Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies
Abstract
1. Introduction
2. Functional Significance of Arginine Methylation
2.1. Type I Protein Arginine Methyltransferases
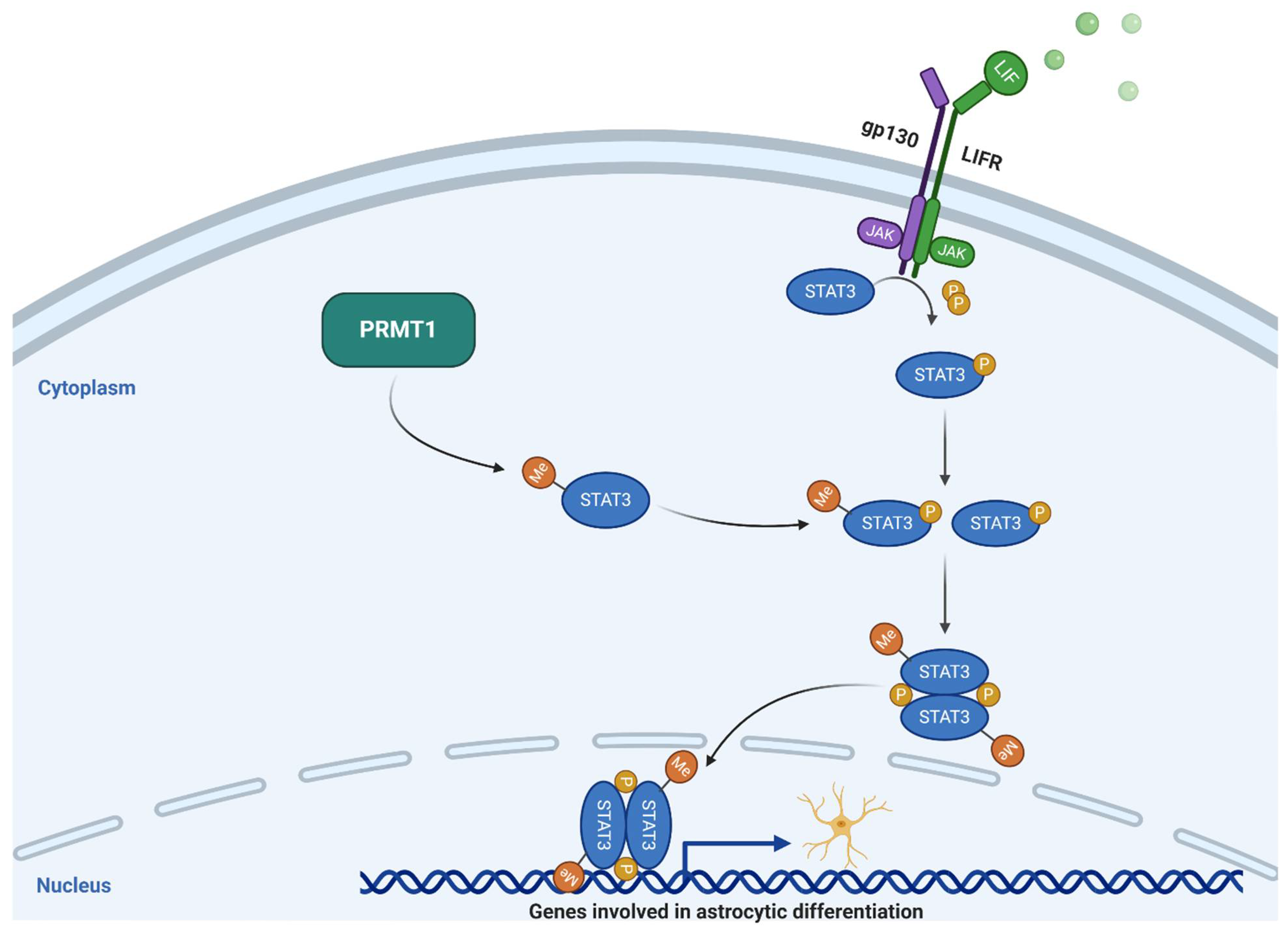
2.1.1. PRMT1
2.1.2. PRMT2
2.1.3. PRMT3
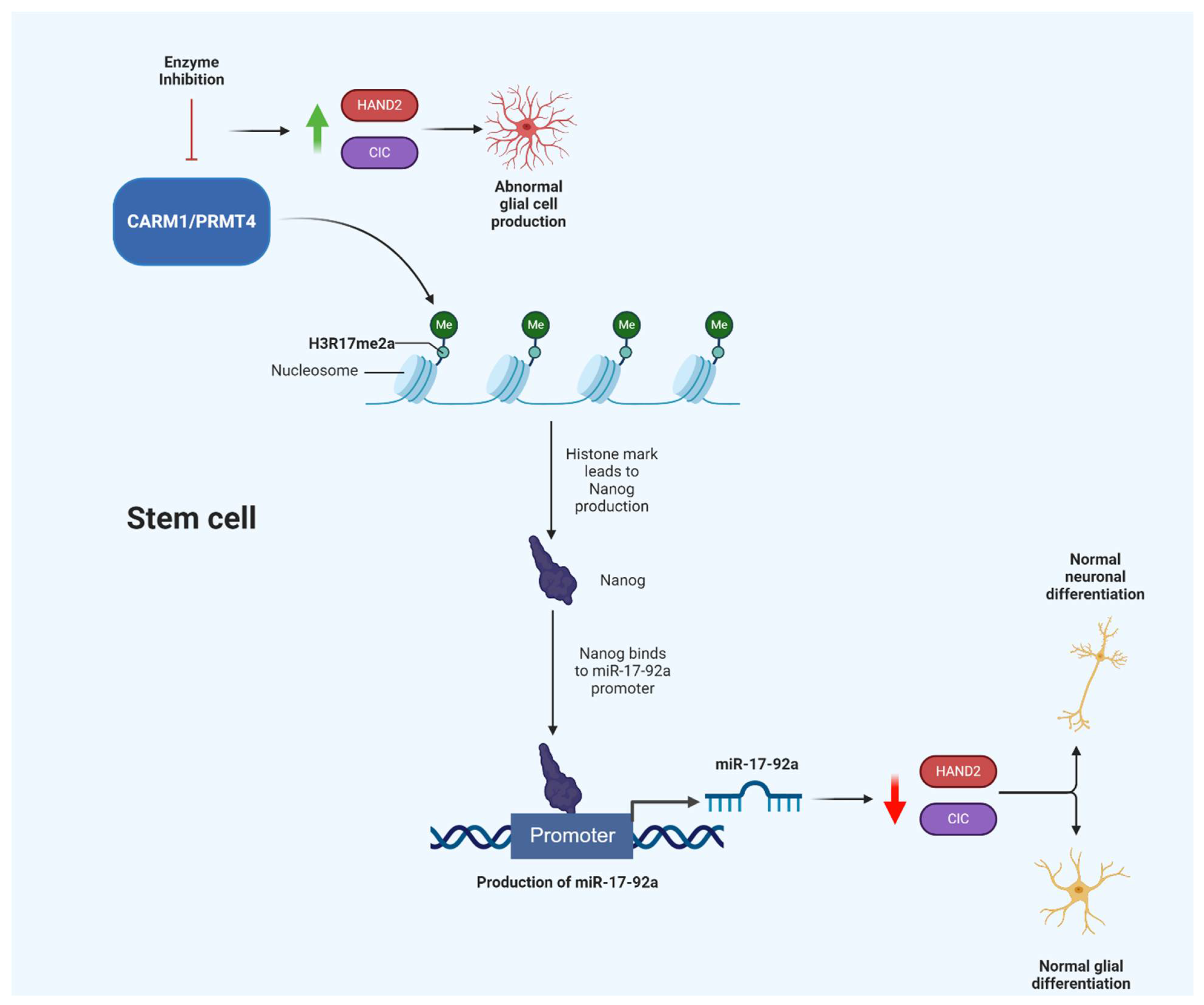
2.1.4. CARM1/PRMT4
2.1.5. PRMT6
2.1.6. PRMT8
2.2. Type II Protein Arginine Methyltransferases
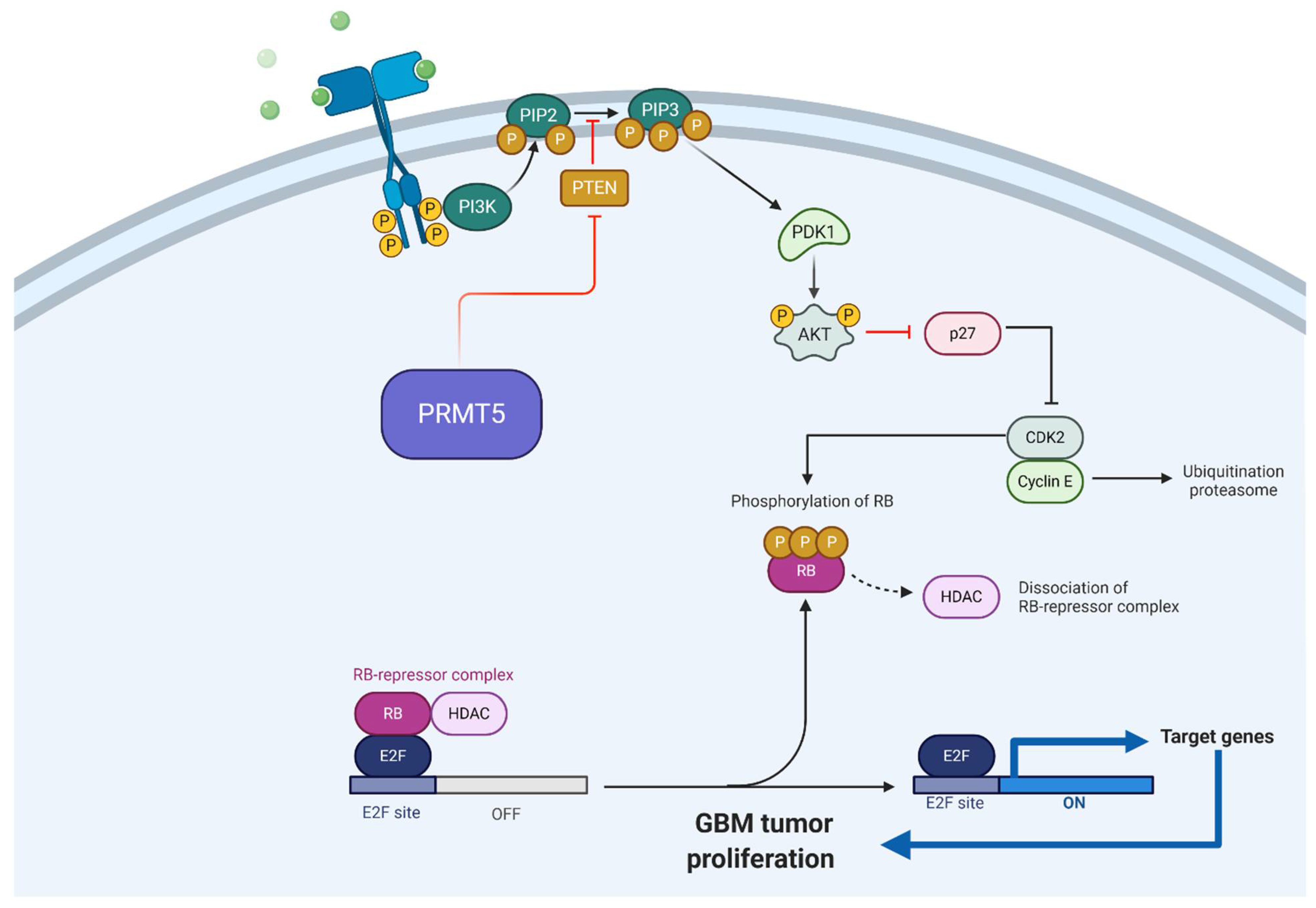
2.2.1. PRMT5
2.2.2. PRMT9
2.3. Type III Protein Arginine Methyltransferases
PRMT7
3. Role of PRMTs in Tumorigenesis
3.1. Role of Arginine Methylation in Oncogenesis
3.2. Role of Arginine Methylation in Brain Tumor Development
3.2.1. Glioma
3.2.2. Glioblastoma
3.2.3. Medulloblastoma
4. PRMT Inhibition and Clinical Applications
4.1. Type I Protein Arginine Methyltransferases Inhibitors
4.1.1. PRMT1
4.1.2. PRMT3
4.1.3. PRMT4/CARM1
4.1.4. PRMT6
4.2. Type II Protein Arginine Methyltransferases
PRMT5
4.3. Type III Protein Arginine Methyltransferases
PRMT7
4.4. Future Directions for Arginine Methylation Inhibition in Brain Tumors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.K.; Kumar, M.; Shanmugam, M.K.; Bhattacharya, A.; Rao, V.J.; Bhat, A.; Vasudevan, M.; Gopinath, K.S.; Mohiyuddin, A.; Chatterjee, A.; et al. Functional interplay between YY1 and CARM1 promotes oral carcinogenesis. Oncotarget 2019, 10, 3709–3724. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Kao, C.; Jeng, M.H.; Eble, J.N.; Koch, M.O.; Gardner, T.A.; Zhang, S.; Li, L.; Pan, C.X.; Hu, Z.; et al. Aberrant expression of CARM1, a transcriptional coactivator of androgen receptor, in the development of prostate carcinoma and androgen-independent status. Cancer 2004, 101, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, F.; Wang, Y.; Hao, L.; Zeng, H.; Jia, C.; Wang, Y.; Liu, P.; Ong, I.M.; Li, B.; et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell Biol. 2017, 19, 1358–1370. [Google Scholar] [CrossRef]
- Osada, S.; Suzuki, S.; Yoshimi, C.; Matsumoto, M.; Shirai, T.; Takahashi, S.; Imagawa, M. Elevated expression of coactivator-associated arginine methyltransferase 1 is associated with early hepatocarcinogenesis. Oncol. Rep. 2013, 30, 1669–1674. [Google Scholar] [CrossRef]
- Sarker, R.S.; John-Schuster, G.; Bohla, A.; Mutze, K.; Burgstaller, G.; Bedford, M.T.; Königshoff, M.; Eickelberg, O.; Yildirim, A. Coactivator-Associated Arginine Methyltransferase-1 Function in Alveolar Epithelial Senescence and Elastase-Induced Emphysema Susceptibility. Am. J. Respir. Cell Mol. Biol. 2015, 53, 769–781. [Google Scholar] [CrossRef]
- Sarker, R.S.J.; Conlon, T.M.; Morrone, C.; Srivastava, B.; Konyalilar, N.; Verleden, S.E.; Bayram, H.; Fehrenbach, H.; Yildirim, A. CARM1 regulates senescence during airway epithelial cell injury in COPD pathogenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L602–L614. [Google Scholar] [CrossRef]
- Wu, D.; He, J.; Zhang, W.; Wang, K.; Jin, S.; Li, J.; Gao, W. CARM1 promotes non-small cell lung cancer progression through upregulating CCNE2 expression. Aging 2020, 12, 10578–10593. [Google Scholar] [CrossRef]
- Syed, N.; Langer, J.; Janczar, K.; Singh, P.; Lo Nigro, C.; Lattanzio, L.; Coley, H.M.; Hatzimichael, E.; Bomalaski, J.; Szlosarek, P.; et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013, 4, e458. [Google Scholar] [CrossRef]
- Takai, H.; Masuda, K.; Sato, T.; Sakaguchi, Y.; Suzuki, T.; Suzuki, T.; Koyama-Nasu, R.; Nasu-Nishimura, Y.; Katou, Y.; Ogawa, H.; et al. 5-Hydroxymethylcytosine plays a critical role in glioblastomagenesis by recruiting the CHTOP-methylosome complex. Cell Rep. 2014, 9, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Mongiardi, M.P.; Savino, M.; Bartoli, L.; Beji, S.; Nanni, S.; Scagnoli, F.; Falchetti, M.L.; Favia, A.; Farsetti, A.; Levi, A.; et al. Myc and Omomyc functionally associate with the Protein Arginine Methyltransferase 5 (PRMT5) in glioblastoma cells. Sci. Rep. 2015, 5, 15494. [Google Scholar] [CrossRef] [PubMed]
- Banasavadi-Siddegowda, Y.K.; Russell, L.; Frair, E.; Karkhanis, V.A.; Relation, T.; Yoo, J.Y.; Zhang, J.; Sif, S.; Imitola, J.; Baiocchi, R.; et al. PRMT5-PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells. Oncogene 2017, 36, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Q.; Yang, C.; Huo, D.; Wang, X.; Ai, C.; Kong, Y.; Sun, X.; Wang, W.; Zhou, Y.; et al. PRMT2 links histone H3R8 asymmetric dimethylation to oncogenic activation and tumorigenesis of glioblastoma. Nat. Commun. 2018, 9, 4552. [Google Scholar] [CrossRef]
- Favia, A.; Salvatori, L.; Nanni, S.; Iwamoto-Stohl, L.K.; Valente, S.; Mai, A.; Scagnoli, F.; Fontanella, R.A.; Totta, P.; Nasi, S.; et al. The Protein Arginine Methyltransferases 1 and 5 affect Myc properties in glioblastoma stem cells. Sci. Rep. 2019, 9, 15925. [Google Scholar] [CrossRef]
- Holmes, B.; Benavides-Serrato, A.; Saunders, J.T.; Landon, K.A.; Schreck, A.J.; Nishimura, R.N.; Gera, J. The protein arginine methyltransferase PRMT5 confers therapeutic resistance to mTOR inhibition in glioblastoma. J. Neuro-Oncol. 2019, 145, 11–22. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, R.; Wang, W.; Fu, D.; Hou, J.; Ji, S.; Chen, B.; Hu, Z.; Shao, X.; Yu, X.; et al. Arginine Methyltransferase 1 in the Nucleus Accumbens Regulates Behavioral Effects of Cocaine. J. Neurosci. 2015, 35, 12890–12902. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, B.C.; Yang, W.S.; Kim, E.; Hong, S.; Cho, J.Y. The Role of Protein Arginine Methyltransferases in Inflammatory Responses. Med. Inflamm. 2016, 2016, 4028353. [Google Scholar] [CrossRef]
- Wolf, S.S. The protein arginine methyltransferase family: An update about function, new perspectives and the physiological role in humans. Cell. Mol. Life Sci. 2009, 66, 2109–2121. [Google Scholar] [CrossRef]
- Eram, M.S.; Shen, Y.; Szewczyk, M.; Wu, H.; Senisterra, G.; Li, F.; Butler, K.V.; Kaniskan, H.; Speed, B.A.; Dela Seña, C.; et al. A Potent, Selective, and Cell-Active Inhibitor of Human Type I Protein Arginine Methyltransferases. ACS Chem. Biol. 2016, 11, 772–781. [Google Scholar] [CrossRef]
- Tewary, S.K.; Zheng, Y.G.; Ho, M.-C. Protein arginine methyltransferases: Insights into the enzyme structure and mechanism at the atomic level. Cell. Mol. Life Sci. 2019, 76, 2917–2932. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Maity, R.; Whitelegge, J.P.; Hadjikyriacou, A.; Li, Z.; Zurita-Lopez, C.; Al-Hadid, Q.; Clark, A.T.; Bedford, M.T.; Masson, J.Y.; et al. Mammalian protein arginine methyltransferase 7 (PRMT7) specifically targets RXR sites in lysine- and arginine-rich regions. J. Biol. Chem. 2013, 288, 37010–37025. [Google Scholar] [CrossRef] [PubMed]
- Goulet, I.; Gauvin, G.; Boisvenue, S.; Côté, J. Alternative splicing yields protein arginine methyltransferase 1 isoforms with distinct activity, substrate specificity, and subcellular localization. J. Biol. Chem. 2007, 282, 33009–33021. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Tsai, Y.J.; Liu, Y.F.; Cheng, Y.C.; Hung, C.M.; Lee, Y.J.; Pan, H.; Li, C. The critical role of protein arginine methyltransferase prmt8 in zebrafish embryonic and neural development is non-redundant with its paralogue prmt1. PLoS ONE 2013, 8, e55221. [Google Scholar] [CrossRef]
- Hadjikyriacou, A.; Yang, Y.; Espejo, A.; Bedford, M.T.; Clarke, S.G. Unique Features of Human Protein Arginine Methyltransferase 9 (PRMT9) and Its Substrate RNA Splicing Factor SF3B2. J. Biol. Chem. 2015, 290, 16723–16743. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Maeda, K. Chapter Four-Germinal Center B-Cell-Associated Nuclear Protein (GANP) Involved in RNA Metabolism for B Cell Maturation. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 131, pp. 135–186. [Google Scholar]
- Lee, J.; Bedford, M.T. PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays. EMBO Rep. 2002, 3, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Auclair, Y.; Richard, S. The role of arginine methylation in the DNA damage response. DNA Repair (Amst.) 2013, 12, 459–465. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, C.; Zhuang, S. Protein arginine methyltransferase 1 mediates renal fibroblast activation and fibrogenesis through activation of Smad3 signaling. Am. J. Physiol. Ren. Physiol. 2020, 318, F375–F387. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, B.-H.; Oh, C.-M.; Lee, J.; Lee, K.; Song, H.; Kim, E.; Yi, K.; Kim, M.-Y.; Kim, H.; et al. PRMT1 Is Required for the Maintenance of Mature β-Cell Identity. Diabetes 2020, 69, 355–368. [Google Scholar] [CrossRef]
- Qiao, X.; Kim, D.-i.; Jun, H.; Ma, Y.; Knights, A.J.; Park, M.-J.; Zhu, K.; Lipinski, J.H.; Liao, J.; Li, Y.; et al. Protein Arginine Methyltransferase 1 Interacts With PGC1α and Modulates Thermogenic Fat Activation. Endocrinology 2019, 160, 2773–2786. [Google Scholar] [CrossRef]
- Hashimoto, M.; Murata, K.; Ishida, J.; Kanou, A.; Kasuya, Y.; Fukamizu, A. Severe Hypomyelination and Developmental Defects Are Caused in Mice Lacking Protein Arginine Methyltransferase 1 (PRMT1) in the Central Nervous System. J. Biol. Chem. 2016, 291, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Nakashima, K.; Katada, S. PRMT1 regulates astrocytic differentiation of embryonic neural stem/precursor cells. J. Neurochem. 2017, 142, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Amano, G.; Matsuzaki, S.; Mori, Y.; Miyoshi, K.; Han, S.; Shikada, S.; Takamura, H.; Yoshimura, T.; Katayama, T. SCYL1 arginine methylation by PRMT1 is essential for neurite outgrowth via Golgi morphogenesis. Mol. Biol. Cell 2020, 31. [Google Scholar] [CrossRef]
- Malbeteau, L.; Poulard, C.; Languilaire, C.; Mikaelian, I.; Flamant, F.; Le Romancer, M.; Corbo, L. PRMT1 Is Critical for the Transcriptional Activity and the Stability of the Progesterone Receptor. iScience 2020, 23, 101236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; O’Neil, M.; Schonfeld, M.; Komatz, A.; Weinman, S.A.; Tikhanovich, I. Hepatocellular Protein Arginine Methyltransferase 1 Suppresses Alcohol-Induced Hepatocellular Carcinoma Formation by Inhibition of Inducible Nitric Oxide Synthase. Hepatol. Commun. 2020, 4, 790–808. [Google Scholar] [CrossRef]
- Hua, Z.Y.; Hansen, J.N.; He, M.; Dai, S.K.; Choi, Y.; Fulton, M.D.; Lloyd, S.M.; Szemes, M.; Sen, J.; Ding, H.F.; et al. PRMT1 promotes neuroblastoma cell survival through ATF5. Oncogenesis 2020, 9, 50. [Google Scholar] [CrossRef]
- Song, C.; Chen, T.; He, L.; Ma, N.; Li, J.A.; Rong, Y.F.; Fang, Y.; Liu, M.; Xie, D.; Lou, W. Author Correction: PRMT1 promotes pancreatic cancer growth and predicts poor prognosis. Cell. Oncol. (Dordr.) 2020, 43, 63–64. [Google Scholar] [CrossRef]
- Wang, S.; Tan, X.; Yang, B.; Yin, B.; Yuan, J.; Qiang, B.; Peng, X. The role of protein arginine-methyltransferase 1 in gliomagenesis. BMB Rep. 2012, 45, 470–475. [Google Scholar] [CrossRef]
- Ganesh, L.; Yoshimoto, T.; Moorthy, N.C.; Akahata, W.; Boehm, M.; Nabel, E.G.; Nabel, G.J. Protein methyltransferase 2 inhibits NF-kappaB function and promotes apoptosis. Mol. Cell. Biol. 2006, 26, 3864–3874. [Google Scholar] [CrossRef]
- Vhuiyan, M.I.; Pak, M.L.; Park, M.A.; Thomas, D.; Lakowski, T.M.; Chalfant, C.E.; Frankel, A. PRMT2 interacts with splicing factors and regulates the alternative splicing of BCL-X. J. Biochem. 2017, 162, 17–25. [Google Scholar] [CrossRef]
- Hou, W.; Nemitz, S.; Schopper, S.; Nielsen, M.L.; Kessels, M.M.; Qualmann, B. Arginine Methylation by PRMT2 Controls the Functions of the Actin Nucleator Cobl. Dev. Cell 2018, 45, 262–275.e8. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yan, C.; Xie, P.; Cao, Y.; Shao, J.; Ge, J. PRMT2 accelerates tumorigenesis of hepatocellular carcinoma by activating Bcl2 via histone H3R8 methylation. Exp. Cell Res. 2020, 394, 112152. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Cao, R.X.; Zu, X.Y.; Hong, T.; Yang, J.; Liu, L.; Xiao, X.H.; Ding, W.J.; Zhao, Q.; Liu, J.H.; et al. Identification and characterization of novel spliced variants of PRMT2 in breast carcinoma. FEBS J. 2012, 279, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Kaniskan, H.; Eram, M.S.; Zhao, K.; Szewczyk, M.M.; Yang, X.; Schmidt, K.; Luo, X.; Xiao, S.; Dai, M.; He, F.; et al. Discovery of Potent and Selective Allosteric Inhibitors of Protein Arginine Methyltransferase 3 (PRMT3). J. Med. Chem. 2018, 61, 1204–1217. [Google Scholar] [CrossRef]
- Miyata, S.; Mori, Y.; Tohyama, M. PRMT3 is essential for dendritic spine maturation in rat hippocampal neurons. Brain Res. 2010, 1352, 11–20. [Google Scholar] [CrossRef]
- Landry-Voyer, A.M.; Bilodeau, S.; Bergeron, D.; Dionne, K.L.; Port, S.A.; Rouleau, C.; Boisvert, F.M.; Kehlenbach, R.H.; Bachand, F. Human PDCD2L Is an Export Substrate of CRM1 That Associates with 40S Ribosomal Subunit Precursors. Mol. Cell. Biol. 2016, 36, 3019–3032. [Google Scholar] [CrossRef]
- Ikenaka, K.; Miyata, S.; Mori, Y.; Koyama, Y.; Taneda, T.; Okuda, H.; Kousaka, A.; Tohyama, M. Immunohistochemical and western analyses of protein arginine N-methyltransferase 3 in the mouse brain. Neuroscience 2006, 141, 1971–1982. [Google Scholar] [CrossRef]
- Xiao, P.F.; Tao, Y.F.; Hu, S.Y.; Cao, L.; Lu, J.; Wang, J.; Feng, X.; Pan, J.; Chai, Y.H. mRNA expression profiling of histone modifying enzymes in pediatric acute monoblastic leukemia. Pharmazie 2017, 72, 177–186. [Google Scholar] [CrossRef]
- Hsu, M.C.; Tsai, Y.L.; Lin, C.H.; Pan, M.R.; Shan, Y.S.; Cheng, T.Y.; Cheng, S.H.; Chen, L.T.; Hung, W.C. Protein arginine methyltransferase 3-induced metabolic reprogramming is a vulnerable target of pancreatic cancer. J. Hematol. Oncol. 2019, 12, 79. [Google Scholar] [CrossRef]
- Singh, V.; Miranda, T.B.; Jiang, W.; Frankel, A.; Roemer, M.E.; Robb, V.A.; Gutmann, D.H.; Herschman, H.R.; Clarke, S.; Newsham, I.F. DAL-1/4.1B tumor suppressor interacts with protein arginine N-methyltransferase 3 (PRMT3) and inhibits its ability to methylate substrates in vitro and in vivo. Oncogene 2004, 23, 7761–7771. [Google Scholar] [CrossRef]
- Sanchez, G.; Bondy-Chorney, E.; Laframboise, J.; Paris, G.; Didillon, A.; Jasmin, B.J.; Côté, J. A novel role for CARM1 in promoting nonsense-mediated mRNA decay: Potential implications for spinal muscular atrophy. Nucleic Acids Res. 2016, 44, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, J.; Xu, C.; Wang, Y.; Sun, L.; Guo, X.; Liu, H. MicroRNA-181 regulates CARM1 and histone arginine methylation to promote differentiation of human embryonic stem cells. PLoS ONE 2013, 8, e53146. [Google Scholar] [CrossRef]
- Lim, C.S.; Alkon, D.L. Inhibition of coactivator-associated arginine methyltransferase 1 modulates dendritic arborization and spine maturation of cultured hippocampal neurons. J. Biol. Chem. 2017, 292, 6402–6413. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Dury, A.Y.; Murray, L.M.; Biondi, O.; Tadesse, H.; El Fatimy, R.; Kothary, R.; Charbonnier, F.; Khandjian, E.W.; Côté, J. A novel function for the survival motoneuron protein as a translational regulator. Hum. Mol. Genet. 2013, 22, 668–684. [Google Scholar] [CrossRef] [PubMed]
- Hubers, L.; Valderrama-Carvajal, H.; Laframboise, J.; Timbers, J.; Sanchez, G.; Côté, J. HuD interacts with survival motor neuron protein and can rescue spinal muscular atrophy-like neuronal defects. Hum. Mol. Genet. 2011, 20, 553–579. [Google Scholar] [CrossRef] [PubMed]
- Selvi, B.R.; Swaminathan, A.; Maheshwari, U.; Nagabhushana, A.; Mishra, R.K.; Kundu, T.K. CARM1 regulates astroglial lineage through transcriptional regulation of Nanog and posttranscriptional regulation by miR92a. Mol. Biol. Cell 2015, 26, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Ke, X.; Peng, W.; Zhao, G.; Peng, S.; Xu, J.; Xu, B.; Cui, H. WDR5-Myc axis promotes the progression of glioblastoma and neuroblastoma by transcriptional activating CARM1. Biochem. Biophys. Res. Commun. 2020, 523, 699–706. [Google Scholar] [CrossRef]
- Stein, C.; Nötzold, R.R.; Riedl, S.; Bouchard, C.; Bauer, U.M. The Arginine Methyltransferase PRMT6 Cooperates with Polycomb Proteins in Regulating HOXA Gene Expression. PLoS ONE 2016, 11, e0148892. [Google Scholar] [CrossRef]
- Scaramuzzino, C.; Casci, I.; Parodi, S.; Lievens, P.M.J.; Polanco, M.J.; Milioto, C.; Chivet, M.; Monaghan, J.; Mishra, A.; Badders, N.; et al. Protein Arginine Methyltransferase 6 Enhances Polyglutamine-Expanded Androgen Receptor Function and Toxicity in Spinal and Bulbar Muscular Atrophy. Neuron 2015, 85, 88–100. [Google Scholar] [CrossRef]
- Park, S.W.; Jun, Y.W.; Choi, H.E.; Lee, J.A.; Jang, D.J. Deciphering the molecular mechanisms underlying the plasma membrane targeting of PRMT8. BMB Rep. 2019, 52, 601–606. [Google Scholar] [CrossRef]
- Solari, C.; Echegaray, C.V.; Luzzani, C.; Cosentino, M.S.; Waisman, A.; Petrone, M.V.; Francia, M.; Sassone, A.; Canizo, J.; Sevlever, G.; et al. Protein arginine Methyltransferase 8 gene is expressed in pluripotent stem cells and its expression is modulated by the transcription factor Sox2. Biochem. Biophys. Res. Commun. 2016, 473, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Simandi, Z.; Czipa, E.; Horvath, A.; Koszeghy, A.; Bordas, C.; Póliska, S.; Juhász, I.; Imre, L.; Szabó, G.; Dezso, B.; et al. PRMT1 and PRMT8 Regulate Retinoic Acid-Dependent Neuronal Differentiation with Implications to Neuropathology. Stem Cells 2015, 33, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Park, K.E.; Ishida, J.; Kako, K.; Hamada, J.; Kani, S.; Takeuchi, M.; Namiki, K.; Fukui, H.; Fukuhara, S.; et al. PRMT8 as a phospholipase regulates Purkinje cell dendritic arborization and motor coordination. Sci. Adv. 2015, 1, e1500615. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fuhrmann, J.; Thompson, P.R. Protein arginine methyltransferase 5 catalyzes substrate dimethylation in a distributive fashion. Biochemistry 2014, 53, 7884–7892. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Luengo, J.I. Nucleoside protein arginine methyltransferase 5 (PRMT5) inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1264–1269. [Google Scholar] [CrossRef]
- Pesiridis, G.S.; Diamond, E.; Van Duyne, G.D. Role of pICLn in methylation of Sm proteins by PRMT5. J. Biol. Chem. 2009, 284, 21347–21359. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Gish, G.; Braunschweig, U.; Li, Y.; Ni, Z.; Schmitges, F.W.; Zhong, G.; Liu, K.; Li, W.; Moffat, J.; et al. SMN and symmetric arginine dimethylation of RNA polymerase II C-terminal domain control termination. Nature 2016, 529, 48–53. [Google Scholar] [CrossRef]
- Grunseich, C.; Wang, I.X.; Watts, J.A.; Burdick, J.T.; Guber, R.D.; Zhu, Z.; Bruzel, A.; Lanman, T.; Chen, K.; Schindler, A.B.; et al. Senataxin Mutation Reveals How R-Loops Promote Transcription by Blocking DNA Methylation at Gene Promoters. Mol. Cell 2018, 69, 426–437.e7. [Google Scholar] [CrossRef]
- Sims, R.J., 3rd; Rojas, L.A.; Beck, D.B.; Bonasio, R.; Schüller, R.; Drury, W.J., 3rd; Eick, D.; Reinberg, D. The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science 2011, 332, 99–103. [Google Scholar] [CrossRef]
- Chittka, A.; Nitarska, J.; Grazini, U.; Richardson, W.D. Transcription factor positive regulatory domain 4 (PRDM4) recruits protein arginine methyltransferase 5 (PRMT5) to mediate histone arginine methylation and control neural stem cell proliferation and differentiation. J. Biol. Chem. 2012, 287, 42995–43006. [Google Scholar] [CrossRef]
- Saha, K.; Adhikary, G.; Eckert, R.L. MEP50/PRMT5 Reduces Gene Expression by Histone Arginine Methylation and this Is Reversed by PKCδ/p38δ Signaling. J. Investig. Dermatol. 2016, 136, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Migliori, V.; Müller, J.; Phalke, S.; Low, D.; Bezzi, M.; Mok, W.C.; Sahu, S.K.; Gunaratne, J.; Capasso, P.; Bassi, C.; et al. Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance. Nat. Struct. Mol. Biol. 2012, 19, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, M.; Teo, S.X.; Muller, J.; Mok, W.C.; Sahu, S.K.; Vardy, L.A.; Bonday, Z.Q.; Guccione, E. Regulation of constitutive and alternative splicing by PRMT5 reveals a role for Mdm4 pre-mRNA in sensing defects in the spliceosomal machinery. Genes Dev. 2013, 27, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Banasavadi-Siddegowda, Y.K.; Welker, A.M.; An, M.; Yang, X.; Zhou, W.; Shi, G.; Imitola, J.; Li, C.; Hsu, S.; Wang, J.; et al. PRMT5 as a druggable target for glioblastoma therapy. Neuro-Oncology 2018, 20, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, R.; Zhang, W.; Yang, X.; Wheeler, C.G.; Friedman, G.K.; Province, P.; Ding, Q.; You, Z.; Fathallah-Shaykh, H.M.; et al. Expression of PRMT5 correlates with malignant grade in gliomas and plays a pivotal role in tumor growth in vitro. J. Neuro-Oncol. 2014, 118, 61–72. [Google Scholar] [CrossRef]
- Yan, F.; Alinari, L.; Lustberg, M.E.; Martin, L.K.; Cordero-Nieves, H.M.; Banasavadi-Siddegowda, Y.; Virk, S.; Barnholtz-Sloan, J.; Bell, E.H.; Wojton, J.; et al. Genetic validation of the protein arginine methyltransferase PRMT5 as a candidate therapeutic target in glioblastoma. Cancer Res. 2014, 74, 1752–1765. [Google Scholar] [CrossRef]
- Huang, J.; Vogel, G.; Yu, Z.; Almazan, G.; Richard, S. Type II arginine methyltransferase PRMT5 regulates gene expression of inhibitors of differentiation/DNA binding Id2 and Id4 during glial cell differentiation. J. Biol. Chem. 2011, 286, 44424–44432. [Google Scholar] [CrossRef]
- Yang, Y.; Hadjikyriacou, A.; Xia, Z.; Gayatri, S.; Kim, D.; Zurita-Lopez, C.; Kelly, R.; Guo, A.; Li, W.; Clarke, S.G.; et al. PRMT9 is a type II methyltransferase that methylates the splicing factor SAP145. Nat. Commun. 2015, 6, 6428. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Z.; Jin, S.; Xu, K.; Zhang, H.; Xu, J.; Sun, Q.; Wang, J.; Xu, J. PRMT9 promotes hepatocellular carcinoma invasion and metastasis via activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Sci. 2018, 109, 1414–1427. [Google Scholar] [CrossRef]
- Karkhanis, V.; Wang, L.; Tae, S.; Hu, Y.J.; Imbalzano, A.N.; Sif, S. Protein arginine methyltransferase 7 regulates cellular response to DNA damage by methylating promoter histones H2A and H4 of the polymerase δ catalytic subunit gene, POLD1. J. Biol. Chem. 2012, 287, 29801–29814. [Google Scholar] [CrossRef]
- Szewczyk, M.M.; Ishikawa, Y.; Organ, S.; Sakai, N.; Li, F.; Halabelian, L.; Ackloo, S.; Couzens, A.L.; Eram, M.; Dilworth, D.; et al. Pharmacological inhibition of PRMT7 links arginine monomethylation to the cellular stress response. Nat. Commun. 2020, 11, 2396. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Vuong, T.A.; So, H.K.; Kim, H.J.; Bin Kim, Y.; Kang, J.S.; Kwon, I.; Cho, H. PRMT7 deficiency causes dysregulation of the HCN channels in the CA1 pyramidal cells and impairment of social behaviors. Exp. Mol. Med. 2020, 52, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wan, L.; Zou, H.; Pan, Z.; Zhou, W.; Lu, X. PRMT7 promotes the growth of renal cell carcinoma through modulating the β-catenin/C-MYC axis. Int. J. Biochem. Cell Biol. 2020, 120, 105686. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, R.; Yosha-Orpaz, N.; Yanoov-Sharav, M.; Kidron, D.; Gur, H.; Yosovich, K.; Lerman-Sagie, T.; Malinger, G.; Lev, D. Prenatal and postnatal presentation of PRMT7 related syndrome: Expanding the phenotypic manifestations. Am. J. Med. Genet. A 2019, 179, 78–84. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.; Zhao, L.; Li, J.; Yang, H.; Zhu, Z.; Liu, J.; Huang, G. Arginine Methylation of SREBP1a via PRMT5 Promotes De Novo Lipogenesis and Tumor Growth. Cancer Res. 2016, 76, 1260–1272. [Google Scholar] [CrossRef]
- Esparza-Moltó, P.B.; Cuezva, J.M. The Role of Mitochondrial H(+)-ATP Synthase in Cancer. Front. Oncol. 2018, 8, 53. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Zhao, L.; Liu, L.; Li, J.; Jia, W.; Liu, J.; Huang, G. PRMT5 competitively binds to CDK4 to promote G1-S transition upon glucose induction in hepatocellular carcinoma. Oncotarget 2016, 7, 72131–72147. [Google Scholar] [CrossRef]
- Tsai, W.W.; Niessen, S.; Goebel, N.; Yates, J.R., 3rd; Guccione, E.; Montminy, M. PRMT5 modulates the metabolic response to fasting signals. Proc. Natl. Acad. Sci. USA 2013, 110, 8870–8875. [Google Scholar] [CrossRef]
- Koh, C.M.; Bezzi, M.; Low, D.H.; Ang, W.X.; Teo, S.X.; Gay, F.P.; Al-Haddawi, M.; Tan, S.Y.; Osato, M.; Sabò, A.; et al. MYC regulates the core pre-mRNA splicing machinery as an essential step in lymphomagenesis. Nature 2015, 523, 96–100. [Google Scholar] [CrossRef]
- Tarighat, S.S.; Santhanam, R.; Frankhouser, D.; Radomska, H.S.; Lai, H.; Anghelina, M.; Wang, H.; Huang, X.; Alinari, L.; Walker, A.; et al. The dual epigenetic role of PRMT5 in acute myeloid leukemia: Gene activation and repression via histone arginine methylation. Leukemia 2016, 30, 789–799. [Google Scholar] [CrossRef]
- Lattouf, H.; Poulard, C.; Le Romancer, M. PRMT5 prognostic value in cancer. Oncotarget 2019, 10, 3151–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ronai, Z.A. PRMT5 function and targeting in cancer. Cell Stress 2020, 4, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Shimizu, D.; Fujii, T.; Tanaka, H.; Shibata, M.; Iwata, N.; Hayashi, M.; Kobayashi, D.; Tanaka, C.; Yamada, S.; et al. Protein arginine methyltransferase 5 is associated with malignant phenotype and peritoneal metastasis in gastric cancer. Int. J. Oncol. 2016, 49, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Shailesh, H.; Zakaria, Z.Z.; Baiocchi, R.; Sif, S. Protein arginine methyltransferase 5 (PRMT5) dysregulation in cancer. Oncotarget 2018, 9, 36705–36718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, S.; Zhu, R.; Hu, C.; Hou, J.; Li, Y.; Zhao, Q.; Shao, X.; Bu, Q.; Li, H.; et al. Targeting protein arginine methyltransferase 5 inhibits colorectal cancer growth by decreasing arginine methylation of eIF4E and FGFR3. Oncotarget 2015, 6, 22799–22811. [Google Scholar] [CrossRef]
- Liu, M.; Yao, B.; Gui, T.; Guo, C.; Wu, X.; Li, J.; Ma, L.; Deng, Y.; Xu, P.; Wang, Y.; et al. PRMT5-dependent transcriptional repression of c-Myc target genes promotes gastric cancer progression. Theranostics 2020, 10, 4437–4452. [Google Scholar] [CrossRef]
- Avasarala, S.; Van Scoyk, M.; Karuppusamy Rathinam, M.K.; Zerayesus, S.; Zhao, X.; Zhang, W.; Pergande, M.R.; Borgia, J.A.; DeGregori, J.; Port, J.D.; et al. PRMT1 Is a Novel Regulator of Epithelial-Mesenchymal-Transition in Non-small Cell Lung Cancer. J. Biol. Chem. 2015, 290, 13479–13489. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Meyer, M.B.; Saha, S.; Yu, M.; Guo, A.; Wisinski, K.B.; Huang, W.; Cai, W.; Pike, J.W.; et al. CARM1 methylates chromatin remodeling factor BAF155 to enhance tumor progression and metastasis. Cancer Cell 2014, 25, 21–36. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, Y.; Liu, X.; Zhang, N.; Liu, Y.; Liu, X.; Lin, C.; Yan, X.; Li, Z.; Wang, G.; et al. Automethylation of protein arginine methyltransferase 7 and its impact on breast cancer progression. FASEB J. 2017, 31, 2287–2300. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, J.Y.; Ha, S.J.; Yu, S.; Shin, J.K.; Kim, H.C. Proteome-wide identification of arginine methylation in colorectal cancer tissues from patients. Proteome Sci. 2020, 18, 6. [Google Scholar] [CrossRef]
- Bednarz-Misa, I.; Fortuna, P.; Fleszar, M.G.; Lewandowski, Ł.; Diakowska, D.; Rosińczuk, J.; Krzystek-Korpacka, M. Esophageal Squamous Cell Carcinoma Is Accompanied by Local and Systemic Changes in L-arginine/NO Pathway. Int. J. Mol. Sci. 2020, 21, 6282. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Matsubara, D.; Yoshimoto, T.; Tamura, T.; Nishino, H.; Mori, Y.; Niki, T. Expression of protein arginine methyltransferase-5 in oral squamous cell carcinoma and its significance in epithelial-to-mesenchymal transition. Pathol. Int. 2018, 68, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, A.; Hansen, J.N.; Koster, J.; Lotta, L.T., Jr.; Wang, S.; Livingstone, E.; Qian, K.; Valentijn, L.J.; Zheng, Y.G.; Schor, N.F.; et al. Protein arginine methyltransferase 1 is a novel regulator of MYCN in neuroblastoma. Oncotarget 2016, 7, 63629–63639. [Google Scholar] [CrossRef] [PubMed]
- Dzieran, J.; Rodriguez Garcia, A.; Westermark, U.K.; Henley, A.B.; Eyre Sánchez, E.; Träger, C.; Johansson, H.J.; Lehtiö, J.; Arsenian-Henriksson, M. MYCN-amplified neuroblastoma maintains an aggressive and undifferentiated phenotype by deregulation of estrogen and NGF signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E1229–E1238. [Google Scholar] [CrossRef]
- Reintjes, A.; Fuchs, J.E.; Kremser, L.; Lindner, H.H.; Liedl, K.R.; Huber, L.A.; Valovka, T. Asymmetric arginine dimethylation of RelA provides a repressive mark to modulate TNFα/NF-κB response. Proc. Natl. Acad. Sci. USA 2016, 113, 4326–4331. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.; Durant, S.T.; Cho, E.-C.; Sheahan, S.; Edelmann, M.; Kessler, B.; La Thangue, N.B. Arginine methylation regulates the p53 response. Nat. Cell Biol. 2008, 10, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Cai, X.L.; Li, Z.Z.; Lv, J.; Xiang, Y.; Chen, J.J.; Chen, W.J.; Sun, W.Y.; Liu, X.M.; Chen, J.B. LncRNA SNHG16 Functions as an Oncogene by Sponging MiR-4518 and Up-Regulating PRMT5 Expression in Glioma. Cell. Physiol. Biochem. 2018, 45, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, K.; Wu, W.; Zhou, Y.; Zhu, J.; Wu, G.; Cao, L.; Zhang, X.; Guan, H.; Yang, Y.; et al. Overexpression of HOXC10 promotes angiogenesis in human glioma via interaction with PRMT5 and upregulation of VEGFA expression. Theranostics 2018, 8, 5143–5158. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, D.; Lin, F.; Wang, X.; Lu, L.; Luo, S.; Chen, J.; Xu, X. LncRNA NNT-AS1 promote glioma cell proliferation and metastases through miR-494-3p/PRMT1 axis. Cell Cycle 2020, 19, 1621–1631. [Google Scholar] [CrossRef]
- Chaturvedi, N.K.; Mahapatra, S.; Kesherwani, V.; Kling, M.J.; Shukla, M.; Ray, S.; Kanchan, R.; Perumal, N.; McGuire, T.R.; Sharp, J.G.; et al. Role of protein arginine methyltransferase 5 in group 3 (MYC-driven) Medulloblastoma. BMC Cancer 2019, 19, 1056. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, Y. Long noncoding RNA LINC00515 promotes cell proliferation and inhibits apoptosis by sponging miR-16 and activating PRMT5 expression in human glioma. Onco Targets 2019, 12, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.F.; Marsden, A.J.; Deepak, S.; Rivero, F.; Greenman, J.; Beltran-Alvarez, P. Inhibiting Arginine Methylation as a Tool to Investigate Cross-Talk with Methylation and Acetylation Post-Translational Modifications in a Glioblastoma Cell Line. Proteomes 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Dominko, T. Novel Protein Arginine Methyltransferase 8 Isoform Is Essential for Cell Proliferation. J. Cell Biochem. 2016, 117, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, T.; Strauss, M.; Hering, S.; Redanz, U.; William, D.; Rosche, Y.; Classen, C.F.; Kreikemeyer, B.; Linnebacher, M.; Maletzki, C. Arginine deprivation by arginine deiminase of Streptococcus pyogenes controls primary glioblastoma growth in vitro and in vivo. Cancer Biol. 2015, 16, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Tejera, D.; Kushnirsky, M.; Gultekin, S.H.; Lu, M.; Steelman, L.; de la Fuente, M.I. Ivosidenib, an IDH1 inhibitor, in a patient with recurrent, IDH1-mutant glioblastoma: A case report from a Phase I study. CNS Oncol. 2020, 9, CNS62. [Google Scholar] [CrossRef]
- Packer, R.J.; Macdonald, T.; Vezina, G.; Keating, R.; Santi, M. Medulloblastoma and primitive neuroectodermal tumors. Handb. Clin. Neurol. 2012, 105, 529–548. [Google Scholar] [CrossRef]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef]
- Cheng, D.; Yadav, N.; King, R.W.; Swanson, M.S.; Weinstein, E.J.; Bedford, M.T. Small molecule regulators of protein arginine methyltransferases. J. Biol. Chem. 2004, 279, 23892–23899. [Google Scholar] [CrossRef]
- Hu, H.; Qian, K.; Ho, M.C.; Zheng, Y.G. Small Molecule Inhibitors of Protein Arginine Methyltransferases. Expert Opin. Investig. Drugs 2016, 25, 335–358. [Google Scholar] [CrossRef]
- Yan, L.; Yan, C.; Qian, K.; Su, H.; Kofsky-Wofford, S.A.; Lee, W.C.; Zhao, X.; Ho, M.C.; Ivanov, I.; Zheng, Y.G. Diamidine compounds for selective inhibition of protein arginine methyltransferase 1. J. Med. Chem. 2014, 57, 2611–2622. [Google Scholar] [CrossRef]
- Smith, E.; Zhou, W.; Shindiapina, P.; Sif, S.; Li, C.; Baiocchi, R.A. Recent advances in targeting protein arginine methyltransferase enzymes in cancer therapy. Expert Opin. Targets 2018, 22, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ouyang, Y.; Ma, H.; Cong, H.; Zhuang, C.; Lok, W.T.; Wang, Z.; Zhu, X.; Sun, Y.; Hong, W.; et al. Design and synthesis of novel PRMT1 inhibitors and investigation of their binding preferences using molecular modelling. Bioorg. Med. Chem. Lett. 2017, 27, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.N.; Zhou, M.; Sun, F.; Huai, M.X.; Zhang, Y.; Qu, C.Y.; Shen, F.; Xu, L.M. Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression. World J. Gastroenterol. 2020, 26, 3737–3749. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, E.M.; Heinke, R.; Spannhoff, A.; Eberlin, A.; Metzger, E.; Cura, V.; Hassenboehler, P.; Cavarelli, J.; Schüle, R.; Bedford, M.T.; et al. Acyl derivatives of p-aminosulfonamides and dapsone as new inhibitors of the arginine methyltransferase hPRMT1. Bioorg. Med. Chem 2011, 19, 3717–3731. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Z.; Yang, H.; Cao, D.; Xu, X.; Zheng, X.; Chen, D.; Wang, Q.; Li, Y.; Li, J.; et al. Design and Synthesis of Potent, Selective Inhibitors of Protein Arginine Methyltransferase 4 against Acute Myeloid Leukemia. J. Med. Chem. 2019, 62, 5414–5433. [Google Scholar] [CrossRef]
- Chan-Penebre, E.; Kuplast, K.G.; Majer, C.R.; Boriack-Sjodin, P.A.; Wigle, T.J.; Johnston, L.D.; Rioux, N.; Munchhof, M.J.; Jin, L.; Jacques, S.L.; et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat. Chem. Biol. 2015, 11, 432–437. [Google Scholar] [CrossRef]
- Watts, J.M.; Bradley, T.J.; Thomassen, A.; Brunner, A.M.; Minden, M.D.; Papadantonakis, N.; Abedin, S.; Baines, A.J.; Barbash, O.; Gorman, S.; et al. A Phase I/II Study to Investigate the Safety and Clinical Activity of the Protein Arginine Methyltransferase 5 Inhibitor GSK3326595 in Subjects with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Blood 2019, 134, 2656. [Google Scholar] [CrossRef]
- Siarheyeva, A.; Senisterra, G.; Allali-Hassani, A.; Dong, A.; Dobrovetsky, E.; Wasney, G.A.; Chau, I.; Marcellus, R.; Hajian, T.; Liu, F.; et al. An allosteric inhibitor of protein arginine methyltransferase 3. Structure 2012, 20, 1425–1435. [Google Scholar] [CrossRef]
- Nahon, J.E.; Groeneveldt, C.; Geerling, J.J.; van Eck, M.; Hoekstra, M. Inhibition of protein arginine methyltransferase 3 activity selectively impairs liver X receptor-driven transcription of hepatic lipogenic genes in vivo. Br. J. Pharm. 2018, 175, 3175–3183. [Google Scholar] [CrossRef]
- Ferreira de Freitas, R.; Eram, M.S.; Smil, D.; Szewczyk, M.M.; Kennedy, S.; Brown, P.J.; Santhakumar, V.; Barsyte-Lovejoy, D.; Arrowsmith, C.H.; Vedadi, M.; et al. Discovery of a Potent and Selective Coactivator Associated Arginine Methyltransferase 1 (CARM1) Inhibitor by Virtual Screening. J. Med. Chem. 2016, 59, 6838–6847. [Google Scholar] [CrossRef]
- Sack, J.S.; Thieffine, S.; Bandiera, T.; Fasolini, M.; Duke, G.J.; Jayaraman, L.; Kish, K.F.; Klei, H.E.; Purandare, A.V.; Rosettani, P.; et al. Structural basis for CARM1 inhibition by indole and pyrazole inhibitors. Biochem. J. 2011, 436, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.H.; Drew, A.E.; Ribich, S.A.; Rioux, N.; Swinger, K.K.; Jacques, S.L.; Lingaraj, T.; Boriack-Sjodin, P.A.; Waters, N.J.; Wigle, T.J.; et al. Aryl Pyrazoles as Potent Inhibitors of Arginine Methyltransferases: Identification of the First PRMT6 Tool Compound. ACS Med. Chem. Lett. 2015, 6, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, W.; Eram, M.S.; Vhuiyan, M.; Dong, A.; Zeng, H.; He, H.; Brown, P.; Frankel, A.; Vedadi, M.; et al. Structural basis of arginine asymmetrical dimethylation by PRMT6. Biochem. J. 2016, 473, 3049–3063. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Szewczyk, M.M.; Eram, M.S.; Smil, D.; Kaniskan, H.; de Freitas, R.F.; Senisterra, G.; Li, F.; Schapira, M.; Brown, P.J.; et al. Discovery of a Potent, Selective, and Cell-Active Dual Inhibitor of Protein Arginine Methyltransferase 4 and Protein Arginine Methyltransferase 6. J. Med. Chem. 2016, 59, 9124–9139. [Google Scholar] [CrossRef]
- Mounir, Z.; Korn, J.M.; Westerling, T.; Lin, F.; Kirby, C.A.; Schirle, M.; McAllister, G.; Hoffman, G.; Ramadan, N.; Hartung, A.; et al. ERG signaling in prostate cancer is driven through PRMT5-dependent methylation of the Androgen Receptor. eLife 2016, 5. [Google Scholar] [CrossRef]
- Alinari, L.; Mahasenan, K.V.; Yan, F.; Karkhanis, V.; Chung, J.-H.; Smith, E.M.; Quinion, C.; Smith, P.L.; Kim, L.; Patton, J.T.; et al. Selective inhibition of protein arginine methyltransferase 5 blocks initiation and maintenance of B-cell transformation. Blood 2015, 125, 2530–2543. [Google Scholar] [CrossRef]
- Zhu, K.; Tao, H.; Song, J.-L.; Jin, L.; Zhang, Y.; Liu, J.; Chen, Z.; Jiang, C.-S.; Luo, C.; Zhang, H. Identification of 5-benzylidene-2-phenylthiazolones as potent PRMT5 inhibitors by virtual screening, structural optimization and biological evaluations. Bioorg. Chem. 2018, 81, 289–298. [Google Scholar] [CrossRef]
- Bonday, Z.Q.; Cortez, G.S.; Grogan, M.J.; Antonysamy, S.; Weichert, K.; Bocchinfuso, W.P.; Li, F.; Kennedy, S.; Li, B.; Mader, M.M.; et al. LLY-283, a Potent and Selective Inhibitor of Arginine Methyltransferase 5, PRMT5, with Antitumor Activity. ACS Med. Chem. Lett. 2018, 9, 612–617. [Google Scholar] [CrossRef]
- Smil, D.; Eram, M.S.; Li, F.; Kennedy, S.; Szewczyk, M.M.; Brown, P.J.; Barsyte-Lovejoy, D.; Arrowsmith, C.H.; Vedadi, M.; Schapira, M. Discovery of a Dual PRMT5-PRMT7 Inhibitor. ACS Med. Chem. Lett. 2015, 6, 408–412. [Google Scholar] [CrossRef]
| Brain Tumor Type | Investigators (Year) | Enzyme | Study Conclusions |
| Glioma | Wang et al. [39] (2012) | PRMT1 | PRMT1 was upregulated in glioma tissues compared to normal cortex tissue. PRMT1 knockdown resulted in G1-S arrest in four glioma cell lines. RNAi greatly reduced tumor growth in vivo. |
| Lu et al. [108] (2018) | PRMT5 | LncRNA SNHG16 knockdown inhibited glioma cell proliferation and induced apoptosis. SNHG16 up-regulated expression miR-4518 targeted gene PRMT5 via sponging of miR-4518. | |
| Tan et al. [109] (2018) | PRMT5 | PRMT5 was required for HOXC10-mediated upregulation of VEGFA. HOXC10 levels and VEGFA expression correlated significantly in human glioma. | |
| Zheng et al. [110] (2020) | PRMT1 | LncRNA NNT-AS1 is significantly up-regulated during the early stages of glioma in vitro. Inhibition of NNT-AS1 led to positive regulation of PRMT1 via miRNA-494-3p. | |
| Glioblastoma | Yan et al. [77] (2014) | PRMT5 | PRMT5 attenuation limited recruitment to the promoter of tumor suppressor ST7. Chromatin immunoprecipitation and genetic profiling showed that the ST7 gene is silenced by PRMT5. PRMT5 overexpression in primary GBM and cell lines correlated positively with cell growth and inversely with overall survival. |
| Han et al. [76] (2014) | PRMT5 | Protein expression profiles revealed that PRMT5 expression was low in low grade glial cell controls and low grade astrocytomas. PRMT5 expression was high in GBM and increased in parallel with malignant progression. | |
| Mongiardi et al. [12] (2015) | PRMT5 | Myc and Omomyc stimulated PRMT5-mediated symmetric dimethylation of H4R3 in human GBM cells. Myc and Omomyc are consistently associated with PRMT5. PRMT5 interference impaired gene activation by Myc. | |
| Banasavadi-Siddegowda et al. [13] (2017) | PRMT5 | PRMT5 depletion caused senescence and apoptosis in the patient-derived primary stem-like cells and differentiated cells respectively. PRMT5 depletion stunted the tumor growth and increased the survival of mice in the intracranial GBM tumor model. | |
| Holmes et al. [16] (2019) | PRMT5 | PRMT5 inhibition by EPZ015666 and PP42 displayed synergistic effects in vitro and a mouse model. | |
| Medulloblastoma | Chaturvedi et al. [111] (2019) | PRMT5 | PRMT5 knockdown significantly decreased medulloblastoma cell growth. PRMT5 inhibition with EPZ015666 suppressed cell growth and induced apoptosis of myc-driven medulloblastoma cells in a dose-dependent manner. |
| Enzyme | Enzyme Target(s) | Inhibitor(s) | Pre-Clinical Testing [REF] | Clinical Trial |
| PRMT1 | H4R3, H2AR3 | Allantodapsone | Breast cancer [125] | |
| E84 | CML, AML | |||
| DB75 | CML, AML, APL [121] | |||
| PT1001B | Pancreatic cancer [124] | |||
| GSK3368715 | NCT03666988 | |||
| PRMT3 | Ribosomal protein-RPS2 | 7 SGC707 | ||
| PRMT4/CARM1 | H3R2, H3R17, H3R26 RNAP II | CMPD-1 CMPD-2 Compound 49 | ||
| SGC2085 TP-064 | AML [126] | |||
| TBBD | ||||
| PRMT5 | H3R2,H3R8, H4R3, H2AR3 Sm proteins Nuclear/cytoplasmic proteins RNAP II | CMP5 | GBM [75] | |
| EPZ015666 | NHL, MCL, GBM, Breast cancer, MM [127] | |||
| GSK3326595 | AML/MDS [128] | NCT02783300, NCT03614728 | ||
| JNJ64619178 | NCT03573310 | |||
| LLY-283 | ||||
| PF-06939999 | NCT03854227 | |||
| PRT811 | NCT04089449 | |||
| PRMT6 | H3R2, H2AR9, H4R3 | EPZ020411 6′-methyleneamine sinefungin (GMS) MS023 MS049 | ||
| PRMT7 | H4R3, H2AR3, H3R2 | DS-437 SGC3027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, J.-P.; Heiss, J.; Banasavadi-Siddegowda, Y.K. Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies. Cells 2021, 10, 124. https://doi.org/10.3390/cells10010124
Bryant J-P, Heiss J, Banasavadi-Siddegowda YK. Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies. Cells. 2021; 10(1):124. https://doi.org/10.3390/cells10010124
Chicago/Turabian StyleBryant, Jean-Paul, John Heiss, and Yeshavanth Kumar Banasavadi-Siddegowda. 2021. "Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies" Cells 10, no. 1: 124. https://doi.org/10.3390/cells10010124
APA StyleBryant, J.-P., Heiss, J., & Banasavadi-Siddegowda, Y. K. (2021). Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies. Cells, 10(1), 124. https://doi.org/10.3390/cells10010124





