Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity
Abstract
1. Introduction
2. Saturated Fatty Acids as Inducers of Membrane Permeabilization
3. Is PA-mPT Based on a General Mechanism of PA/Ca2+-Induced Permeabilization of Lipid Membranes?
4. Molecular Mechanism of PA-mPT
5. Properties of PA-mPTP and Its Regulation
6. Differences between PA-mPT and Classical mPT
7. The Mechanism of Formation of PA-mPT Pores
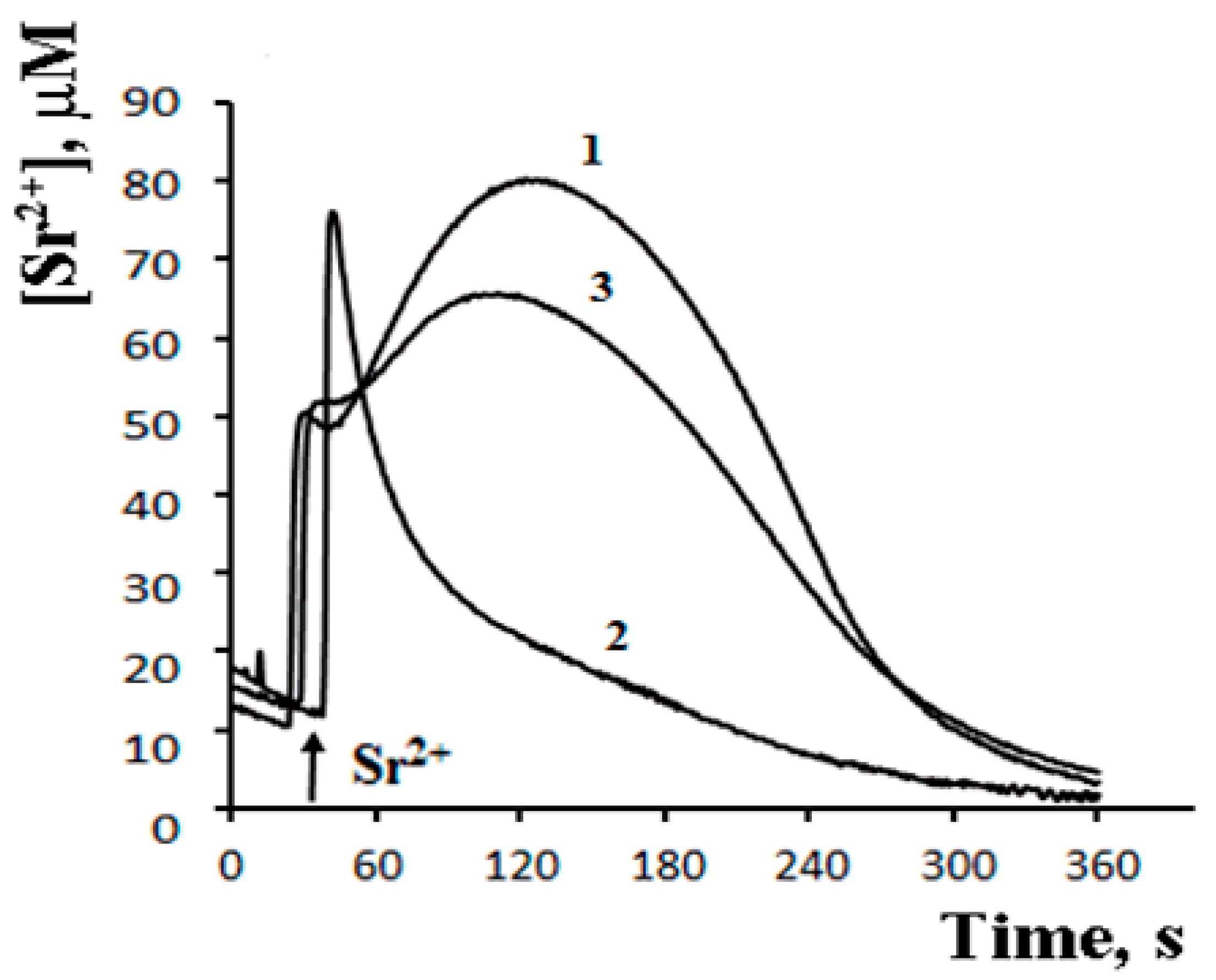
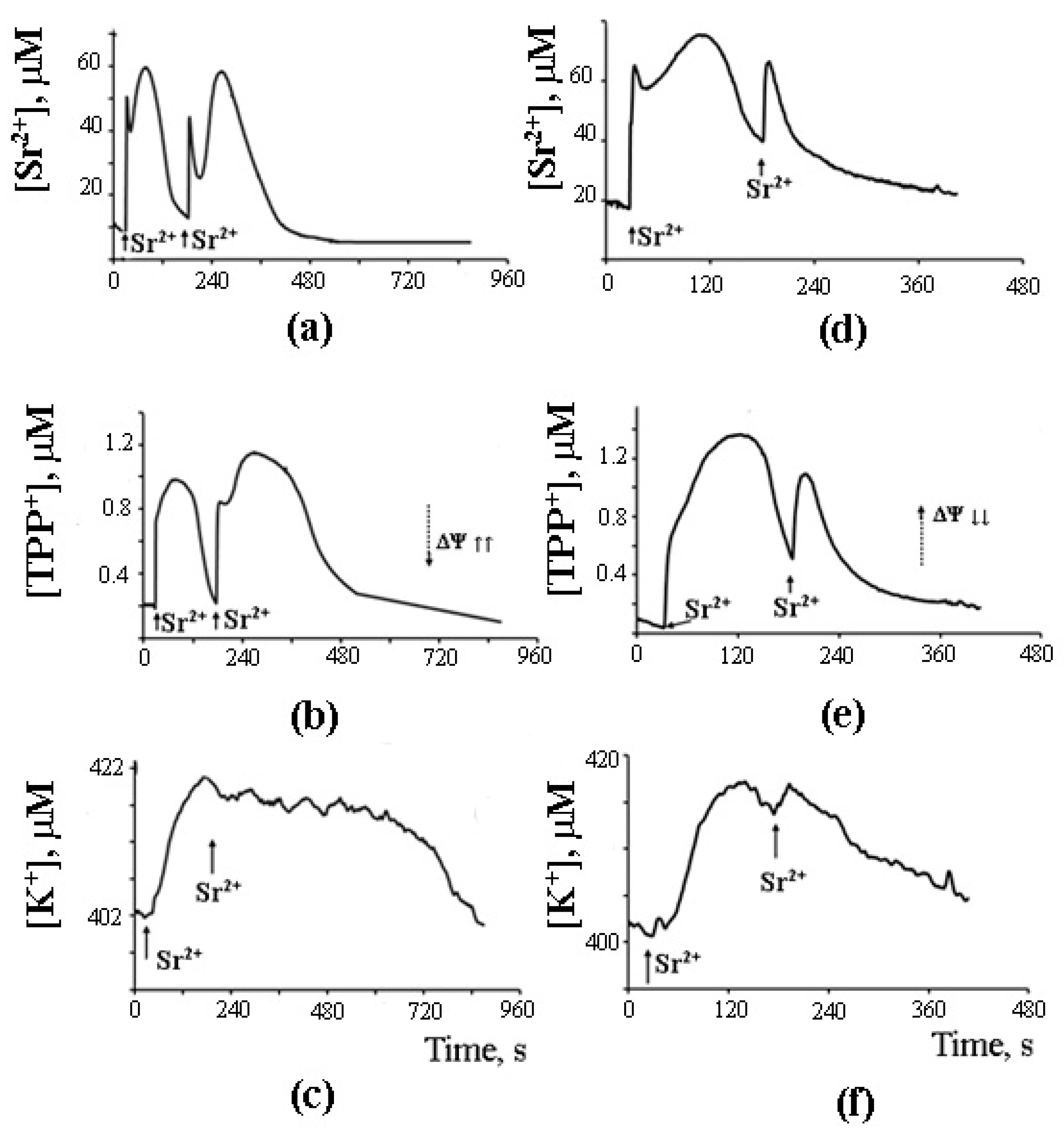
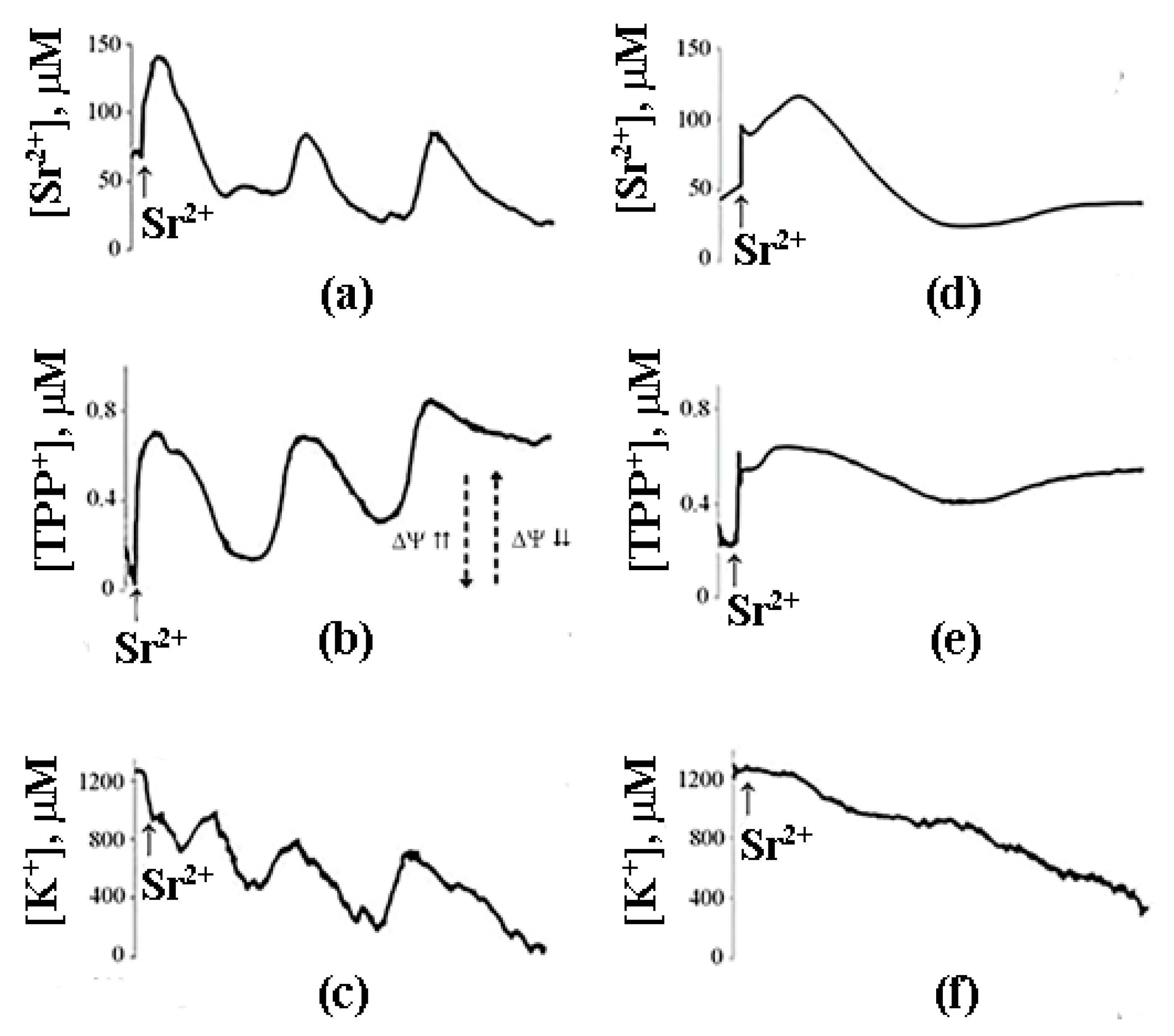
8. A Possible Role of PA-mPT in the Emergency Release of Ca2+ from Mitochondria and Maintenance of Ion Homeostasis
9. The Mechanism of PA-mPT-Mediated Protection against Ca2+ Overload and mPTP Activation
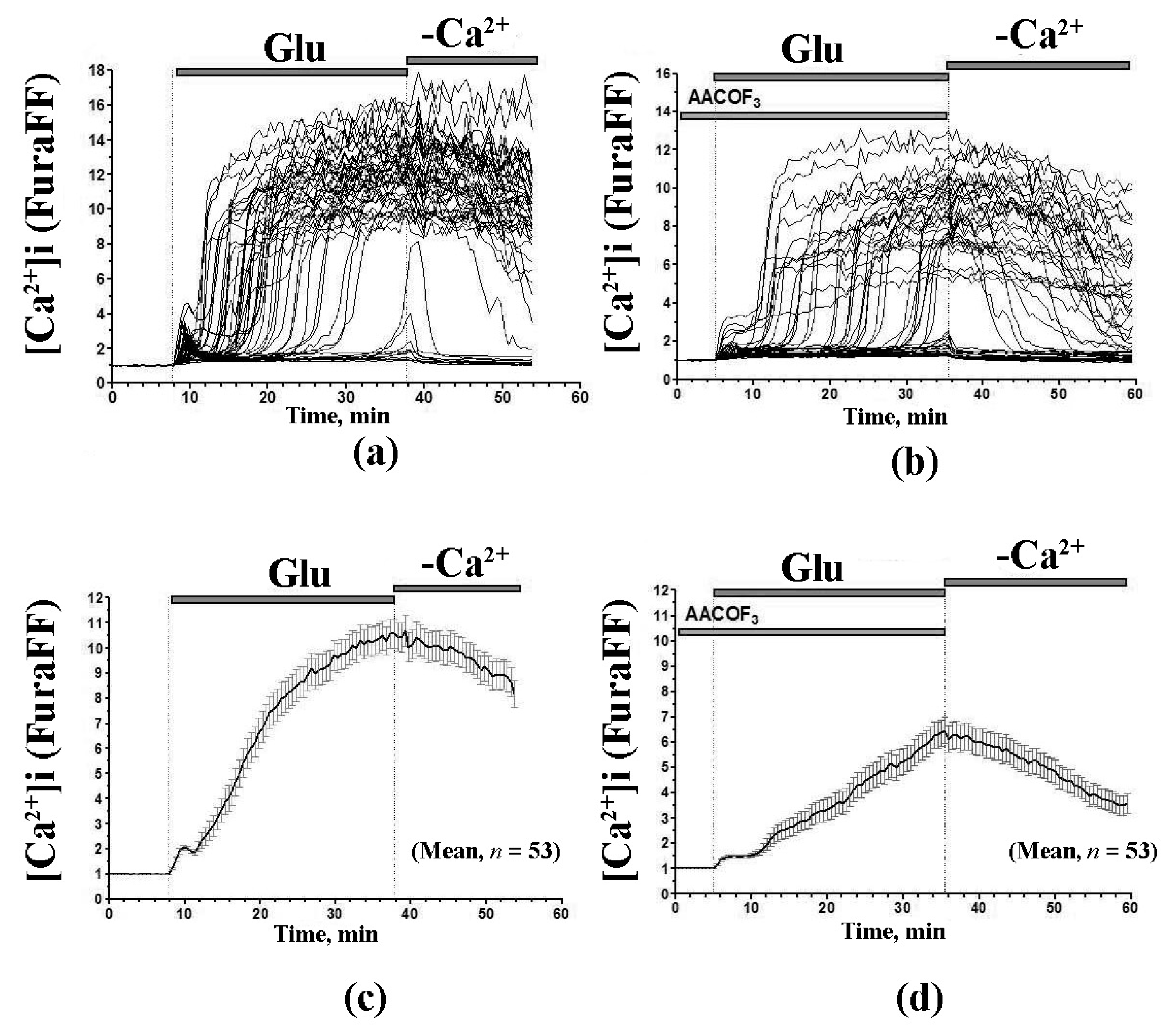
10. A Possible Role of PA-mPT in the Glutamate-Induced Neurotoxicity
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carraro, M.; Carrer, A.; Urbani, A.; Bernardi, P. Molecular nature and regulation of the mitochondrial permeability transition pore(s), drug target(s) in cardioprotection. J. Mol. Cell. Cardiol. 2020, 144, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Bround, M.J.; Bers, D.M.; Molkentin, J.D. A 20/20 view of ANT function in mitochondrial biology and necrotic cell death. J. Mol. Cell. Cardiol. 2020, 144, A3–A13. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanyan, N.; Jonas, E.A. ATP synthase c-subunit ring as the channel of mitochondrial permeability transition: Regulator of metabolism in development and degeneration. J. Mol. Cell. Cardiol. 2020, 144, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Petronilli, V.; Miotto, G.; Canton, M.; Brini, M.; Colonna, R.; Bernardi, P.; Di Lisa, F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 1999, 76, 725–734. [Google Scholar] [CrossRef]
- Bernardi, P.; von Stockum, S. The permeability transition pore as a Ca(2+) release channel: New answers to an old question. Cell Calcium 2012, 52, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanyan, N.; Llaguno, M.C.; Yang, Y.; Yan, Y.; Weber, J.; Sigworth, F.J.; Jonas, E.A. A mitochondrial megachannel resides in monomeric F1FO ATP synthase. Nat. Commun. 2019, 10, 5823. [Google Scholar] [CrossRef]
- Neginskaya, M.A.; Solesio, M.E.; Berezhnaya, E.V.; Amodeo, G.F.; Mnatsakanyan, N.; Jonas, E.A.; Pavlov, E.V. ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel, but Lack the Permeability Transition Pore. Cell Rep. 2019, 26, 11–17. [Google Scholar] [CrossRef]
- Urbani, A.; Giorgio, V.; Carrer, A.; Franchin, C.; Arrigoni, G.; Jiko, C.; Abe, K.; Maeda, S.; Shinzawa-Itoh, K.; Bogers, J.F.M.; et al. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat. Commun. 2019, 10, 4341. [Google Scholar] [CrossRef]
- Karch, J.; Bround, M.J.; Khalil, H.; Sargent, M.A.; Latchman, N.; Terada, N.; Peixoto, P.M.; Molkentin, J.D. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 2019, 5, eaaw4597. [Google Scholar] [CrossRef]
- Walker, J.E.; Carroll, J.; He, J. Reply to Bernardi: The mitochondrial permeability transition pore and the ATP synthase. Proc. Natl. Acad. Sci. USA 2020, 117, 2745–2746. [Google Scholar] [CrossRef]
- Bernardi, P. Mechanisms for Ca(2+)-dependent permeability transition in mitochondria. Proc. Natl. Acad. Sci. USA 2020, 117, 2743–2744. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.D.; Lazareva, A.; Gateau-Roesch, O.; Tyynela, J.; Pavlov, Y.; Vanier, M.; Saris, N.E. Oscillating Ca2+-induced channel activity obtained in BLM with a mitochondrial membrane component. J. Bioenerg. Biomembr. 1997, 29, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.D.; Gateau-Roesch, O.; Levrat, C.; Gritsenko, E.; Pavlov, E.; Lazareva, A.V.; Limarenko, E.; Rey, C.; Louisot, P.; Saris, N.E. Palmitic and stearic acids bind Ca2+ with high affinity and form nonspecific channels in black-lipid membranes. Possible relation to Ca2+-activated mitochondrial pores. J. Bioenerg. Biomembr. 2001, 33, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Gateau-Roesch, O.; Pavlov, E.; Lazareva, A.V.; Limarenko, E.A.; Levrat, C.; Saris, N.E.; Louisot, P.; Mironova, G.D. Calcium-binding properties of the mitochondrial channel-forming hydrophobic component. J. Bioenerg. Biomembr. 2000, 32, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, A.V.; Gritsenko, E.N.; Shlyapnikova, E.A.; Kharakoz, D.P.; Belosludtseva, N.V.; Lezhnev, E.I.; Saris, N.E.; Mironova, G.D. Ca2+-induced phase separation in the membrane of palmitate-containing liposomes and its possible relation to membrane permeabilization. J. Membr. Biol. 2007, 215, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, A.; Gritsenko, E.; Belosludtsev, K.; Kovalev, A.; Gateau-Roesch, O.; Saris, N.E.; Mironova, G.D. A permeability transition in liposomes induced by the formation of Ca2+/palmitic acid complexes. Biochim. Biophys. Acta 2003, 1609, 153–160. [Google Scholar] [CrossRef]
- Mironova, G.D.; Gritsenko, E.; Gateau-Roesch, O.; Levrat, C.; Agafonov, A.; Belosludtsev, K.; Prigent, A.F.; Muntean, D.; Dubois, M.; Ovize, M. Formation of palmitic acid/Ca2+ complexes in the mitochondrial membrane: A possible role in the cyclosporin-insensitive permeability transition. J. Bioenerg. Biomembr. 2004, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.D.; Belosludtsev, K.N.; Belosludtseva, N.V.; Gritsenko, E.N.; Khodorov, B.I.; Saris, N.E. Mitochondrial Ca2+ cycle mediated by the palmitate-activated cyclosporin A-insensitive pore. J. Bioenerg. Biomembr. 2007, 39, 167–174. [Google Scholar] [CrossRef]
- Sultan, A.; Sokolove, P.M. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 2001, 386, 37–51. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Agafonov, A.V.; Mironova, G.D. Palmitic acid induces the opening of a Ca2+-dependent pore in the plasma membrane of red blood cells: The possible role of the pore in erythrocyte lysis. J. Membr. Biol. 2010, 237, 13–19. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Mironova, G.D. Possible mechanism for formation and regulation of the palmitate-induced cyclosporin A-insensitive mitochondrial pore. Biochemistry 2005, 70, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Belosludtseva, N.V.; Belosludtsev, K.N.; Agafonov, A.V.; Mironova, G.D. [Effect of cholesterol on the formation of palmitate/Ca(2+)-activated pore in mitochondria and liposomes]. Biofizika 2009, 54, 464–470. [Google Scholar] [PubMed]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Mironova, G.D. [The role of a mitochondrial palmitate/Ca(2+)-activated pore in palmitate-induced apoptosis]. Biofizika 2008, 53, 967–971. [Google Scholar] [PubMed]
- Belosludtsev, K.; Saris, N.E.; Andersson, L.C.; Belosludtseva, N.; Agafonov, A.; Sharma, A.; Moshkov, D.A.; Mironova, G.D. On the mechanism of palmitic acid-induced apoptosis: The role of a pore induced by palmitic acid and Ca2+ in mitochondria. J. Bioenerg. Biomembr. 2006, 38, 113–120. [Google Scholar] [CrossRef]
- Antonov, V.F.; Shevchenko, E.V. Lipid pores and stability of cell membranes. Vestn. Ross. Akad. Med. Nauk. 1995, 10, 48–55. [Google Scholar]
- Antonov, V.F.; Shevchenko, E.V.; Smirnova, E.; Yakovenko, E.V.; Frolov, A.V. Stable cupola-shaped bilayer lipid membranes with mobile Plateau-Gibbs border: Expansion-shrinkage of membrane due to thermal transitions. Chem. Phys. Lipids 1992, 61, 219–224. [Google Scholar] [CrossRef]
- Rand, R.P. Mechanical Properties of the Red Cell Membrane. Ii. Viscoelastic Breakdown of the Membrane. Biophys. J. 1964, 4, 303–316. [Google Scholar] [CrossRef]
- Schmidt, G.; Knoll, W. Densitometric Characterization of Aqueous Lipid Dispersions. Ber. Bunsen. Phys. Chem. 1985, 89, 36–43. [Google Scholar] [CrossRef]
- Jacobson, K.; Papahadjopoulos, D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry 1975, 14, 152–161. [Google Scholar] [CrossRef]
- Abidor, I.G.; Chernomordik, L.V.; Sukharev, S.I.; Chizmadzhev, Y.A. The Reversible Electrical Breakdown of Bilayer Lipid-Membranes Modified by Uranyl Ions. Bioelectroch. Bioener. 1982, 9, 141–148. [Google Scholar] [CrossRef]
- Benz, R.; Beckers, F.; Zimmermann, U. Reversible Electrical Breakdown of Lipid Bilayer Membranes—Charge-Pulse Relaxation Study. J. Membr. Biol. 1979, 48, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Bodrova, M.E.; Dedukhova, V.I.; Samartsev, V.N.; Mokhova, E.N. Role of the ADP/ATP-antiporter in fatty acid-induced uncoupling of Ca2+-loaded rat liver mitochondria. Iubmb. Life 2000, 50, 189–194. [Google Scholar] [PubMed]
- Sultan, A.; Sokolove, P.M. Free fatty acid effects on mitochondrial permeability: An overview. Arch. Biochem. Biophys. 2001, 386, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 1993, 262, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E. Stimulation of phospholipase A2 activity in mitochondria by magnesium and polyamines. Magnes. Res. 1994, 7, 5–10. [Google Scholar] [PubMed]
- Glover, S.; de Carvalho, M.S.; Bayburt, T.; Jonas, M.; Chi, E.; Leslie, C.C.; Gelb, M.H. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J. Biol. Chem. 1995, 270, 15359–15367. [Google Scholar] [CrossRef] [PubMed]
- Loo, R.W.; Conde-Frieboes, K.; Reynolds, L.J.; Dennis, E.A. Activation, inhibition, and regiospecificity of the lysophospholipase activity of the 85-kDa group IV cytosolic phospholipase A2. J. Biol. Chem. 1997, 272, 19214–19219. [Google Scholar] [CrossRef] [PubMed]
- Thorne, T.E.; Voelkel-Johnson, C.; Casey, W.M.; Parks, L.W.; Laster, S.M. The activity of cytosolic phospholipase A2 is required for the lysis of adenovirus-infected cells by tumor necrosis factor. J. Virol. 1996, 70, 8502–8507. [Google Scholar] [CrossRef]
- Venediktova, N.; Shigaeva, M.; Belova, S.; Belosludtsev, K.; Belosludtseva, N.; Gorbacheva, O.; Lezhnev, E.; Lukyanova, L.; Mironova, G. Oxidative phosphorylation and ion transport in the mitochondria of two strains of rats varying in their resistance to stress and hypoxia. Mol. Cell Biochem. 2013, 383, 261–269. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Gylkhandanyan, A.V.; Evtodienko, Y.V.; Zhabotinsky, A.M.; Kondrashova, M.N. Continuous Sr2+-induced oscillations of the ionic fluxes in mitochondria. FEBS Lett. 1976, 66, 44–47. [Google Scholar] [CrossRef]
- Ichas, F.; Jouaville, L.S.; Sidash, S.S.; Mazat, J.P.; Holmuhamedov, E.L. Mitochondrial calcium spiking: A transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994, 348, 211–215. [Google Scholar] [CrossRef]
- Holmuhamedov, E.L.; Teplova, V.V.; Chukhlova, E.A.; Evtodienko, Y.V.; Ulrich, R.G. Strontium excitability of the inner mitochondrial membrane: Regenerative strontium-induced strontium release. Biochem. Mol. Biol. Int. 1995, 36, 39–49. [Google Scholar] [PubMed]
- Mironova, G.D.; Saris, N.E.; Belosludtseva, N.V.; Agafonov, A.V.; Elantsev, A.B.; Belosludtsev, K.N. Involvement of palmitate/Ca2+(Sr2+)-induced pore in the cycling of ions across the mitochondrial membrane. Biochim. Biophys. Acta 2015, 1848, 488–495. [Google Scholar] [CrossRef][Green Version]
- Pfeiffer, D.R.; Schmid, P.C.; Beatrice, M.C.; Schmid, H.H. Intramitochondrial phospholipase activity and the effects of Ca2+ plus N-ethylmaleimide on mitochondrial function. J. Biol. Chem. 1979, 254, 11485–11494. [Google Scholar] [CrossRef]
- Medvedev, B.I.; Severina, E.P.; Gogvadze, V.G.; Chukhlova, E.A.; Evtodienko Yu, V. Participation of endogenous fatty acids in Ca2+ release activation from mitochondria. Gen. Physiol. Biophys. 1985, 4, 549–556. [Google Scholar]
- De Villiers, M.; Lochner, A. Mitochondrial Ca2+ fluxes: Role of free fatty acids, acyl-CoA and acylcarnitine. Biochim. Biophys. Acta 1986, 876, 309–317. [Google Scholar] [CrossRef]
- Harris, E.J.; Cooper, M.B. Calcium and magnesium ion losses in response to stimulants of efflux applied to heart, liver and kidney mitochondria. Biochem. Biophys. Res. Commun. 1981, 103, 788–796. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Saris, N.E.; Belosludtseva, N.V.; Trudovishnikov, A.S.; Lukyanova, L.D.; Mironova, G.D. Physiological aspects of the mitochondrial cyclosporin A-insensitive palmitate/Ca2+-induced pore: Tissue specificity, age profile and dependence on the animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2009, 41, 395–401. [Google Scholar] [CrossRef]
- Mironova, G.D.; Shigaeva, M.I.; Gritsenko, E.N.; Murzaeva, S.V.; Gorbacheva, O.S.; Germanova, E.L.; Lukyanova, L.D. Functioning of the mitochondrial ATP-dependent potassium channel in rats varying in their resistance to hypoxia. Involvement of the channel in the process of animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2010, 42, 473–481. [Google Scholar] [CrossRef]
- Bernardi, P.; Petronilli, V. The permeability transition pore as a mitochondrial calcium release channel: A critical appraisal. J. Bioenerg. Biomembr. 1996, 28, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Severina, E.P.; Evtodienko Iu, V. [Phospholipase A2 localization in mitochondria]. Biokhimiia 1981, 46, 1199–1201. [Google Scholar] [PubMed]
- Murakami, M.; Taketomi, Y.; Miki, Y.; Sato, H.; Hirabayashi, T.; Yamamoto, K. Recent progress in phospholipase A(2) research: From cells to animals to humans. Prog. Lipid Res. 2011, 50, 152–192. [Google Scholar] [CrossRef] [PubMed]
- Pshennikova, M.G.; Belkina, L.M.; Bakhtina, L.Y.; Baida, L.A.; Smirin, B.V.; Malyshev, I.Y. HSP70 stress proteins, nitric oxide, and resistance of August and Wistar rats to myocardial infarction. Bull. Exp. Biol. Med. 2001, 132, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Gadd, M.E.; Broekemeier, K.M.; Crouser, E.D.; Kumar, J.; Graff, G.; Pfeiffer, D.R. Mitochondrial iPLA2 activity modulates the release of cytochrome c from mitochondria and influences the permeability transition. J. Biol. Chem. 2006, 281, 6931–6939. [Google Scholar] [CrossRef] [PubMed]
- Khodorov, B. Glutamate-induced deregulation of calcium homeostasis and mitochondrial dysfunction in mammalian central neurones. Prog. Biophys. Mol. Biol. 2004, 86, 279–351. [Google Scholar] [CrossRef] [PubMed]
- Budd, S.L.; Nicholls, D.G. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurochem. 1996, 67, 2282–2291. [Google Scholar] [CrossRef]
- Bolshakov, A.P.; Mikhailova, M.M.; Szabadkai, G.; Pinelis, V.G.; Brustovetsky, N.; Rizzuto, R.; Khodorov, B.I. Measurements of mitochondrial pH in cultured cortical neurons clarify contribution of mitochondrial pore to the mechanism of glutamate-induced delayed Ca2+ deregulation. Cell Calcium 2008, 43, 602–614. [Google Scholar] [CrossRef]
- Mironova, G.D.; Belosludtsev, K.N.; Surin, A.M.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Pinelis, V.G.; Krasilnikova, I.A.; Khodorov, B.I. Mitochondrial Lipid Pore in the Mechanism of Glutamate-Induced Calcium Deregulation of Brain Neurons. Biochem. Mosc. Suppl. S 2012, 6, 45–55. [Google Scholar] [CrossRef]
- Crompton, M. Mitochondrial intermembrane junctional complexes and their role in cell death. J. Physiol. 2000, 529 Pt 1, 11–21. [Google Scholar] [CrossRef]
- Sparagna, G.C.; Hickson-Bick, D.L.; Buja, L.M.; McMillin, J.B. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2124–H2132. [Google Scholar] [CrossRef] [PubMed]
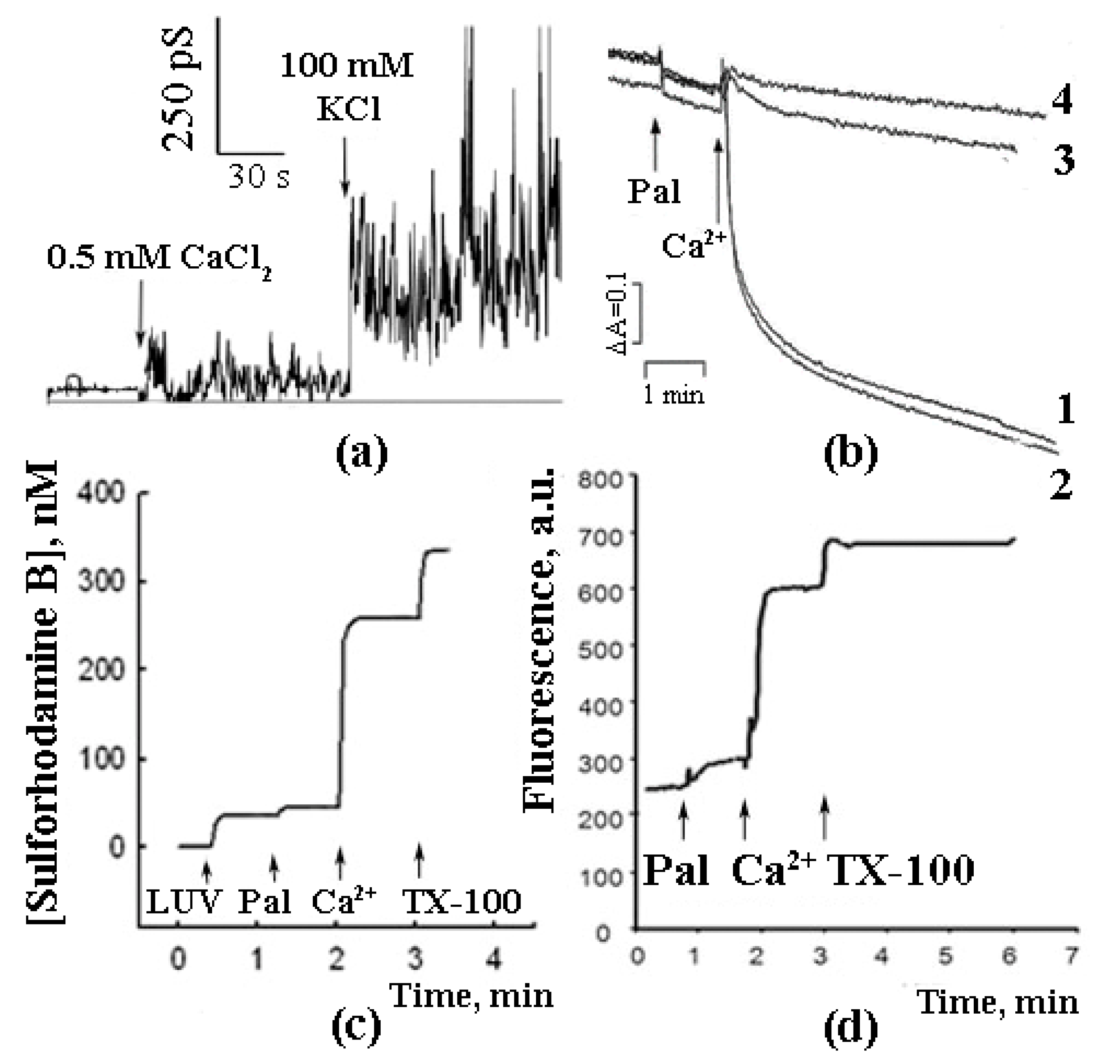
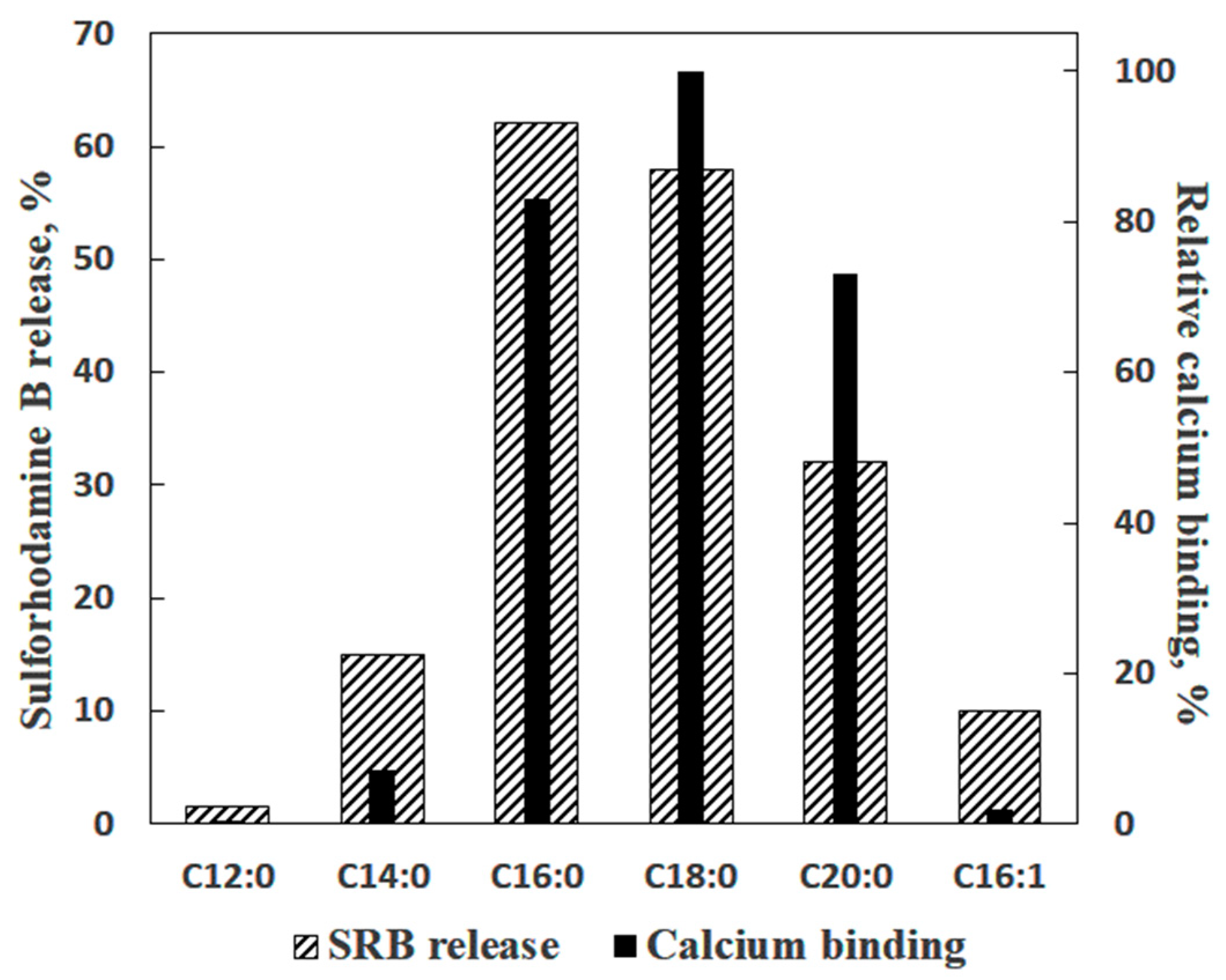
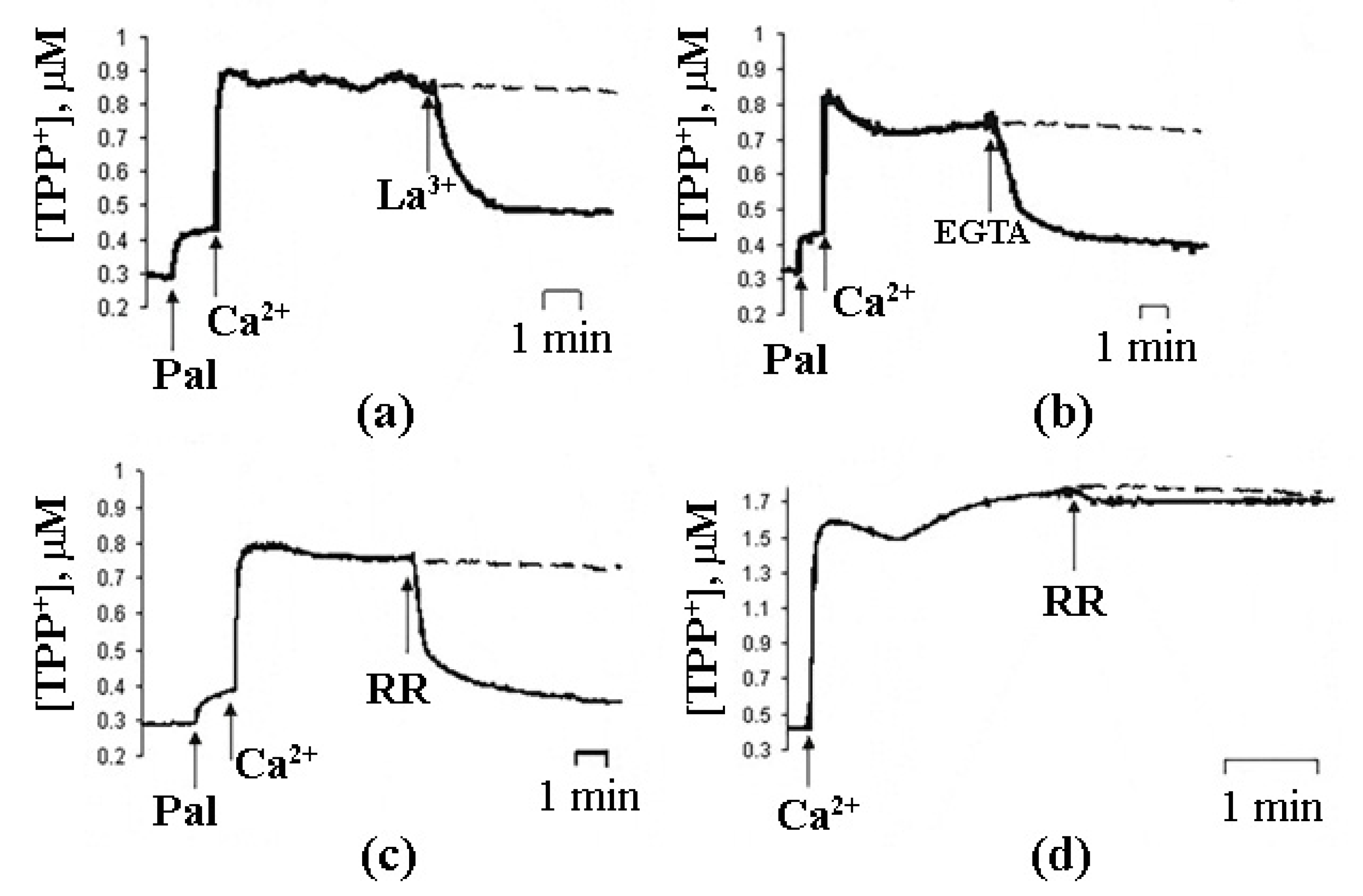

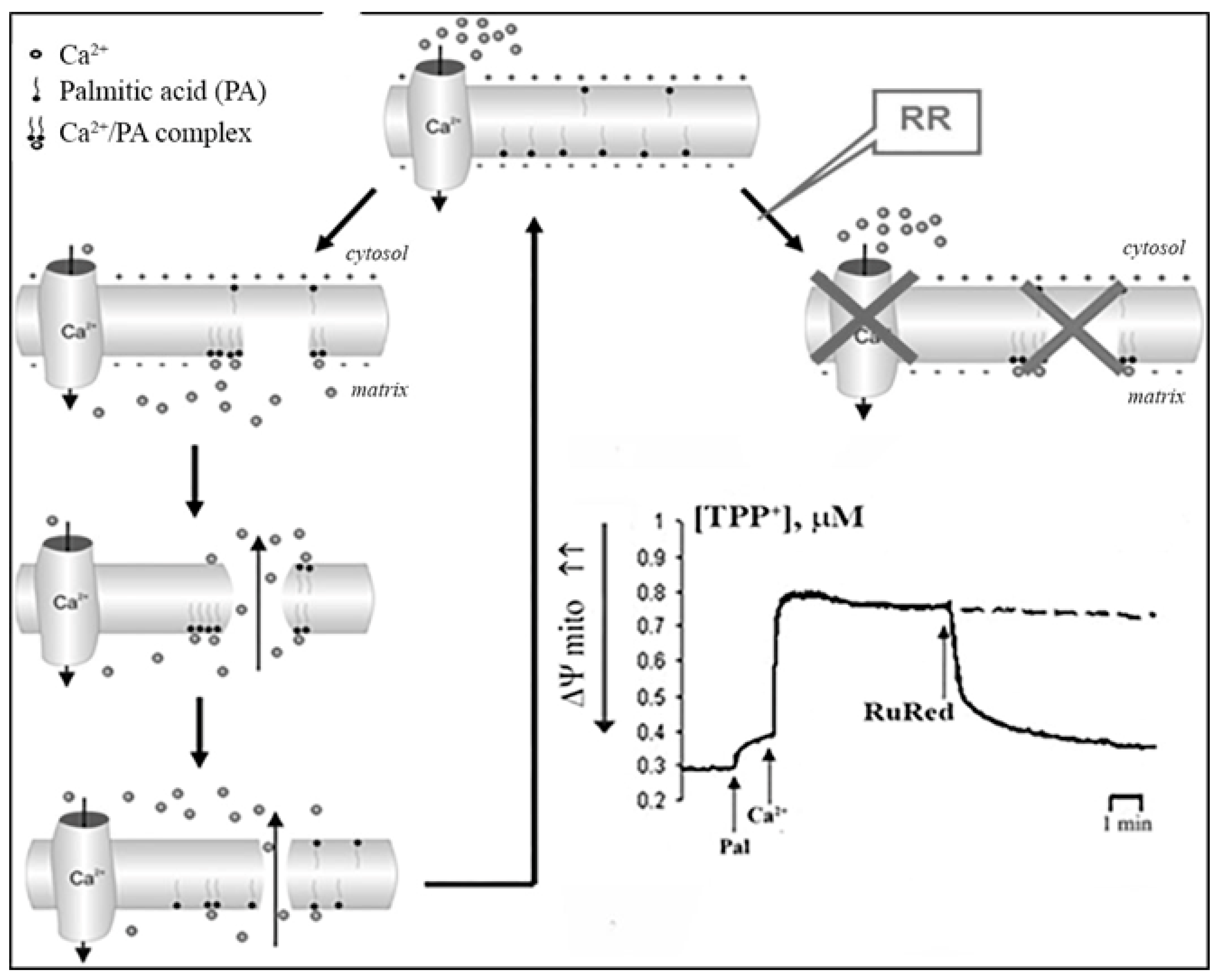
| Lipids | Relative Ca2+ Binding |
|---|---|
| Lauric acid (12:0) | 0.50 ± 0.03 |
| Myristic acid (14:0) | 7.30 ± 0.25 |
| Palmitic acid (16:0) | 83.00 ± 0.75 |
| Stearic acid (18:0) | 100.00 |
| Eicosanoic acid (20:0) | 73.00 ± 2.5 |
| Docosanoic acid (22:0) | 44.00 ± 1.2 |
| Lignoceric acid (24:0) | 15.00 ± 0.35 |
| Palmitoleic acid (16:1) | 1.90 ± 0.08 |
| Oleic acid | 5.70 ± 0.12 |
| Linoleic acid (18:2) | 0.65 ± 0.04 |
| Linoleinic acid (18:3) | 0.87 ± 0.05 |
| Arachidonic acid (20:4) | 1.10 ± 0.05 |
| 1-Palmitoyl-lysophosphatidylcholine | 0.43 ± 0.02 |
| 1-Stearoyl-lysophosphatidylcholine | 0.47 ± 0.02 |
| 1-Lauroyl-lysophosphatidylcholine | 0.43 ± 0.01 |
| Lysophosphatidylserine | 0.54 ± 0.03 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine | 0.40 ± 0.2 |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphatidylethanolamine | 0.22 ± 0.01 |
| 1-Palmitoyl-sn-glycero-1-3-phosphatidylethanolamine | 0.76 ± 0.03 |
| Palmitoil-CoA | 0.43 ± 0.02 |
| Cardiolipin | 0.60 ± 0.03 |
| L-α-phosphatidic acid | 19.50 ± 0.8 |
| Cholesterol | 0.33 ± 0.01 |
| Cerebrosides | 0.20 ± 0.01 |
| Sphingomyelin | 0.30 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironova, G.D.; Pavlov, E.V. Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity. Cells 2021, 10, 125. https://doi.org/10.3390/cells10010125
Mironova GD, Pavlov EV. Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity. Cells. 2021; 10(1):125. https://doi.org/10.3390/cells10010125
Chicago/Turabian StyleMironova, Galina D., and Evgeny V. Pavlov. 2021. "Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity" Cells 10, no. 1: 125. https://doi.org/10.3390/cells10010125
APA StyleMironova, G. D., & Pavlov, E. V. (2021). Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity. Cells, 10(1), 125. https://doi.org/10.3390/cells10010125





