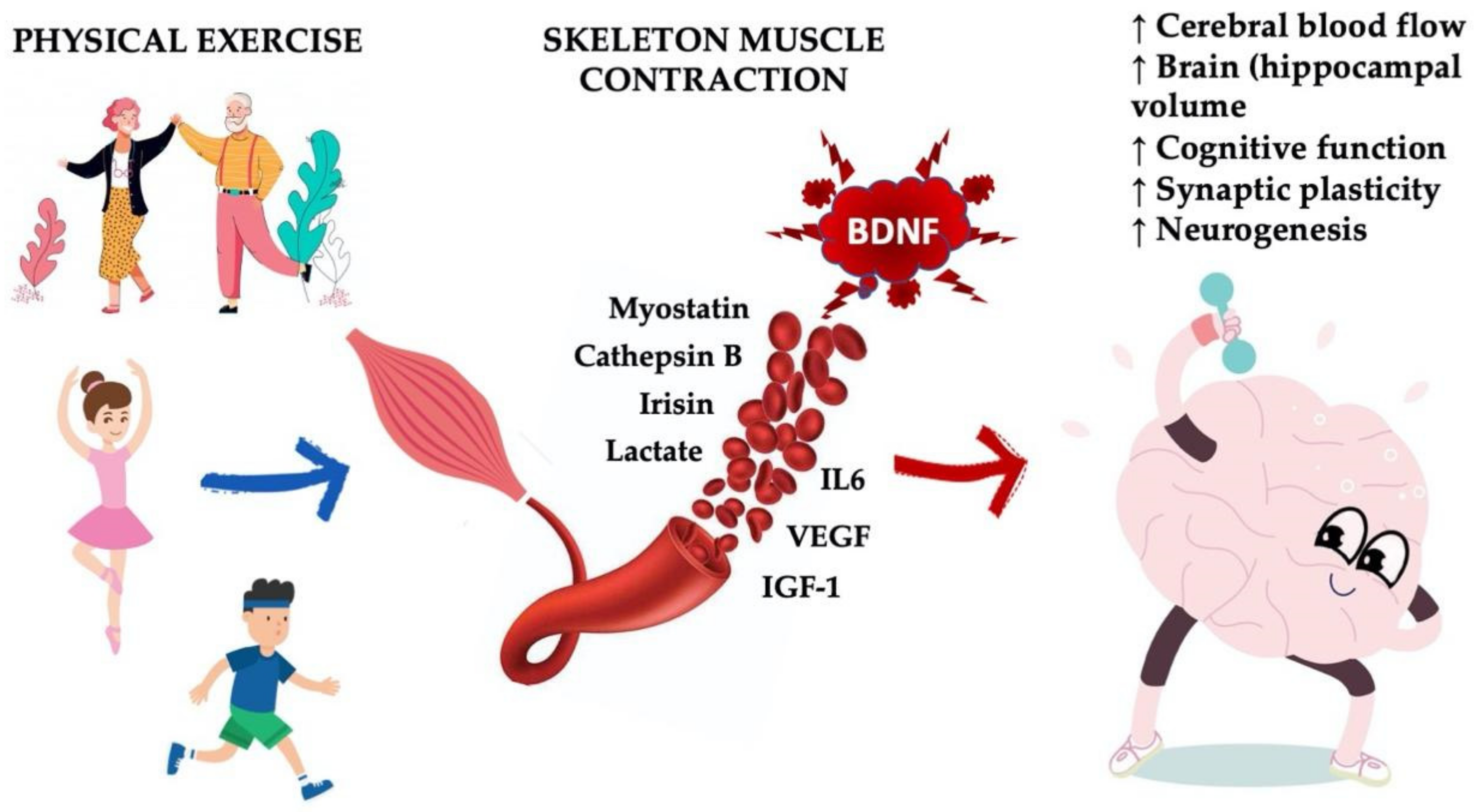
4. Boosting Endogenous BDNF: Sport
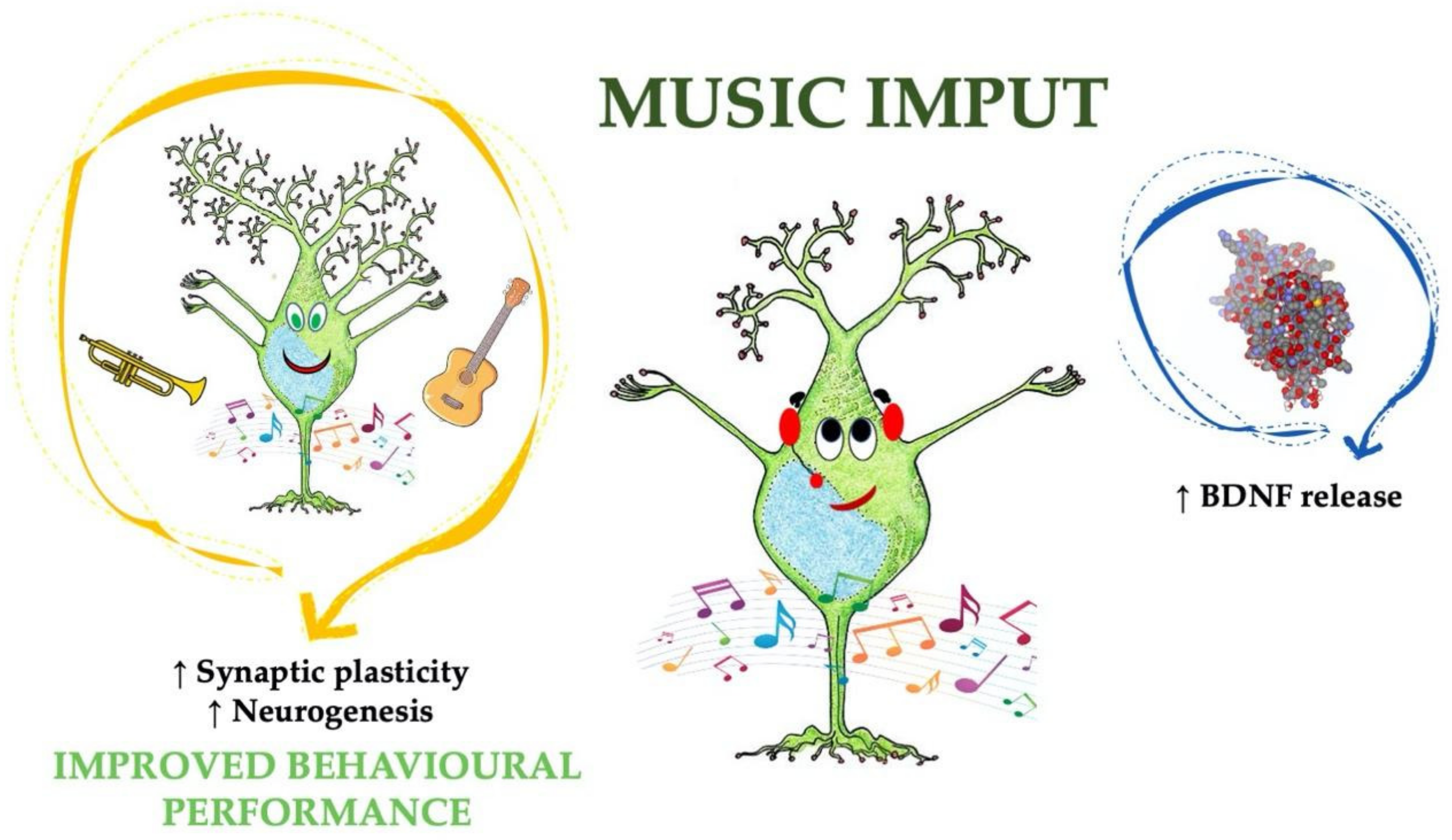
5. Boosting Endogenous BDNF: Music
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda:, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2018, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Figurov, A.; Lu, B. Recent progress in studies of neurotrophic factors and their clinical implications. J. Mol. Med. (Berl.) 1997, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Matrone, C.; Brattico, E. The power of music on Alzheimer’s Disease and the need to understand the underlying molecular mechanisms. J. Alzheimers Dis. Parkinsonism 2015, 5, 3. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Neeper, S.A.; Gómez-Pinilla, F.; Choi, J.; Cotman, C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996, 726, 49–56. [Google Scholar] [CrossRef]
- Venezia, A.C.; Quinlan, E.; Roth, S.M. A single bout of exercise increases hippocampal Bdnf: Influence of chronic exercise and noradrenaline. Genes Brain Behav. 2017, 16, 800–811. [Google Scholar] [CrossRef]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 1985, 121, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Oztasyonar, Y. Interaction between different sports branches such as taekwondo, box, athletes and serum brain derived neurotrophic factor (BDNF) levels. J. Sports Med. Phys. Fit. 2017, 57, 457–460. [Google Scholar]
- Chaban, V. Music and brain plasticity: How sounds trigger neurogenerative adaptations. In Neuroplasticity—Insights of Neural Reorganization; Reybrouck, M., Vuust, P., Brattico, E., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Alluri, V.; Toiviainen, P.; Burunat, I.; Kliuchko, M.; Vuust, P.; Brattico, E. Connectivity patterns during music listening: Evidence for action-based processing in musicians. Hum. Brain Mapp. 2017, 38, 2955–2970. [Google Scholar] [CrossRef] [PubMed]
- Palomar-García, M.-Á.; Zatorre, R.J.; Ventura-Campos, N.; Bueichekú, E.; Ávila, C. Modulation of functional connectivity in auditory–motor networks in musicians compared with nonmusicians. Cereb. Cortex 2017, 27, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Guo, Z.-W.; Lai, Y.-X.; Liao, W.; Liu, Q.; Kendrick, K.M.; Yao, D.; Li, H. Musical training induces functional plasticity in perceptual and motor networks: Insights from resting-state fMRI. PLoS ONE 2012, 7, e36568. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Rowe, J.B. Feeling the beat: Premotor and striatal interactions in musicians and nonmusicians during beat perception. J. Neurosci. 2009, 29, 7540–7548. [Google Scholar] [CrossRef] [PubMed]
- Burunat, I.; Brattico, E.; Puoliväli, T.; Ristaniemi, T.; Sams, M.; Toiviainen, P. Action in perception: Prominent visuo-motor functional symmetry in musicians during music listening. PLoS ONE 2015, 10, e0138238. [Google Scholar] [CrossRef]
- Moore, E.; Schaefer, R.S.; Bastin, M.E.; Roberts, N.; Overy, K. Can musical training influence brain connectivity? evidence from diffusion tensor MRI. Brain Sci. 2014, 4, 405–427. [Google Scholar] [CrossRef]
- Schlaug, G. Musicians and music making as a model for the study of brain plasticity. Prog. Brain Res. 2015, 217, 37–55. [Google Scholar] [CrossRef]
- Krampe, R.T.; Ericsson, K.A. Maintaining excellence: Deliberate practice and elite performance in young and older pianists. J. Exp. Psychol. Gen. 1996, 125, 331–359. [Google Scholar] [CrossRef]
- Hyde, K.L.; Lerch, J.; Norton, A.; Forgeard, M.; Winner, E.; Evans, A.C.; Schlaug, G. Musical training shapes structural brain development. J. Neurosci. 2009, 29, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.C.; Glerean, E.; Gold, B.P.; Sheng, D.; Sams, M.; Vuust, P.; Rauschecker, J.P.; Brattico, E. Inter-subject similarity of brain activity in expert musicians after multimodal learning: A behavioral and neuroimaging study on learning to play a piano sonata. Neuroscience 2020, 441, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Zentner, M.; Eerola, T. Rhythmic engagement with music in infancy. Proc. Natl. Acad. Sci. USA 2010, 107, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Iversen, J.R.; Bregman, M.R.; Schulz, I. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Curr. Biol. 2009, 19, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Lappe, C.; Herholz, S.C.; Trainor, L.J.; Pantev, C. Cortical plasticity induced by short-term unimodal and multimodal musical training. J. Neurosci. 2008, 28, 9632–9639. [Google Scholar] [CrossRef]
- Archakov, D.; DeWitt, I.; Kuśmierek, P.; Ortiz-Rios, M.; Cameron, D.; Cui, D.; Morin, E.L.; VanMeter, J.W.; Sams, M.; Jääskeläinen, I.P.; et al. Auditory representation of learned sound sequences in motor regions of the macaque brain. Proc. Natl. Acad. Sci. USA 2020, 117, 15242–15252. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Arevalo, J.C.; Wu, S.H. Neurotrophin signaling: Many exciting surprises! Cell. Mol. Life Sci. 2006, 63, 1523–1537. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Gabriele, B.; Enrico, T. BDNF splice variants from the second promoter cluster support cell survival of differentiated neuroblastoma upon cytotoxic stress. J. Cell Sci. 2009, 122, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and ratBDNF gene structure and expression revisited. J. Neurosci. Res. 2007, 85, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Evicario, A.; Ecolliva, A.; Eratti, A.; Edavidovic, L.; Ebaj, G.; Egricman, L.; Ecolombrita, C.; Epallavicini, A.; Jones, K.R.; Ebardoni, B.; et al. Dendritic targeting of short and long 3′ UTR BDNF mRNA is regulated by BDNF or NT-3 and distinct sets of RNA-binding proteins. Front. Mol. Neurosci. 2015, 8, 62. [Google Scholar] [CrossRef]
- Ma, B.; Culver, B.P.; Baj, G.; Tongiorgi, E.; Chao, M.V.; Tanese, N. Localization of BDNF mRNA with the Huntington’s disease protein in rat brain. Mol. Neurodegener. 2010, 5, 22. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Mallei, A.; Baj, G.; Ieraci, A.; Corna, S.; Musazzi, L.; Lee, F.S.; Tongiorgi, E.; Popoli, M. Expression and dendritic trafficking of bdnf-6 splice variant are impaired in knock-in mice carrying human BDNF Val66Met polymorphism. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef]
- Karpova, N.N. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology 2014, 76 Pt C, 709–718. [Google Scholar] [CrossRef]
- Roth, T.L.; Zoladz, P.R.; Sweatt, J.D.; Diamond, D.M. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J. Psychiatr. Res. 2011, 45, 919–926. [Google Scholar] [CrossRef]
- Thompson Ray, M.; Weickert, C.S.; Wyatt, E.; Webster, M.J. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011, 36, 195–203. [Google Scholar] [CrossRef]
- Dunham, J.S.; Deakin, J.F.W.; Miyajima, F.; Payton, T.; Toro, C.T. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J. Psychiatr. Res. 2009, 43, 1175–1184. [Google Scholar] [CrossRef]
- Guilloux, J.P.; Douillard-Guilloux, G.; Kota, R.; Wang, X.; Gardier, A.M.; Martinowich, K.; Tseng, G.C.; Lewis, D.A.; Sibille, E. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 2012, 17, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Sobreviela, T.; Pagcatipunan, M.; Kroin, J.S.; Mufson, E.J. Retrograde transport of brain-derived neurotrophic factor (BDNF) following infusion in neo- and limbic cortex in rat: Relationship to BDNF mRNA expressing neurons. J. Comp. Neurol. 1996, 375, 417–444. [Google Scholar] [CrossRef]
- Tang, S.; Machaalani, R.; Waters, K.A. Immunolocalization of pro- and mature-brain derived neurotrophic factor (BDNF) and receptor TrkB in the human brainstem and hippocampus. Brain Res. 2010, 1354, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A.; Cai, N.; Bliven, T.; Juhasz, M.; Conner, J.M.; Acheson, A.L.; Lindsay, R.M.; Wiegand, S.J. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997, 389, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Tongiorgi, E. Activity-dependent expression of brain-derived neurotrophic factor in dendrites: Facts and open questions. Neurosci. Res. 2008, 61, 335–346. [Google Scholar] [CrossRef]
- Tongiorgi, E.; Baj, G. Functions and mechanisms of BDNF mRNA trafficking. Novartis Found. Symp. 2008, 289, 136–147. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef]
- Ghassabian, A.; Sundaram, R.; Chahal, N.; McLain, A.; Bell, E.; Lawrence, D.A.; Yeung, E.H. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev. Psychopathol. 2017, 29, 1499–1511. [Google Scholar] [CrossRef]
- Hempstead, B.L. Deciphering proneurotrophin actions. Handb. Exp. Pharmacol. 2014, 220, 17–32. [Google Scholar] [CrossRef]
- Je, H.S.; Yang, F.; Ji, Y.; Nagappan, G.; Hempstead, B.L.; Lu, B. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc. Natl. Acad. Sci. USA 2012, 109, 15924–15929. [Google Scholar] [CrossRef] [PubMed]
- Orefice, L.L.; Shih, C.-C.; Xu, H.; Waterhouse, E.G.; Xu, B. Control of spine maturation and pruning through proBDNF synthesized and released in dendrites. Mol. Cell. Neurosci. 2016, 71, 66–79. [Google Scholar] [CrossRef][Green Version]
- Taylor, A.R.; Gifondorwa, D.J.; Robinson, M.B.; Strupe, J.L.; Prevette, D.; Johnson, J.E.; Hempstead, B.; Oppenheim, R.W.; Milligan, C.E. Motoneuron programmed cell death in response to proBDNF. Dev. Neurobiol. 2012, 72, 699–712. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef]
- Nagappan, G.; Lu, B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005, 28, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Calissano, P.; Matrone, C.; Amadoro, G. Apoptosis and in vitro Alzheimer’s disease neuronal models. Commun. Integr. Biol. 2009, 2, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Calissano, P.; Amadoro, G.; Matrone, C.; Ciafrè, S.; Marolda, R.; Corsetti, V.; Ciotti, M.T.; Mercanti, D.; Di Luzio, A.; Severini, C.; et al. Does the term ‘trophic’ actually mean anti-amyloidogenic? The case of NGF. Cell Death Differ. 2010, 17, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.N.; Wu, Y.W.; Bammler, T.K.; Comstock, B.; Mathur, A.; McKinstry, R.C.; Chang, T.; Mayock, D.E.; Mulkey, S.B.; Van Meurs, K.; et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 2018, 194, 67–75.e61. [Google Scholar] [CrossRef]
- Rodrigues-Amorim, D.; Rivera-Baltanás, T.; Bessa, J.; Sousa, N.; Vallejo-Curto, M.D.C.; Rodríguez-Jamardo, C.; de Las Heras, M.E.; Díaz, R.; Agís-Balboa, R.C.; Olivares, J.M.; et al. The neurobiological hypothesis of neurotrophins in the pathophysiology of schizophrenia: A meta-analysis. J. Psychiatr. Res. 2018, 106, 43–53. [Google Scholar] [CrossRef]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. NY Acad. Sci. 2008, 1144, 97–112. [Google Scholar] [CrossRef]
- Murray, P.S.; Holmes, P.V. An Overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. Int. J. Pept. 2011, 2011, 654085. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Goodman, J.; MacLeod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Pencea, V.; Bingaman, K.D.; Wiegand, S.J.; Luskin, M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001, 21, 6706–6717. [Google Scholar] [CrossRef]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Connor, B.; Young, D.; Yan, Q.; Faull, R.; Synek, B.; Dragunow, M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res. Mol. Brain Res. 1997, 49, 71–81. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.; Tam, W.; Kua, E.H.; Ho, R.C. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s Disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, S.H.; Graham, P.L.; Angelucci, F.; Lucia, A.; Pareja-Galeano, H.; Leyhe, T.; Turana, Y.; Lee, I.R.; Yoon, J.H.; et al. Peripheral brain-derived neurotrophic factor levels in Alzheimer’s Disease and mild cognitive impairment: A comprehensive systematic review and meta-analysis. Mol. Neurobiol. 2016, 54, 7297–7311. [Google Scholar] [CrossRef]
- Chao, M.V.; Rajagopal, R.; Lee, F.S. Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 2006, 110, 167–173. [Google Scholar] [CrossRef]
- Mariga, A.; Zavadil, J.; Ginsberg, S.D.; Chao, M.V. Withdrawal of BDNF from hippocampal cultures leads to changes in genes involved in synaptic function. Dev. Neurobiol. 2015, 75, 173–192. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog. Neurobiol. 2007, 81, 294–330. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Croll, S.D.; Gall, C.M.; Scharfman, H.E. BDNF and epilepsy: Too much of a good thing? Trends Neurosci. 2001, 24, 47–53. [Google Scholar] [CrossRef]
- Peng, Q.; Masuda, N.; Jiang, M.; Li, Q.; Zhao, M.; Ross, C.A.; Duan, W. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington’s disease mouse model. Exp. Neurol. 2008, 210, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Braschi, C.; Capsoni, S.; Narducci, R.; Poli, A.; Sansevero, G.; Brandi, R.; Maffei, L.; Cattaneo, A.; Berardi, N. Intranasal delivery of BDNF rescues memory deficits in AD11 mice and reduces brain microgliosis. Aging Clin. Exp. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; De Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Nikokalam Nazif, N.; Khosravi, M.; Ahmadi, R.; Bananej, M.; Majd, A. Effect of treadmill exercise on catalepsy and the expression of the BDNF gene in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine -induced Parkinson in male. Iran. J. Basic Med. Sci. 2020, 23, 483–493. [Google Scholar] [CrossRef]
- Schneider, A.; Sari, A.T.; Alhaddad, H.; Sari, Y. Overview of therapeutic drugs and methods for the treatment of Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2020, 19, 195–206. [Google Scholar] [CrossRef]
- Miranda-Lourenço, C.; Ribeiro-Rodrigues, L.; Fonseca-Gomes, J.; Tanqueiro, S.R.; Belo, R.F.; Ferreira, C.B.; Rei, N.; Ferreira-Manso, M.; De Almeida-Borlido, C.; Costa-Coelho, T.; et al. Challenges of BDNF-based therapies: From common to rare diseases. Pharmacol. Res. 2020, 162, 105281. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Feng, Z.; Feng, M. BDNF-overexpressing human umbilical cord mesenchymal stem cell-derived motor neurons improve motor function and prolong survival in amyotrophic lateral sclerosis mice. Neurol. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Pawlukowska, W.; Baumert, B.; Gołąb-Janowska, M.; Pius-Sadowska, E.; Litwińska, Z.; Kotowski, M.; Meller, A.; Rotter, I.; Peregud-Pogorzelski, J.; Nowacki, P. Articulation recovery in ALS patients after lineage-negative adjuvant cell therapy—preliminary report. Int. J. Med Sci. 2020, 17, 1927–1935. [Google Scholar] [CrossRef]
- Eyileten, C.; Sharif, L.; Wicik, Z.; Jakubik, D.; Jarosz-Popek, J.; Soplinska, A.; Postula, M.; Czlonkowska, A.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D. The Relation of the brain-derived neurotrophic factor with microRNAs in neurodegenerative diseases and ischemic stroke. Mol. Neurobiol. 2021, 58, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xiong, R.; Gong, X. Memantine, NMDA receptor antagonist, attenuates ox-LDL-induced inflammation and oxidative stress via activation of BDNF/TrkB signaling pathway in HUVECs. Inflammation 2020. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Singh, A.K.; Tiwari, V.; Thacker, A.K. Brain-derived neurotrophic factor levels in acute stroke and its clinical implications. Brain Circ. 2020, 6, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Nakamura, T.; Kamijo, Y.-I.; Arakawa, H.; Umemoto, Y.; Kinoshita, T.; Sakurai, Y.; Tajima, F. Increased serum levels of brain-derived neurotrophic factor following wheelchair half marathon race in individuals with spinal cord injury. J. Spinal Cord. Med. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marchionne, F.; Krupka, A.J.; Smith, G.M.; Lemay, M.A. Intrathecal delivery of BDNF into the lumbar cistern re-engages locomotor stepping after spinal cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2459–2467. [Google Scholar] [CrossRef]
- Xue, M.; Sun, Y.-L.; Xia, Y.-Y.; Huang, Z.-H.; Xing, G.-G.; Huang, C. Electroacupuncture modulates spinal BDNF/TrκB signaling pathway and ameliorates the sensitization of dorsal horn WDR neurons in spared nerve injury rats. Int. J. Mol. Sci. 2020, 21, 6524. [Google Scholar] [CrossRef]
- Goldhardt, M.G.; Andreia, A.; Dorneles, G.P.; Da Silva, I.R.; Pochmann, D.; Peres, A.; Elsner, V.R. Does a single bout of exercise impacts BDNF, oxidative stress and epigenetic markers in spinal cord injury patients? Funct. Neurol. 2019, 34, 158–166. [Google Scholar]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Zuccato, C.; Liber, D.; Ramos, C.; Tarditi, A.; Rigamonti, D.; Tartari, M.; Valenza, M.; Cattaneo, E. Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol. Res. 2005, 52, 133–139. [Google Scholar] [CrossRef]
- Narisawa-Saito, M.; Wakabayashi, K.; Tsuji, S.; Takahashi, H.; Nawa, H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimerʼs disease. NeuroReport 1996, 7, 2925–2928. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fukumoto, H.; Orne, J.; Klucken, J.; Raju, S.; Vanderburg, C.; Irizarry, M.C.; Hyman, B.T.; Ingelsson, M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005, 194, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef]
- Murer, M.G.; Boissiere, F.; Yan, Q.; Hunot, S.; Villares, J.; Faucheux, B.; Agid, Y.; Hirsch, E.; Raisman-Vozari, R. An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer’s disease. Neuroscience 1999, 88, 1015–1032. [Google Scholar] [CrossRef]
- Baquet, Z.C.; Bickford, P.C.; Jones, K.R. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J. Neurosci. 2005, 25, 6251–6259. [Google Scholar] [CrossRef] [PubMed]
- Parain, K.; Murer, M.G.; Yan, Q.; Faucheux, B.; Agid, Y.; Hirsch, E.; Raisman-Vozari, R. Reduced expression of brain-derived neurotrophic factor protein in Parkinsonʼs disease substantia nigra. NeuroReport 1999, 10, 557–561. [Google Scholar] [CrossRef]
- Anastasia, A.; Deinhardt, K.; Chao, M.V.; Will, N.E.; Irmady, K.; Lee, F.S.; Hempstead, B.L.; Bracken, C. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat. Commun. 2013, 4, 2490. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003, 23, 6690–6694. [Google Scholar] [CrossRef]
- Cagni, F.C.; Campêlo, C.L.D.C.; Coimbra, D.G.; Barbosa, M.R.; Júnior, L.G.O.; Neto, A.B.S.; Ribeiro, A.M.; Júnior, C.O.G.; Gomes de Andrade, T.; Silva, R.H. Association of BDNF Val66MET polymorphism with Parkinson’s disease and depression and anxiety symptoms. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 142–147. [Google Scholar] [CrossRef]
- Soliman, F.; Glatt, C.E.; Bath, K.G.; Levita, L.; Jones, R.M.; Pattwell, S.S.; Jing, D.; Tottenham, N.; Amso, D.; Somerville, L.H.; et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 2010, 327, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Su, X.; Pan, L.; Li, C. BDNF Val66Met polymorphism and cognitive impairment in Parkinson’s disease—a meta-analysis. Neurol. Sci. 2019, 40, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.L.; Ng, T.; Tan, M.; Tan, A.; Chan, A. Impact of brain-derived neurotrophic factor genetic polymorphism on cognition: A systematic review. Brain Behav. 2018, 8, e01009. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chang, H.; Xiao, X. BDNF Val66Met polymorphism and bipolar disorder in European populations: A risk association in case-control, family-based and GWAS studies. Neurosci. Biobehav. Rev. 2016, 68, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.; Underwood, M.D.; Huang, Y.-Y.; Hsiung, S.-C.; Liu, Y.; Simpson, N.R.; Bakalian, M.J.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int. J. Neuropsychopharmacol. 2018, 21, 528–538. [Google Scholar] [CrossRef]
- Fukumoto, N.; Fujii, T.; Combarros, O.; Kamboh, M.I.; Tsai, S.-J.; Matsushita, S.; Nacmias, B.; Comings, D.E.; Arboleda, H.; Ingelsson, M.; et al. Sexually dimorphic effect of the Val66Met polymorphism ofBDNFon susceptibility to Alzheimer’s disease: New data and meta-analysis. Am. J. Med Genet. B Neuropsychiatr. Genet. 2010, 153B, 235–242. [Google Scholar] [CrossRef]
- Kambeitz, J.P.; Bhattacharyya, S.; Kambeitz-Ilankovic, L.M.; Valli, I.; Collier, D.A.; McGuire, P. Effect of BDNF val66met polymorphism on declarative memory and its neural substrate: A meta-analysis. Neurosci. Biobehav. Rev. 2012, 36, 2165–2177. [Google Scholar] [CrossRef]
- Franzmeier, N.; Ren, J.; Damm, A.; Monté-Rubio, G.; Boada, M.; Ruiz, A.; Ramirez, A.; Jessen, F.; Düzel, E.; Rodriguez Gómez, O.; et al. The BDNFVal66Met SNP modulates the association between beta-amyloid and hippocampal disconnection in Alzheimer’s disease. Mol. Psychiatry 2019, 1–15. [Google Scholar] [CrossRef]
- Olin, D.; MacMurray, J.; Comings, D.E. Risk of late-onset Alzheimer’s disease associated with BDNF C270T polymorphism. Neurosci. Lett. 2005, 381, 275–278. [Google Scholar] [CrossRef]
- Metzger, S.; Bauer, P.; Tomiuk, J.; Laccone, F.; DiDonato, S.; Gellera, C.; Mariotti, C.; Lange, H.W.; Weirich-Schwaiger, H.; Wenning, G.K.; et al. Genetic analysis of candidate genes modifying the age-at-onset in Huntington’s disease. Hum. Genet. 2006, 120, 285–292. [Google Scholar] [CrossRef]
- Wright, G.E.B.; Caron, N.S.; Ng, B.; Casal, L.; Casazza, W.; Xu, X.; Ooi, J.; Pouladi, M.A.; Mostafavi, S.; Ross, C.J.; et al. Gene expression profiles complement the analysis of genomic modifiers of the clinical onset of Huntington disease. Hum. Mol. Genet. 2020, 29, 2788–2802. [Google Scholar] [CrossRef] [PubMed]
- Alberch, J.; Lopez, M.; Badenas, C.; Carrasco, J.L.; Mila, M.; Munoz, E.; Canals, J.M. Association between BDNF Val66Met polymorphism and age at onset in Huntington disease. Neurol. 2005, 65, 964–965. [Google Scholar] [CrossRef]
- Mai, M.; Akkad, A.D.; Wieczorek, S.; Saft, C.; Andrich, J.; Kraus, P.H.; Epplen, J.T.; Arning, L. No association between polymorphisms in the BDNF gene and age at onset in Huntington disease. BMC Med Genet. 2006, 7, 79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kishikawa, S.; Li, J.-L.; Gillis, T.; Hakky, M.M.; Warby, S.; Hayden, M.R.; Macdonald, M.E.; Myers, R.H.; Gusella, J.F. Brain-derived neurotrophic factor does not influence age at neurologic onset of Huntington’s disease. Neurobiol. Dis. 2006, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Deflesselle, E.; Colle, R.; Rigal, L.; David, D.J.; Vievard, A.; Martin, S.; Becquemont, L.; Verstuyft, C.; Corruble, E. The TRKB rs2289656 genetic polymorphism is associated with acute suicide attempts in depressed patients: A transversal case control study. PLoS ONE 2018, 13, e0205648. [Google Scholar] [CrossRef]
- Green, C.R.; Corsi-Travali, S.; Neumeister, A. The role of BDNF-TrkB signaling in the pathogenesis of PTSD. J. Depress. Anxiety 2013, 2013, 006. [Google Scholar] [CrossRef]
- Shi, S.-S.; Shao, S.-H.; Yuan, B.-P.; Pan, F.; Li, Z.-L. Acute stress and chronic stress change brain-derived neurotrophic factor (BDNF) and tyrosine kinase-coupled receptor (TrkB) expression in both young and aged rat hippocampus. Yonsei Med J. 2010, 51, 661–671. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Radecki, D.T.; Brown, L.M.; Martinez, J.; Teyler, T. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus 2005, 15, 246–253. [Google Scholar] [CrossRef]
- Houlton, J.; Abumaria, N.; Hinkley, S.F.R.; Clarkson, A.N. Therapeutic potential of neurotrophins for repair after brain injury: A helping hand from biomaterials. Front. Neurosci. 2019, 13, 790. [Google Scholar] [CrossRef]
- Alcalá-Barraza, S.R.; Lee, M.S.; Hanson, L.R.; McDonald, A.A.; Frey, W.H., II; McLoon, L.K. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J. Drug Target. 2010, 18, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Sansevero, G.; Baroncelli, L.; Scali, M.; Sale, A. Intranasal BDNF administration promotes visual function recovery in adult amblyopic rats. Neuropharmacology 2019, 145, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Toccaceli, G.; Barbagallo, G.M.V.; Peschillo, S. Low-intensity focused ultrasound for the treatment of brain diseases: Safety and feasibility. Theranostics 2019, 9, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, G.Z.X.; Getachew, H.; Acosta, C.; Sierra Sánchez, C.; Konofagou, E.E. Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor. Sci. Rep. 2016, 6, 28599. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Smith, L.M.; Niimi, Y.; Karakatsani, M.E.M.; Murillo, M.F.; Jackson-Lewis, V.; Przedborski, S.; Konofagou, E.E. Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model. Sci. Rep. 2019, 9, 19402. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- Fukuchi, M.; Okuno, Y.; Nakayama, H.; Nakano, A.; Mori, H.; Mitazaki, S.; Nakano, Y.; Toume, K.; Jo, M.; Takasaki, I.; et al. Screening inducers of neuronal BDNF gene transcription using primary cortical cell cultures from BDNF-luciferase transgenic mice. Sci. Rep. 2019, 9, 11833. [Google Scholar] [CrossRef]
- Simmons, D.A.; Belichenko, N.P.; Yang, T.; Condon, C.; Monbureau, M.; Shamloo, M.; Jing, D.; Massa, S.M.; Longo, F.M. A small molecule TrkB ligand reduces motor impairment and neuropathology in R6/2 and BACHD mouse models of Huntington’s disease. J. Neurosci. 2013, 33, 18712–18727. [Google Scholar] [CrossRef]
- Jang, S.-W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.S.; Chao, M.V. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc. Natl. Acad. Sci. USA 2001, 98, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Diógenes, M.J.; Fernandes, C.C.; Sebastião, A.M.; Ribeiro, J.A. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J. Neurosci. 2004, 24, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Ekblom, Ö.; Ekblom, M.; Lebedev, A.; Tarassova, O.; Moberg, M.; Lövdén, M. Acute increases in brain-derived neurotrophic factor in plasma following physical exercise relates to subsequent learning in older adults. Sci. Rep. 2020, 10, 4395. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chang, Y.; Gao, X.L.; Li, H.; Zhao, P. Dynamic Expression and the Role of BDNF in exercise-induced skeletal muscle regeneration. Int. J. Sports. Med. 2017, 38, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xia, Y.; Kendrick, K.; Liu, X.; Wang, M.; Wu, D.; Yang, H.; Jing, W.; Guo, D.; Yao, D. Mozart, Mozart rhythm and retrograde Mozart effects: Evidences from behaviours and neurobiology bases. Sci. Rep. 2016, 6, 18744. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Fiore, M.; Ricci, E.; Padua, L.; Sabino, A.; Tonali, P.A. Investigating the neurobiology of music: Brain-derived neurotrophic factor modulation in the hippocampus of young adult mice. Behav. Pharmacol. 2007, 18, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Ricci, E.; Padua, L.; Sabino, A.; Tonali, P.A. Music exposure differentially alters the levels of brain-derived neurotrophic factor and nerve growth factor in the mouse hypothalamus. Neurosci. Lett. 2007, 429, 152–155. [Google Scholar] [CrossRef]
- Chikahisa, S.; Sei, H.; Morishima, M.; Sano, A.; Kitaoka, K.; Nakaya, Y.; Morita, Y. Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav. Brain Res. 2006, 169, 312–319. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, W.; Wang, Y.; Jing, W.; Gao, S.; Guo, D.; Xia, Y.; Yao, D. Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res. Bull. 2016, 121, 131–137. [Google Scholar] [CrossRef]
- Rauscher, F.H.; Shaw, G.L.; Ky, K.N. Music and spatial task performance. Nature 1993, 365, 611. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, F.; Robinson, D.; Jens, J. Improved maze learning through early music exposure in rats. Neurol. Res. 1998, 20, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Aoun, P.; Jones, T.; Shaw, G.L.; Bodner, M. Long-term enhancement of maze learning in mice via a generalized Mozart effect. Neurol. Res. 2005, 27, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Bodner, M.; Muftuler, L.T.; Nalcioglu, O.; Shaw, G.L. fMRI study relevant to the Mozart effect: Brain areas involved in spatial–temporal reasoning. Neurol. Res. 2001, 23, 683–690. [Google Scholar] [CrossRef]
- Lints, A.; Gadbois, S. Is Listening to Mozart the only way to enhance spatial reasoning? Percept. Mot. Ski. 2003, 97, 1163–1174. [Google Scholar] [CrossRef]
- Thompson, W.F.; Schellenberg, E.G.; Husain, G. Arousal, mood, and the Mozart effect. Psychol. Sci. 2001, 12, 248–251. [Google Scholar] [CrossRef]
- MacDonald, R.; Kreutz, G.; Mitchell, L. Cognitive performance after listening to music: A review of the Mozart effect. In Music, Health, and Wellbeing; Schellenberg, E.G., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 325–338. [Google Scholar] [CrossRef]
- Habibi, A.; Damasio, A.; Ilari, B.; Elliott Sachs, M.; Damasio, H. Music training and child development: A review of recent findings from a longitudinal study. Ann. N. Y. Acad. Sci. 2018, 1423, 73–81. [Google Scholar] [CrossRef]
- Sachs, M.; Kaplan, J.; Der Sarkissian, A.; Habibi, A. Increased engagement of the cognitive control network associated with music training in children during an fMRI Stroop task. PLoS ONE 2017, 12, e0187254. [Google Scholar] [CrossRef]
- Ilari, B.S.; Keller, P.; Damasio, H.; Habibi, A. The Development of musical skills of underprivileged children over the course of 1 year: A study in the context of an El Sistema-inspired program. Front. Psychol. 2016, 7, 62. [Google Scholar] [CrossRef]
- Saarikivi, K.A.; Putkinen, V.; Tervaniemi, M.; Huotilainen, M. Cognitive flexibility modulates maturation and music-training-related changes of neural sound discrimination. Eur. J. Neurosci. 2016, 44, 1815–1825. [Google Scholar] [CrossRef]
- Rauscher, F.; Shaw, G.; Levine, L.; Wright, E.; Dennis, W.; Newcomb, R. Music training causes long-term enhancement of preschool children’s spatial–temporal reasoning. Neurol. Res. 1997, 19, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Bonetti, L.; Särkämö, T.; Kliuchko, M.; Brattico, E. On the association between musical training, intelligence and executive functions in adulthood. Front. Psychol. 2019, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Wadhwa, S. Prenatal auditory stimulation alters the levels of CREB mRNA, p-CREB and BDNF expression in chick hippocampus. Int. J. Dev. Neurosci. 2009, 27, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kathpalia, P.; Nag, T.C.; Chattopadhyay, P.; Sharma, A.; Bhat, M.A.; Roy, T.S.; Wadhwa, S. In ovo sound stimulation mediated regulation of BDNF in the auditory cortex and hippocampus of neonatal chicks. Neuroscience 2019, 408, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Yu, H.; Yang, J.-M.; Gao, J.; Jiang, H.; Feng, M.; Zhao, Y.-X.; Chen, Z.-Y. Anxiolytic effect of music exposure on BDNFMet/Met transgenic mice. Brain Res. 2010, 1347, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liang, T.; Zhou, F.H.; Cao, Y.; Wang, C.; Wang, F.-Y.; Li, F.; Zhou, X.-F.; Zhang, J.-Y.; Li, C.-Q. Regular music exposure in juvenile rats facilitates conditioned fear extinction and reduces anxiety after foot shock in adulthood. BioMed Res. Int. 2019, 2019, 8740674. [Google Scholar] [CrossRef]
- Oikkonen, J.; Onkamo, P.; Järvelä, I.; Kanduri, C. Convergent evidence for the molecular basis of musical traits. Sci. Rep. 2016, 6, 39707. [Google Scholar] [CrossRef]
- Nair, P.S.; Raijas, P.; Ahvenainen, M.; Philips, A.K.; Ukkola-Vuoti, L.; Jarvela, I. Music-listening regulates human microRNA expression. Epigenetics 2020, 1–13. [Google Scholar] [CrossRef]
- Minutillo, A.; Panza, G.; Mauri, M.C. Musical practice and BDNF plasma levels as a potential marker of synaptic plasticity: An instrument of rehabilitative processes. Neurol. Sci. 2020. [Google Scholar] [CrossRef]
- Kanduri, C.; Kuusi, T.; Ahvenainen, M.; Philips, A.K.; Lähdesmäki, H.; Jarvela, I. The effect of music performance on the transcriptome of professional musicians. Sci. Rep. 2015, 5, 9506. [Google Scholar] [CrossRef]
- Bonetti, L.; Brattico, E.; Carlomagno, F.; Cabral, J.; Stevner, A.; Deco, G.; Whybrow, P.C.; Pearce, M.; Pantazis, D.; Vuust, P.; et al. Spatiotemporal brain dynamics during recognition of the music of Johann Sebastian Bach. bioRxiv 2020. [Google Scholar] [CrossRef]
| Sport | Music |
|---|---|
| Similarities | |
| Moving body | Moving limbs and fingers, and occasionally the whole body |
| Watching activates action observation areas in experts | Listening activates action areas in experts |
| Watching sport activates reward brain circuits | Listening to upbeat music causes the drive to move and hence activates motor and reward areas |
| Behavioural studies show cognitive, mood, and health benefits | Behavioural studies show cognitive, mood, and health benefits |
| Differences | |
| Sport activities require body mobility | Musical activities do not require body mobility |
| Sport activities require awareness | Musical activities do not require awareness and can be proposed even to vegetative patients |
| BDNF studies on animals and humans | BDNF studies only on animals |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brattico, E.; Bonetti, L.; Ferretti, G.; Vuust, P.; Matrone, C. Putting Cells in Motion: Advantages of Endogenous Boosting of BDNF Production. Cells 2021, 10, 183. https://doi.org/10.3390/cells10010183
Brattico E, Bonetti L, Ferretti G, Vuust P, Matrone C. Putting Cells in Motion: Advantages of Endogenous Boosting of BDNF Production. Cells. 2021; 10(1):183. https://doi.org/10.3390/cells10010183
Chicago/Turabian StyleBrattico, Elvira, Leonardo Bonetti, Gabriella Ferretti, Peter Vuust, and Carmela Matrone. 2021. "Putting Cells in Motion: Advantages of Endogenous Boosting of BDNF Production" Cells 10, no. 1: 183. https://doi.org/10.3390/cells10010183
APA StyleBrattico, E., Bonetti, L., Ferretti, G., Vuust, P., & Matrone, C. (2021). Putting Cells in Motion: Advantages of Endogenous Boosting of BDNF Production. Cells, 10(1), 183. https://doi.org/10.3390/cells10010183





