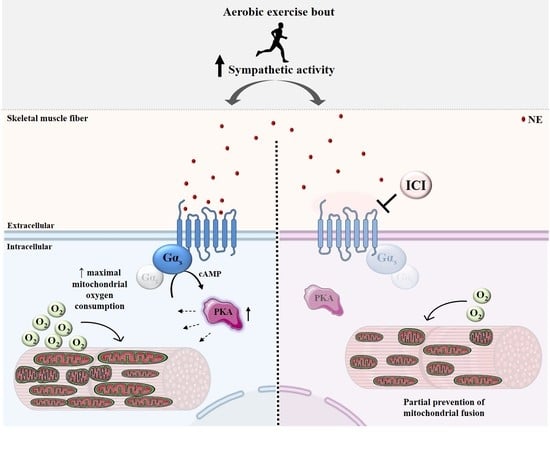
β2-Adrenergic Signaling Modulates Mitochondrial Function and Morphology in Skeletal Muscle in Response to Aerobic Exercise
Abstract
1. Introduction
2. Materials and Methods
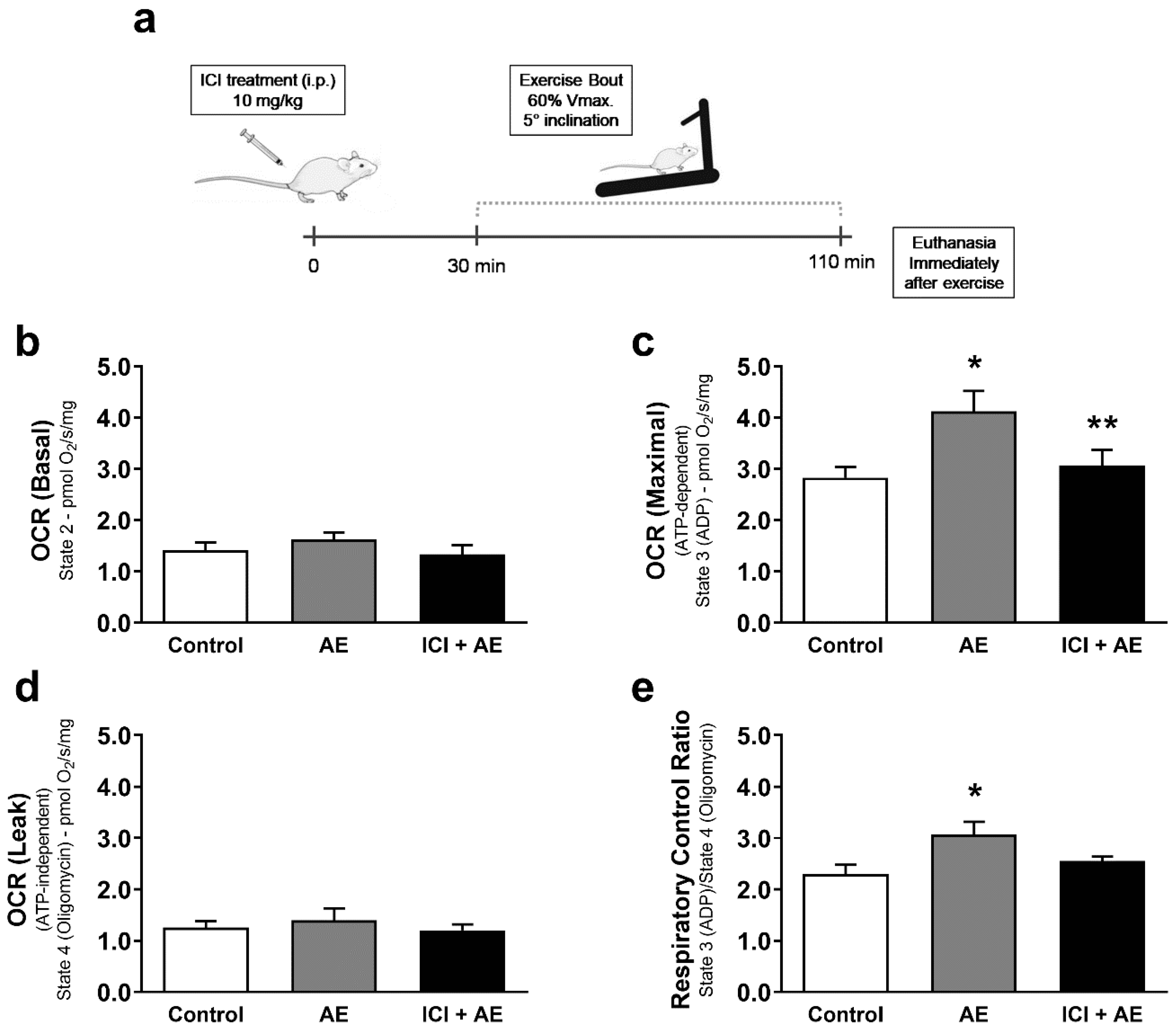
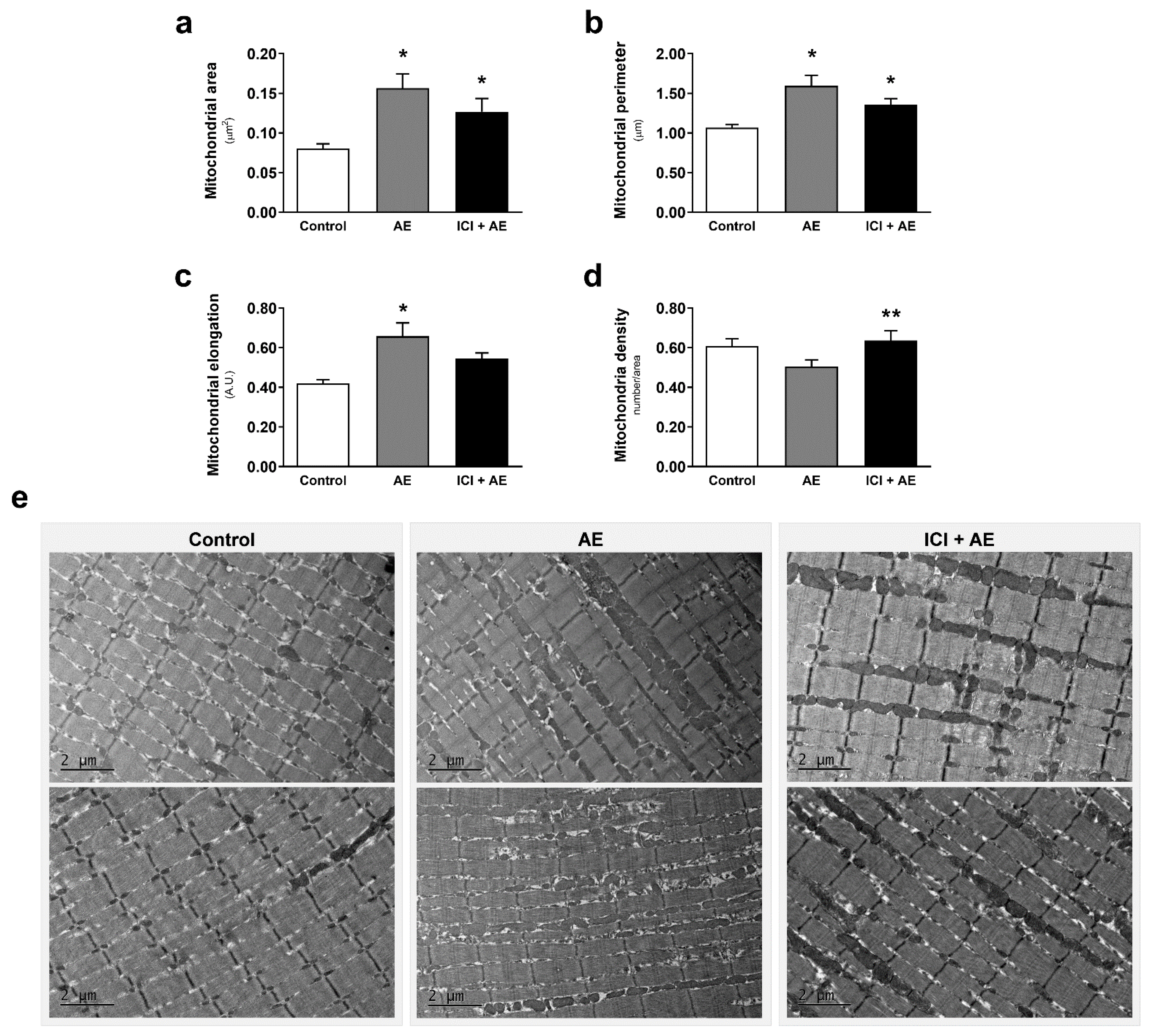
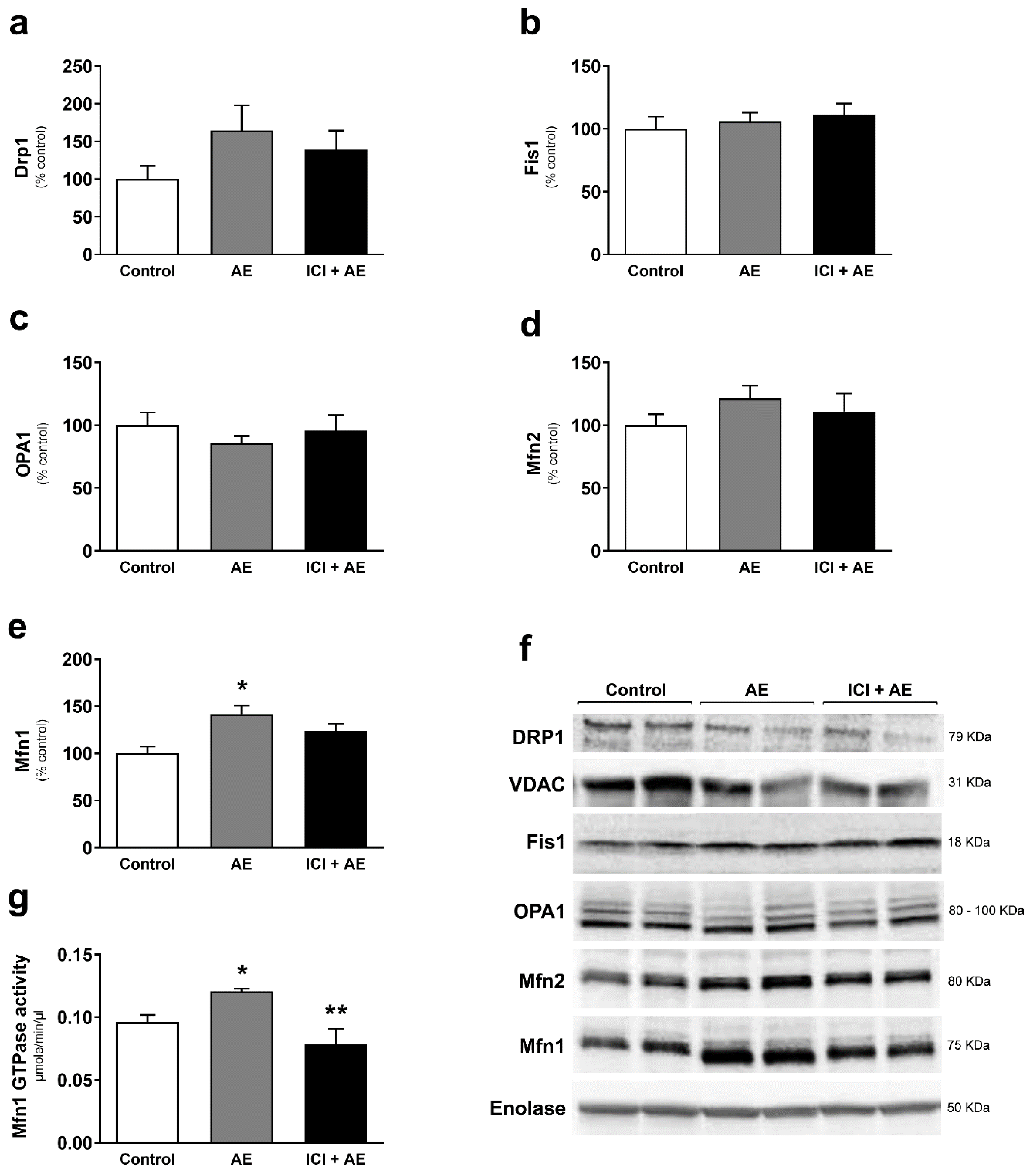
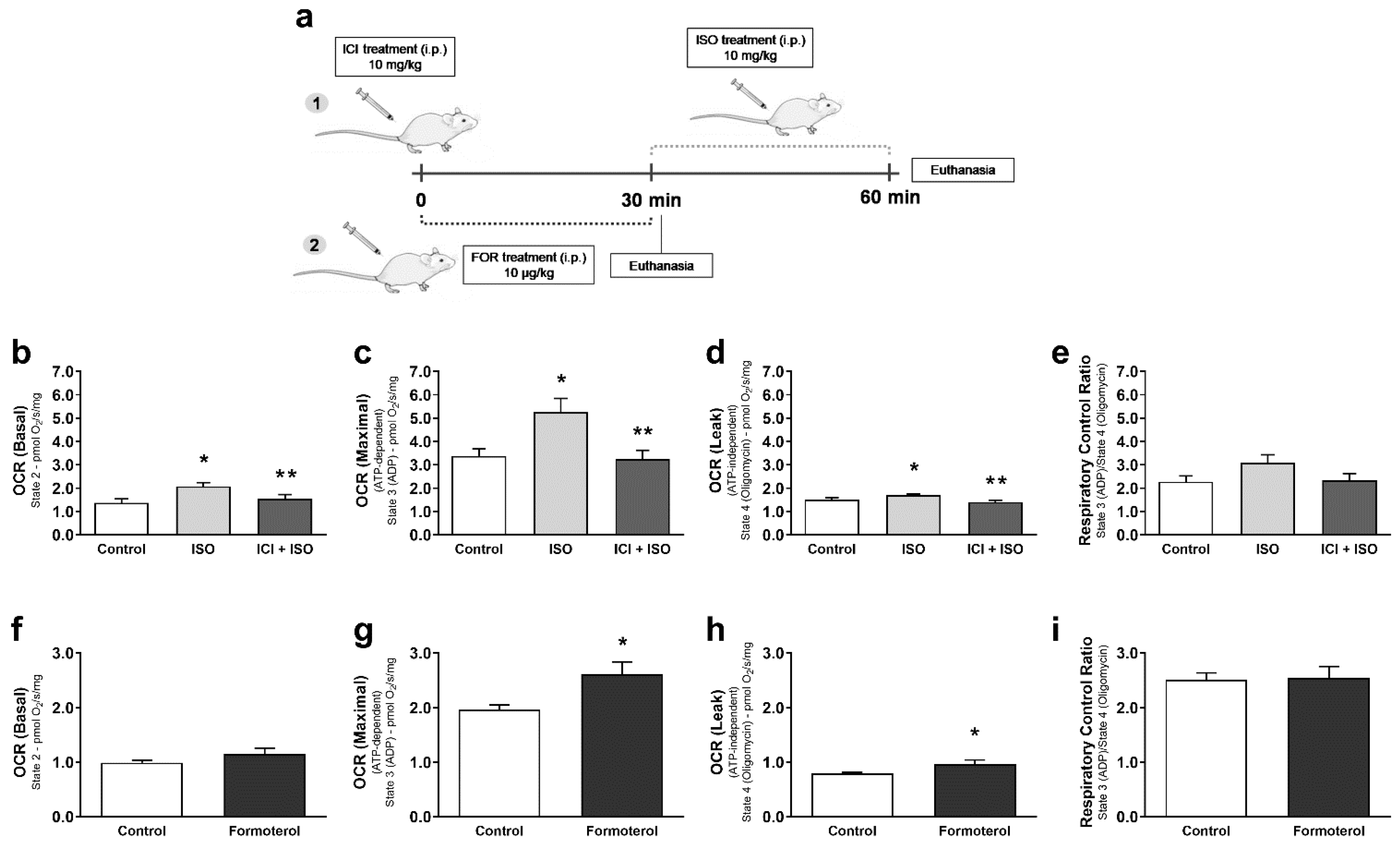
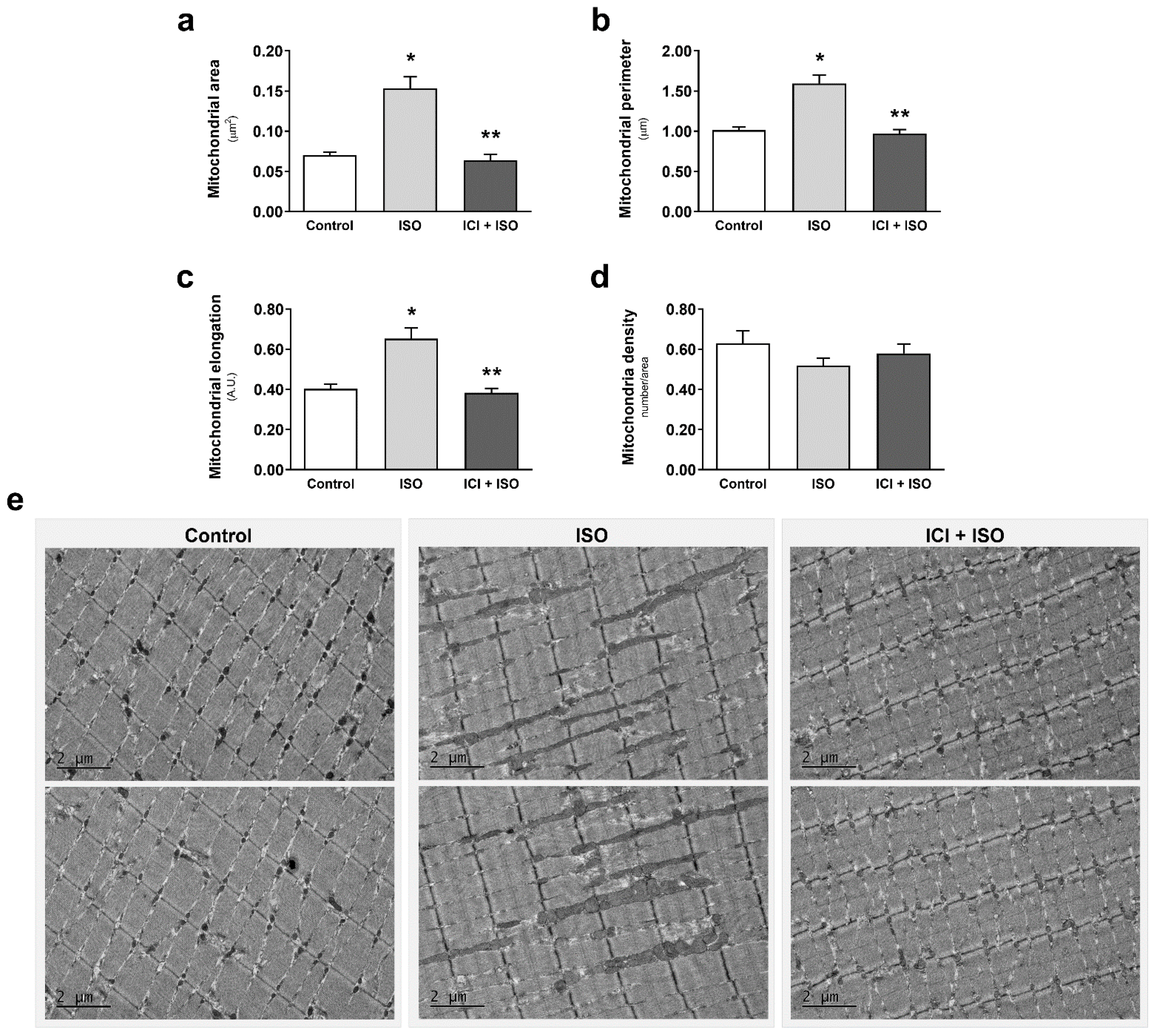
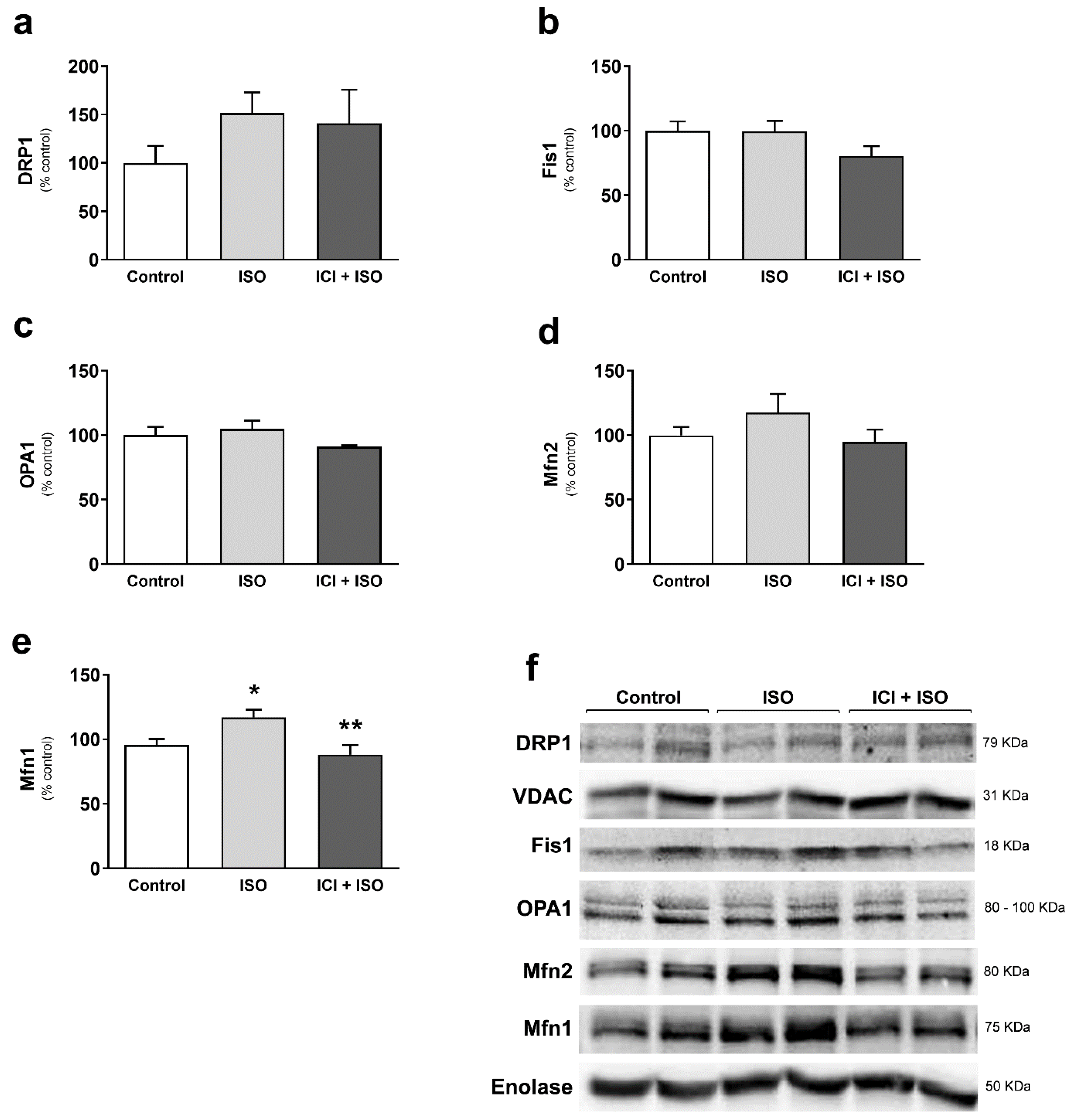
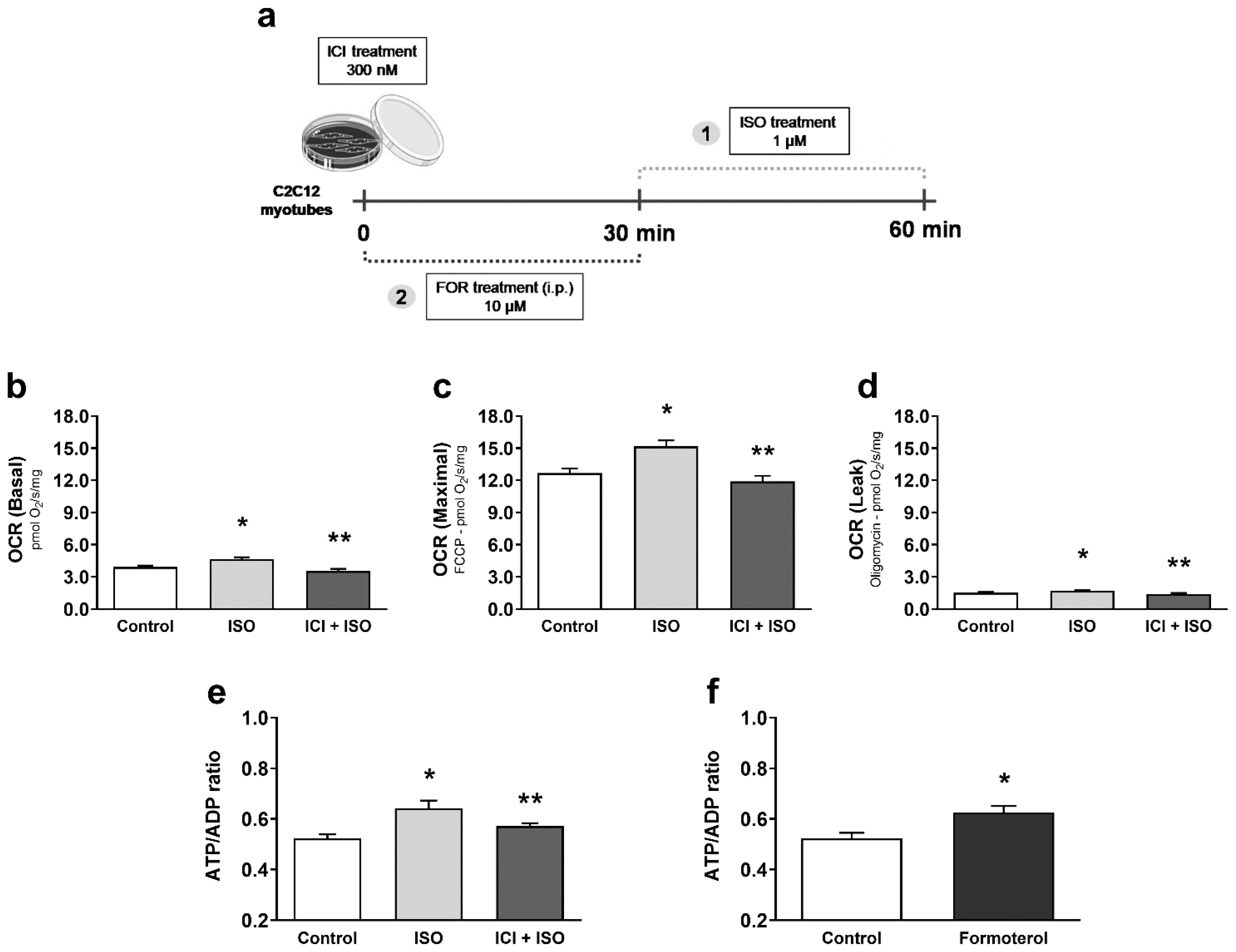
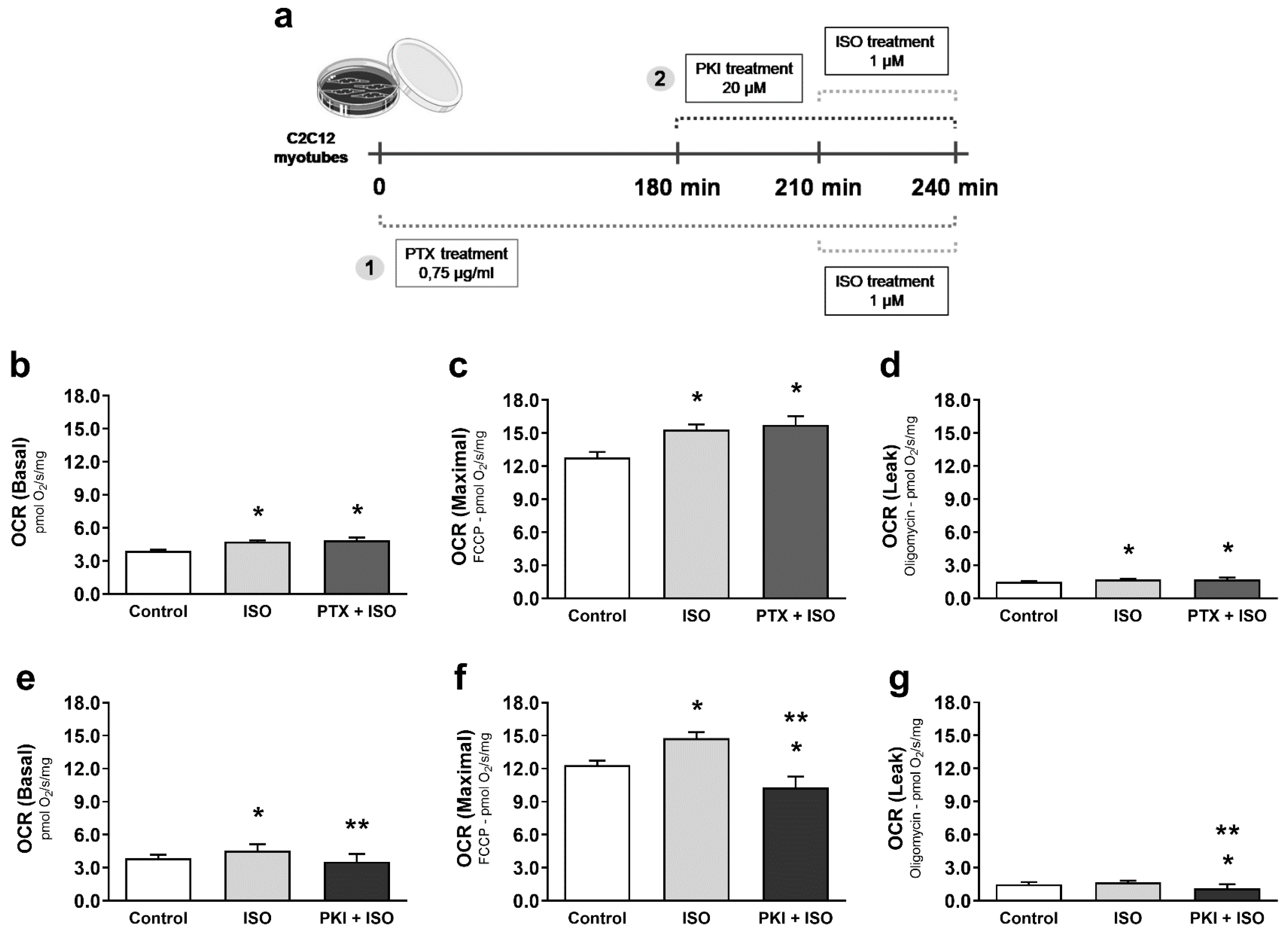
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef]
- Hood, D. A Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 465–472. [Google Scholar] [CrossRef]
- Cartoni, R.; Léger, B.; Hock, M.B.; Praz, M.; Crettenand, A.; Pich, S.; Ziltener, J.-L.; Luthi, F.; Dériaz, O.; Zorzano, A.; et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J. Physiol. 2005, 567, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Jiang, N.; Liu, H.; Liu, X.; Liu, D.; Zhao, F.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim. Biophys. Acta 2010, 1800, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Fortin, D.; Zoll, J.; N’Guessan, B.; Mettauer, B.; Lampert, E.; Veksler, V.; Ventura-Clapier, R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Baehr, L.M.; Gomes, K.M.S.; Bechara, L.R.G.; Voltarelli, V.A.; Bozi, L.H.M.; Ribeiro, M.A.C.; Ferreira, N.D.; Moreira, J.B.N.; Brum, P.C.; et al. Exercise prevents impaired autophagy and proteostasis in a model of neurogenic myopathy. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Campos, J.C.; Queliconi, B.B.; Bozi, L.H.M.; Bechara, L.R.G.; Dourado, P.M.M.; Andres, A.M.; Jannig, P.R.; Gomes, K.M.S.; Zambelli, V.O.; Rocha-Resende, C.; et al. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 2017. [Google Scholar] [CrossRef]
- Miura, S.; Kai, Y.; Kamei, Y.; Ezaki, O. Isoform-Specific Increases in Murine Skeletal Muscle Peroxisome Proliferator-Activated Receptor- Coactivator-1 (PGC-1) mRNA in Response to 2-Adrenergic Receptor Activation and Exercise. Endocrinology 2008, 149, 4527–4533. [Google Scholar] [CrossRef]
- Wills, L.P.; Trager, R.E.; Beeson, G.C.; Lindsey, C.C.; Peterson, Y.K.; Beeson, C.C.; Schnellmann, R.G. The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 2012. [Google Scholar] [CrossRef]
- Campos, J.C.; Baehr, L.M.; Ferreira, N.D.; Bozi, L.H.M.; Andres, A.M.; Ribeiro, M.A.C.; Gottlieb, R.A.; Bodine, S.C.; Ferreira, J.C.B. β2-adrenoceptor activation improves skeletal muscle autophagy in neurogenic myopathy. FASEB J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Lustrino, D.; Silveira, W.A.; Wild, F.; Straka, T.; Issop, Y.; O’Connor, E.; Cox, D.; Reischl, M.; Marquardt, T.; et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. USA 2016. [Google Scholar] [CrossRef]
- Elfellah, M.; Dalling, R.; Kantola, I.; Reid, J. Beta-adrenoceptors and human skeletal muscle characterisation of receptor subtype and effect of age. Br. J. Clin. Pharmacol. 1989. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.J.; Dyck, D.J.; Bonen, A.; Spriet, L.L. Effects of epinephrine on lipid metabolism in resting skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 1998. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Barnes, W.S. The positive inotropic effect of epinephrine on skeletal muscle: A brief review. Muscle Nerve 1989. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.; Fajardo, G.; Nguyen, K.; Zhao, M.; Kooiker, K.; Jung, G.; Hu, D.Q.; Reddy, S.; Sandoval, E.; Stotland, A.; et al. Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circ. Res. 2018. [Google Scholar] [CrossRef]
- Ferreira, J.C.B.; Rolim, N.P.L.; Bartholomeu, J.B.; Gobatto, C.A.; Kokubun, E.; Brum, P.C. Maximal lactate steady state in running mice: Effect of exercise training. Clin. Exp. Pharmacol. Physiol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Urhausen, A.; Weiler, B.; Coen, B.; Kindermann, W. Plasma catecholamines during endurance exercise of different intensities as related to the individual anaerobic threshold. Eur. J. Appl. Physiol. Occup. Physiol. 1994. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sport. Med. 2008, 38, 401–423. [Google Scholar] [CrossRef]
- Menconi, M.; Gonnella, P.; Petkova, V.; Lecker, S.; Hasselgren, P.O. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J. Cell. Biochem. 2008. [Google Scholar] [CrossRef]
- Ryall, J.G.; Sillence, M.N.; Lynch, G.S. Systemic administration of beta2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. Br. J. Pharmacol. 2006, 147, 587–595. [Google Scholar] [CrossRef]
- Richards, D.A.; Bao, W.; Rambo, M.V.; Burgert, M.; Jucker, B.M.; Lenhard, S.C. Examining the relationship between exercise tolerance and isoproterenol-based cardiac reserve in murine models of heart failure. J. Appl. Physiol. 2013. [Google Scholar] [CrossRef][Green Version]
- Engle, S.K.; Jordan, W.H.; Pritt, M.L.; Chiang, A.Y.; Davis, M.A.; Zimmermann, J.L.; Rudmann, D.G.; Heinz-Taheny, K.M.; Irizarry, A.R.; Yamamoto, Y.; et al. Qualification of Cardiac Troponin I Concentration in Mouse Serum Using Isoproterenol and Implementation in Pharmacology Studies to Accelerate Drug Development. Toxicol. Pathol. 2009. [Google Scholar] [CrossRef]
- Brooks, W.W.; Conrad, C.H. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: Structural and functional correlates. Comp. Med. 2009, 59, 339–343. [Google Scholar]
- Burniston, J.G.; Tan, L.B.; Goldspink, D.F. Relative myotoxic and haemodynamic effects of the β-agonists fenoterol and clenbuterol measured in conscious unrestrained rats. Exp. Physiol. 2006. [Google Scholar] [CrossRef]
- Shivachar, A.C.; Eikenburg, D.C. Differential effects of epinephrine and norepinephrine on cAMP response and G(i3)α protein expression in cultured sympathetic neurons. J. Pharmacol. Exp. Ther. 1999, 291, 258–264. [Google Scholar] [PubMed]
- Xiang, Y.; Kobilka, B. The PDZ-binding motif of the β2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2003. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Michel, M.C.; Insel, P.A.; Ennis, C.; Ziegler, M.G.; Phillips, C. Pertussis toxin treatment of whole blood: A novel approach to assess g protein function in congestive heart failure. Circulation 1990. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Pearson, T.; Caporossi, D.; Jackson, M.J. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res. Notes 2012. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J. The Localization of Cholinesterase Activity in Rat Cardiac Muscle by Electron Microscopy. J. Cell Biol. 1964. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Ertbjerg, P.; Djurhuus, M.S.; Pedersen, P.K. Calcium content and respiratory control index of skeletal muscle mitochondria during exercise and recovery. Am. J. Physiol. Endocrinol. Metab. 1996. [Google Scholar] [CrossRef] [PubMed]
- Tonkonogi, M.; Harris, B.; Sahlin, K. Mitochondrial oxidative function in human saponin-skinned muscle fibres: Effects of prolonged exercise. J. Physiol. 1998. [Google Scholar] [CrossRef] [PubMed]
- Tonkonogi, M.; Walsh, B.; Tiivel, T.; Saks, V.; Sahlin, K. Mitochondrial function in human skeletal muscle is not impaired by high intensity exercise. Pflugers Arch. Eur. J. Physiol. 1999. [Google Scholar] [CrossRef] [PubMed]
- Fernström, M.; Tonkonogi, M.; Sahlin, K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J. Physiol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, G.; Bo, H.; Qu, J.; Ma, G.; Cao, D.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Upregulation of uncoupling protein-3 in skeletal muscle during exercise: A potential antioxidant function. Free Radic. Biol. Med. 2009. [Google Scholar] [CrossRef]
- Ryall, J.G.; Gregorevic, P.; Plant, D.R.; Sillence, M.N.; Lynch, G.S. β2-agonist fenoterol has greater effects on contractile function of rat skeletal muscles than clenbuterol. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002. [Google Scholar] [CrossRef]
- Navegantes, L.C.C.; Resano, N.M.Z.; Baviera, A.M.; Migliorini, R.H.; Kettelhut, I.C. Effect of sympathetic denervation on the rate of protein synthesis in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sainz, R.D.; Summers, R.J.; Molenaar, P. Cimaterol reduces beta-adrenergic receptor density in rat skeletal muscles. J. Anim. Sci. 1992. [Google Scholar] [CrossRef]
- Jensen, J.; Brennesvik, E.O.; Bergersen, L.H.; Oseland, H.; Jebens, E.; Brørs, O. Quantitative determination of cell surface β-adrenoceptors in different rat skeletal muscles. Pflugers Arch. Eur. J. Physiol. 2002. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.S.; Ryall, J.G. Role of β-adrenoceptor signaling in skeletal muscle: Implications for muscle wasting and disease. Physiol. Rev. 2008, 88, 729–767. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P.; Borrani, F. β-Adrenergic modulation of skeletal muscle contraction: Key role of excitation-contraction coupling. J. Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Voltarelli, V.A.; Bechara, L.R.G.; Bacurau, A.V.N.; Mattos, K.C.; Dourado, P.M.M.; Bueno, C.R.; Casarini, D.E.; Negrao, C.E.; Brum, P.C. Lack of β2-adrenoceptors aggravates heart failure-induced skeletal muscle myopathy in mice. J. Cell. Mol. Med. 2014, 18. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 2011. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Schrauwen, P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R285–R296. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X. Inter-organ regulation of adipose tissue browning. Cell. Mol. Life Sci. 2017, 74, 1765–1776. [Google Scholar] [CrossRef]
- Sprague, J.E.; Yang, X.; Sommers, J.; Gilman, T.L.; Mills, E.M. Roles of norepinephrine, free fatty acids, thyroid status, and skeletal muscle uncoupling protein 3 expression in sympathomimetic-induced thermogenesis. J. Pharmacol. Exp. Ther. 2007. [Google Scholar] [CrossRef]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 1833–1838. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Qi, Y.; Xu, H. Mitochondrial cAMP signaling. Cell. Mol. Life Sci. 2016, 73, 4577–4590. [Google Scholar] [CrossRef]
- Eisner, V.; Lenaers, G.; Hajnóczky, G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J. Cell Biol. 2014. [Google Scholar] [CrossRef]
- Moore, T.M.; Zhou, Z.; Cohn, W.; Norheim, F.; Lin, A.J.; Kalajian, N.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol. Metab. 2019. [Google Scholar] [CrossRef]
- Picard, M.; Gentil, B.J.; McManus, M.J.; White, K.; St Louis, K.; Gartside, S.E.; Wallace, D.C.; Turnbull, D.M. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 2013, 115, 1562–1571. [Google Scholar] [CrossRef]
- Huertas, J.R.; Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Nordsborg, N.B.; Martín-Albo, J.; Rueda-Robles, A.; Casuso, R.A. Human muscular mitochondrial fusion in athletes during exercise. FASEB J. 2019. [Google Scholar] [CrossRef]
- Skulachev, V.P. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem. Sci. 2001, 26, 23–29. [Google Scholar] [CrossRef]
- Drake, J.C.; Wilson, R.J.; Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016, 30, 13–22. [Google Scholar] [CrossRef]
- Ferreira, J.C.B.; Campos, J.C.; Qvit, N.; Qi, X.; Bozi, L.H.M.; Bechara, L.R.G.; Lima, V.M.; Queliconi, B.B.; Disatnik, M.H.; Dourado, P.M.M.; et al. A selective inhibitor of mitofusin 1-βIIPKC association improves heart failure outcome in rats. Nat. Commun. 2019. [Google Scholar] [CrossRef]
- Bozi, L.H.M.; Campos, J.C.; Zambelli, V.O.; Ferreira, N.D.; Ferreira, J.C.B. Mitochondrially-targeted treatment strategies. Mol. Aspects Med. 2020, 71, 100836. [Google Scholar] [CrossRef]
- Herzig, S.; Long, F.; Jhala, U.S.; Hedrick, S.; Quinn, R.; Bauer, A.; Rudolph, D.; Schutz, G.; Yoon, C.; Puigserver, P.; et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 2001. [Google Scholar] [CrossRef]
- Sebastián, D.; Hernández-Alvarez, M.I.; Segalés, J.; Sorianello, E.; Muñoz, J.P.; Sala, D.; Waget, A.; Liesa, M.; Paz, J.C.; Gopalacharyulu, P.; et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl. Acad. Sci. USA 2012. [Google Scholar] [CrossRef]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 145–161. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019. [Google Scholar] [CrossRef]
- Chang, C.R.; Blackstone, C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007. [Google Scholar] [CrossRef]
- Cribbs, J.T.; Strack, S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007, 8, 939–944. [Google Scholar] [CrossRef]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011. [Google Scholar] [CrossRef]
- García-Bermúdez, J.; Sánchez-Aragó, M.; Soldevilla, B.; del Arco, A.; Nuevo-Tapioles, C.; Cuezva, J.M. PKA Phosphorylates the ATPase Inhibitory Factor 1 and Inactivates Its Capacity to Bind and Inhibit the Mitochondrial H+-ATP Synthase. Cell Rep. 2015. [Google Scholar] [CrossRef]
- Acin-Perez, R.; Salazar, E.; Kamenetsky, M.; Buck, J.; Levin, L.R.; Manfredi, G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009, 9, 265–276. [Google Scholar] [CrossRef]
- Valsecchi, F.; Ramos-Espiritu, L.S.; Buck, J.; Levin, L.R.; Manfredi, G. cAMP and mitochondria. Physiology 2013, 28, 199–209. [Google Scholar] [CrossRef]
- Feliciello, A.; Gottesman, M.E.; Avvedimento, E.V. cAMP-PKA signaling to the mitochondria: Protein scaffolds, mRNA and phosphatases. Cell. Signal. 2005, 17, 279–287. [Google Scholar] [CrossRef]
- Lark, D.S.; Reese, L.R.; Ryan, T.E.; Torres, M.J.; Smith, C.D.; Lin, C.T.; Neufer, P.D. Protein kinase A governs oxidative phosphorylation kinetics and oxidant emitting potential at complex I. Front. Physiol. 2015. [Google Scholar] [CrossRef]
- Dagda, R.K.; Gusdon, A.M.; Pien, I.; Strack, S.; Green, S.; Li, C.; Van Houten, B.; Cherra, S.J.; Chu, C.T. Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson’s disease. Cell Death Differ. 2011, 18, 1914–1923. [Google Scholar] [CrossRef]
- De Rasmo, D.; Gattoni, G.; Papa, F.; Santeramo, A.; Pacelli, C.; Cocco, T.; Micelli, L.; Sardaro, N.; Larizza, M.; Scivetti, M.; et al. The β-adrenoceptor agonist isoproterenol promotes the activity of respiratory chain complex I and lowers cellular reactive oxygen species in fibroblasts and heart myoblasts. Eur. J. Pharmacol. 2011, 652, 15–22. [Google Scholar] [CrossRef]
- Lefkimmiatis, K.; Leronni, D.; Hofer, A.M. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J. Cell Biol. 2013, 202, 453–462. [Google Scholar] [CrossRef]
- Negrao, C.E.; Middlekauff, H.R. Adaptations in autonomic function during exercise training in heart failure. Heart Fail. Rev. 2008, 13, 51–60. [Google Scholar] [CrossRef]
- Negrão, C.E.; Moreira, E.D.; Brum, P.C.; Denadai, M.L.; Krieger, E.M. Vagal and sympathetic control of heart rate during exercise by sedentary and exercise-trained rats. Braz. J. Med. Biol. Res. 1992, 25, 1045–1052. [Google Scholar]
- Fernandez-Gonzalo, R.; Lundberg, T.R.; Tesch, P.A. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol. 2013. [Google Scholar] [CrossRef]
- Chapman, M.A.; Arif, M.; Emanuelsson, E.B.; Reitzner, S.M.; Lindholm, M.E.; Mardinoglu, A.; Sundberg, C.J. Skeletal Muscle Transcriptomic Comparison between Long-Term Trained and Untrained Men and Women. Cell Rep. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo Voltarelli, V.; Coronado, M.; Gonçalves Fernandes, L.; Cruz Campos, J.; Jannig, P.R.; Batista Ferreira, J.C.; Fajardo, G.; Chakur Brum, P.; Bernstein, D. β2-Adrenergic Signaling Modulates Mitochondrial Function and Morphology in Skeletal Muscle in Response to Aerobic Exercise. Cells 2021, 10, 146. https://doi.org/10.3390/cells10010146
Azevedo Voltarelli V, Coronado M, Gonçalves Fernandes L, Cruz Campos J, Jannig PR, Batista Ferreira JC, Fajardo G, Chakur Brum P, Bernstein D. β2-Adrenergic Signaling Modulates Mitochondrial Function and Morphology in Skeletal Muscle in Response to Aerobic Exercise. Cells. 2021; 10(1):146. https://doi.org/10.3390/cells10010146
Chicago/Turabian StyleAzevedo Voltarelli, Vanessa, Michael Coronado, Larissa Gonçalves Fernandes, Juliane Cruz Campos, Paulo Roberto Jannig, Julio Cesar Batista Ferreira, Giovanni Fajardo, Patricia Chakur Brum, and Daniel Bernstein. 2021. "β2-Adrenergic Signaling Modulates Mitochondrial Function and Morphology in Skeletal Muscle in Response to Aerobic Exercise" Cells 10, no. 1: 146. https://doi.org/10.3390/cells10010146
APA StyleAzevedo Voltarelli, V., Coronado, M., Gonçalves Fernandes, L., Cruz Campos, J., Jannig, P. R., Batista Ferreira, J. C., Fajardo, G., Chakur Brum, P., & Bernstein, D. (2021). β2-Adrenergic Signaling Modulates Mitochondrial Function and Morphology in Skeletal Muscle in Response to Aerobic Exercise. Cells, 10(1), 146. https://doi.org/10.3390/cells10010146