The Effect of Abiotic Factors on Abundance and Photosynthetic Performance of Airborne Cyanobacteria and Microalgae Isolated from the Southern Baltic Sea Region
Abstract
1. Introduction
2. Results and Discussion
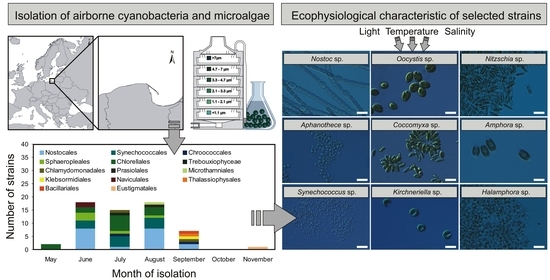
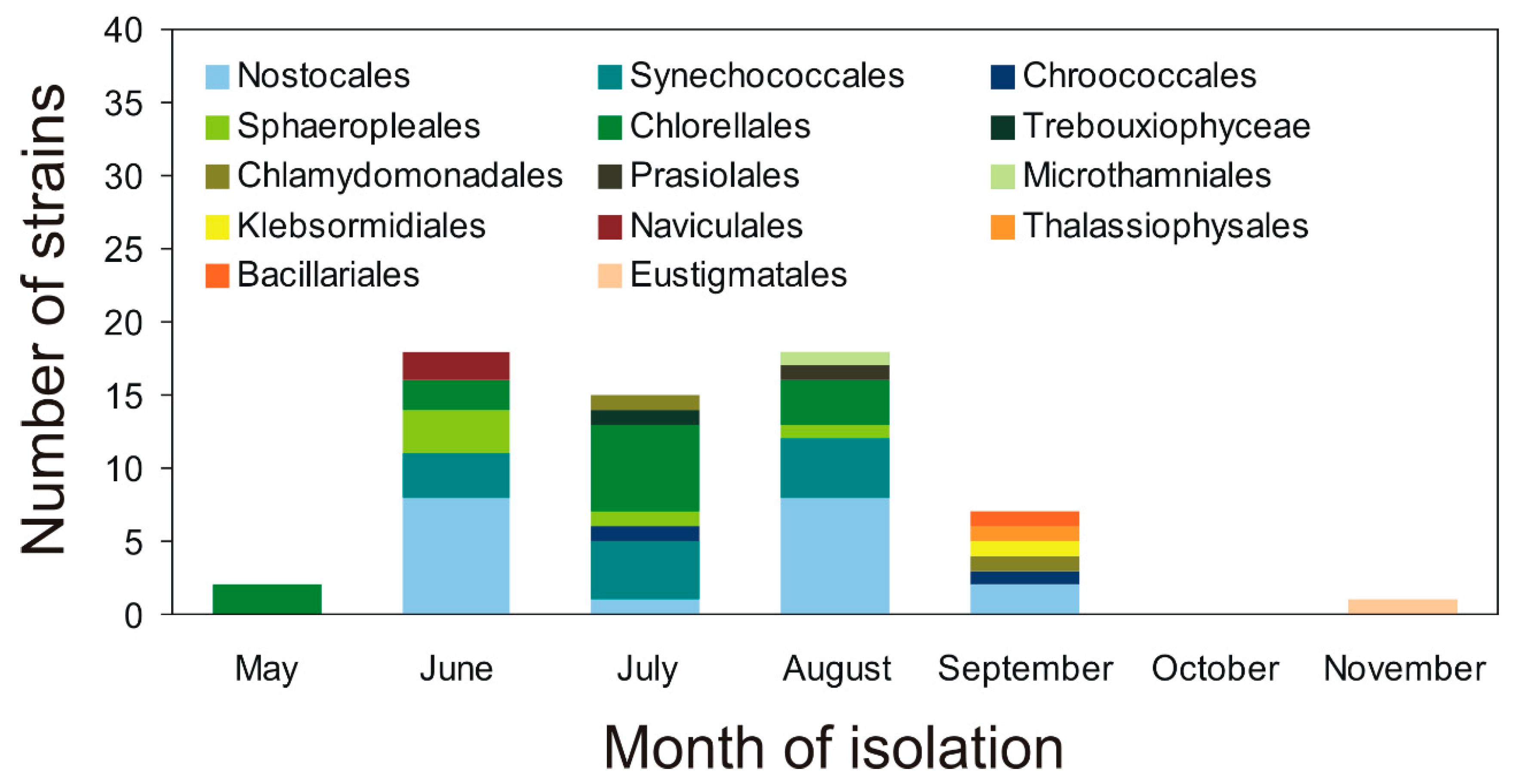
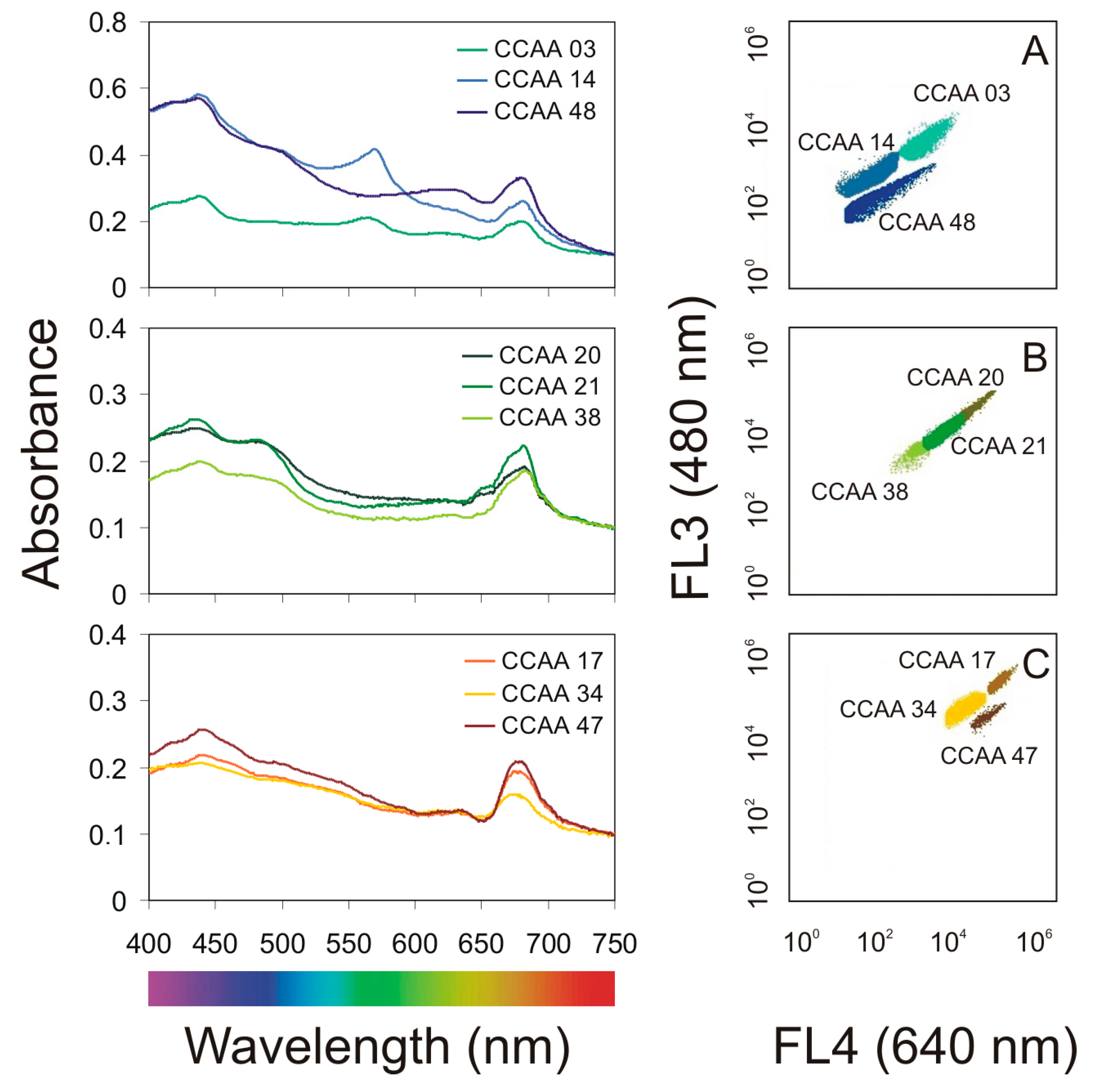
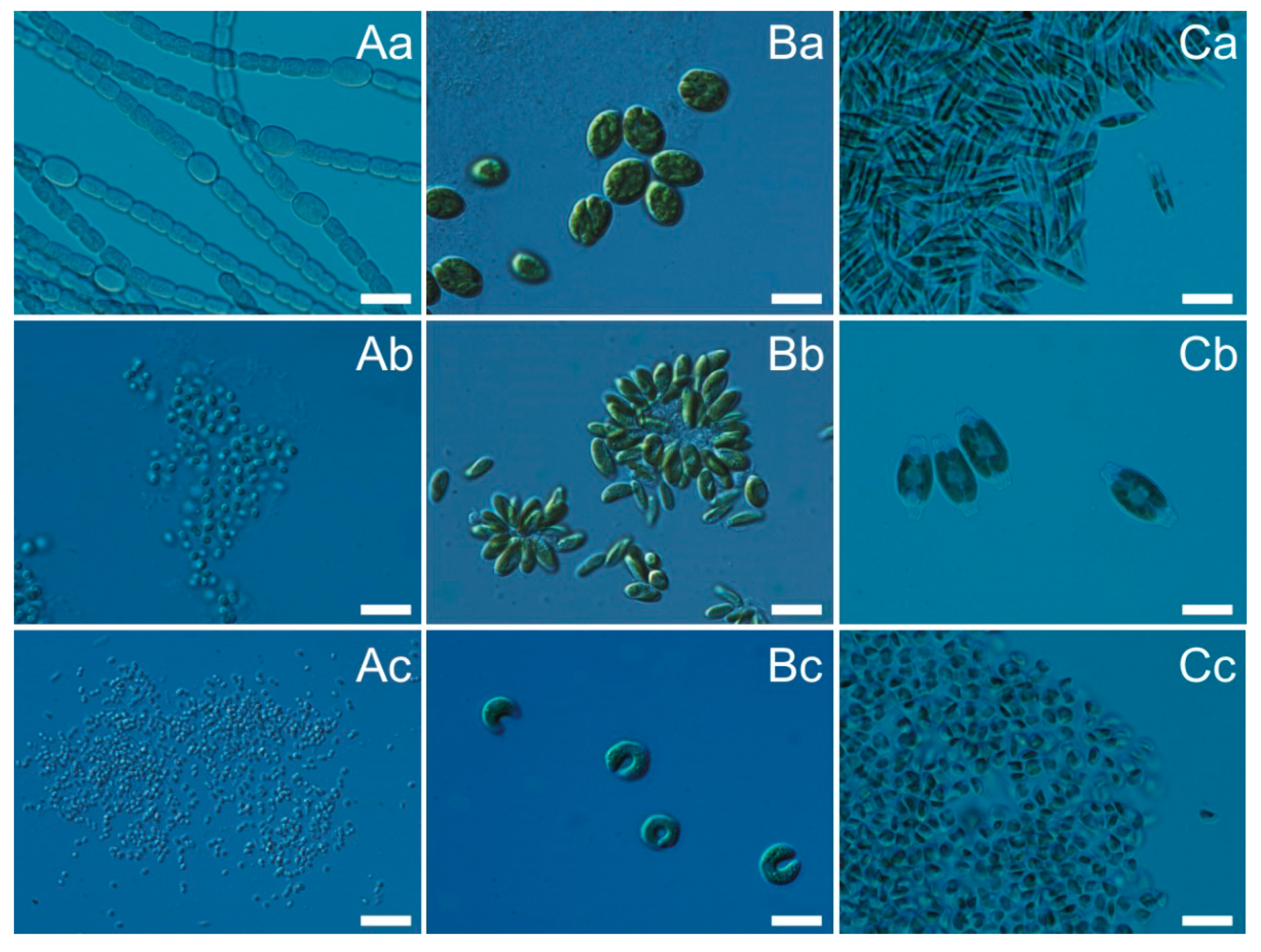
2.1. Characterization of Airborne Cyanobacteria and Microalgae Strains
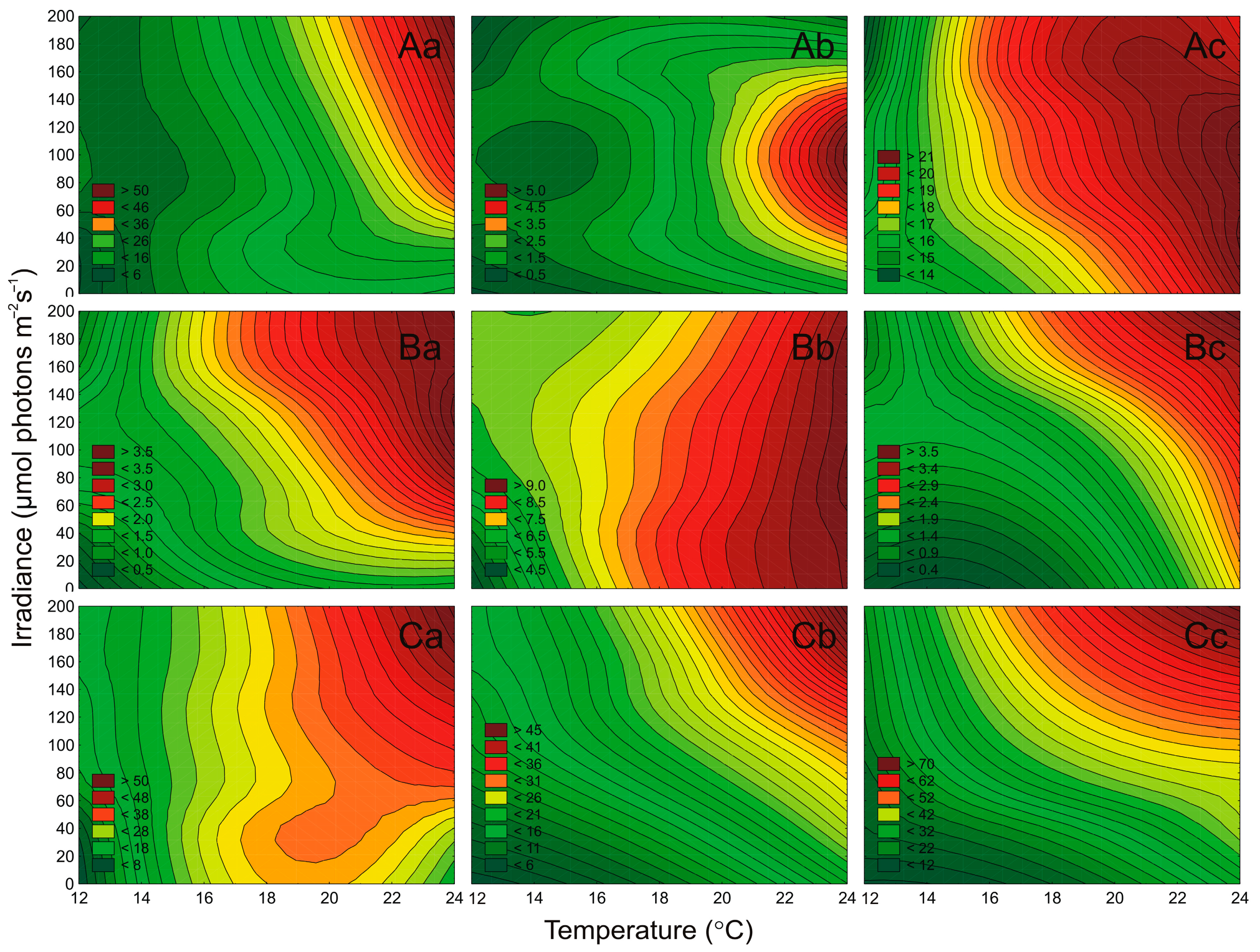
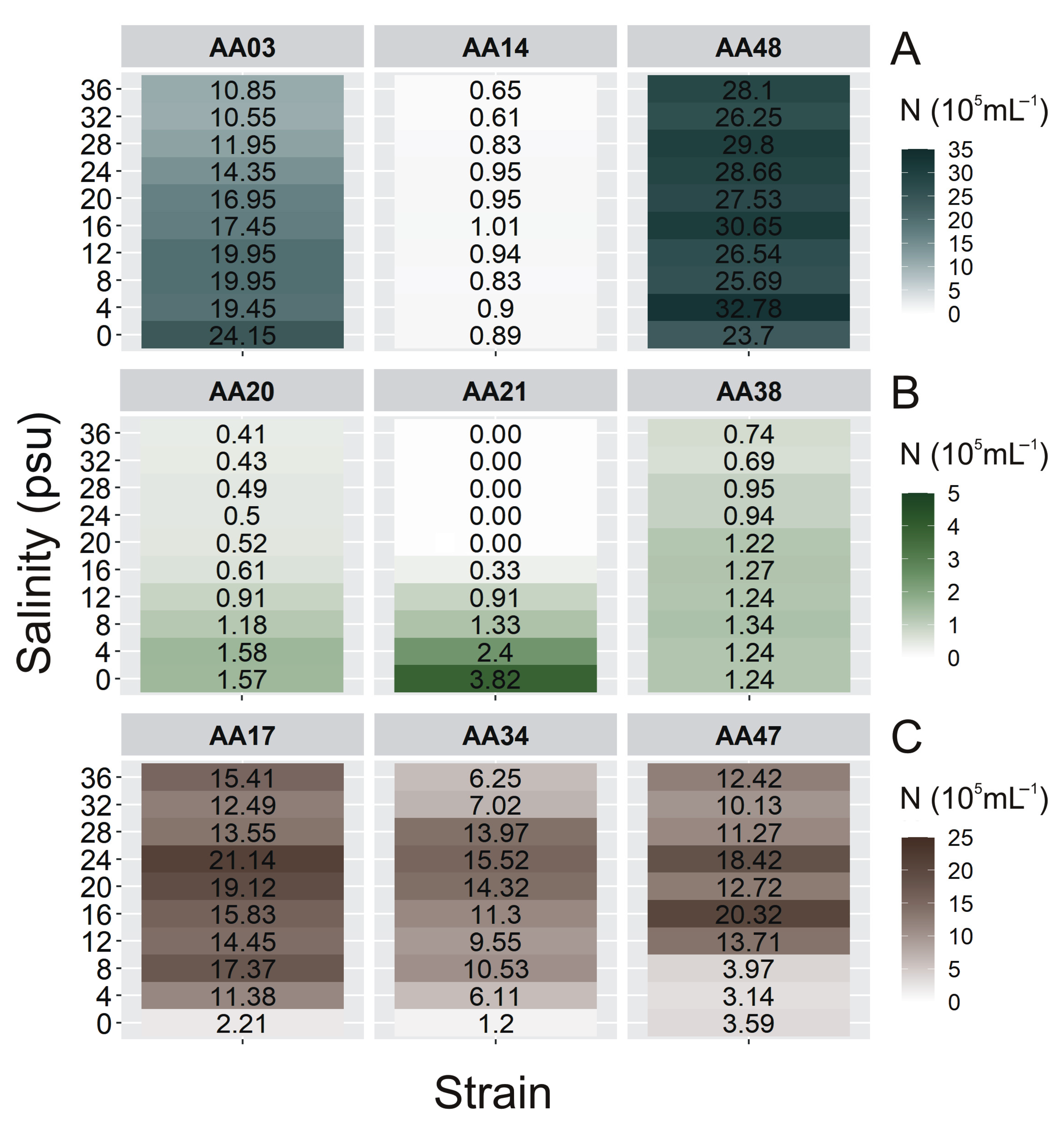
2.2. The Cell Concentration of Airborne Strains under Different Culture Conditions
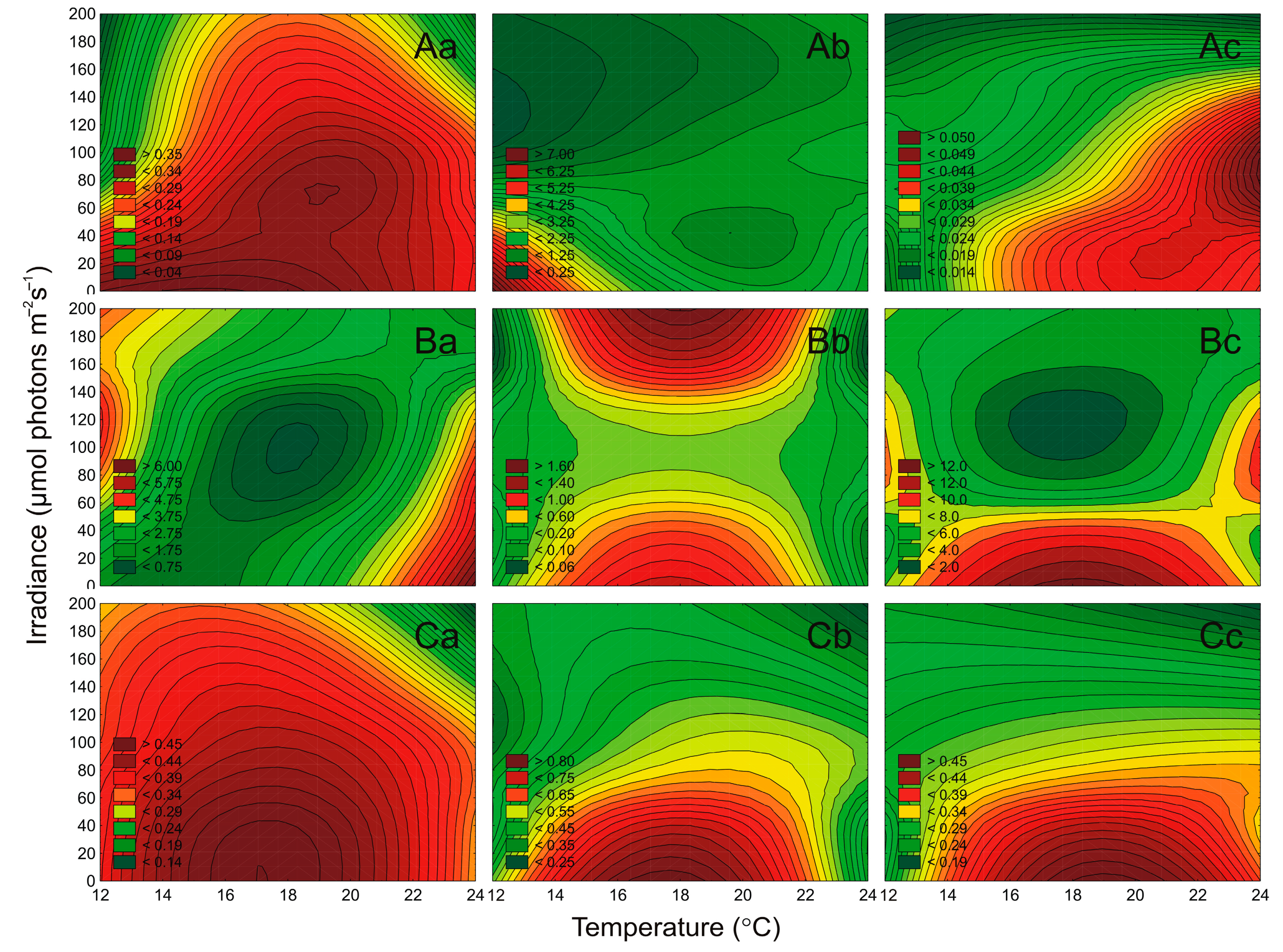
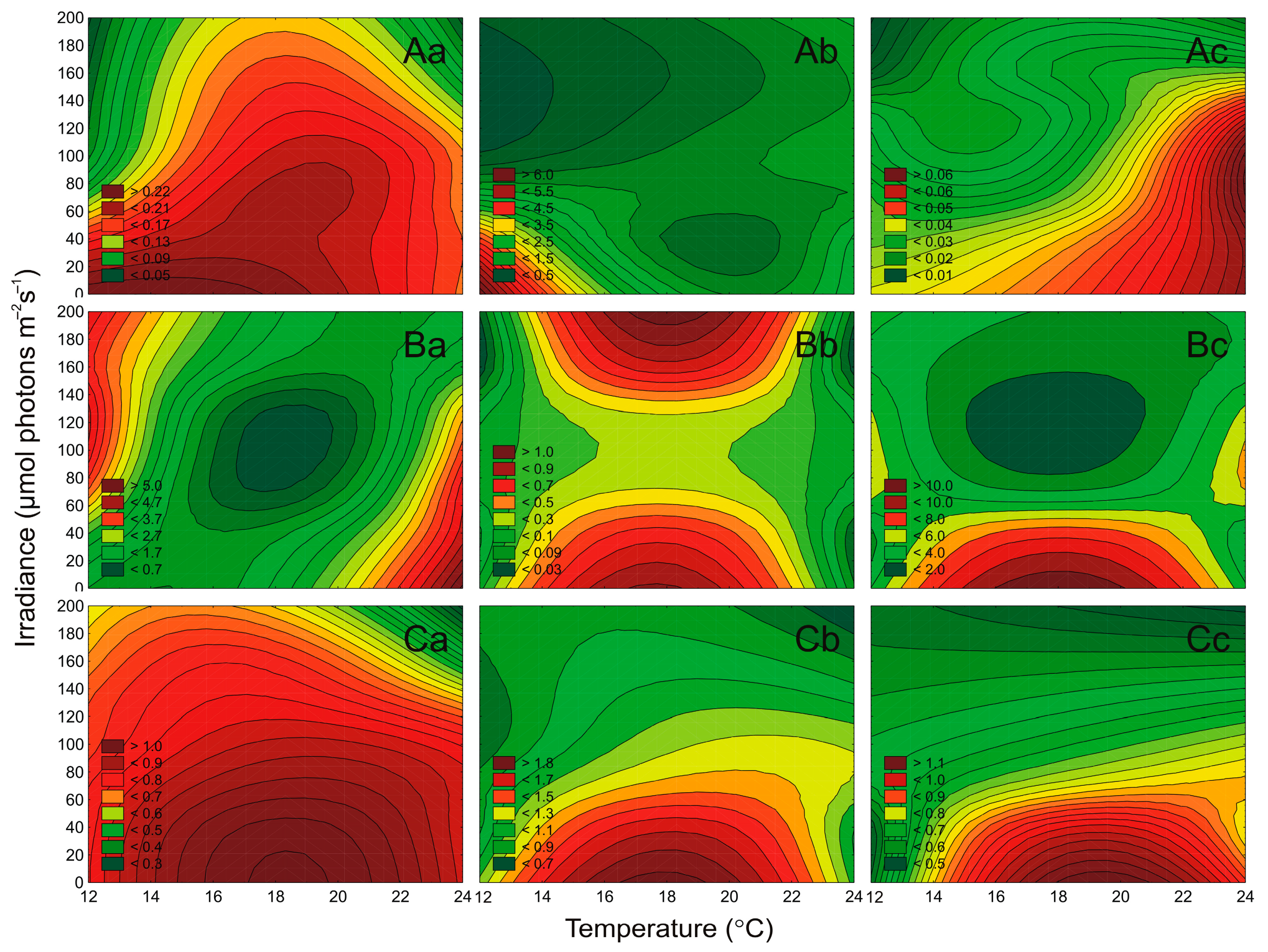
2.3. Effect of Irradiance and Temperature on Pigments Content
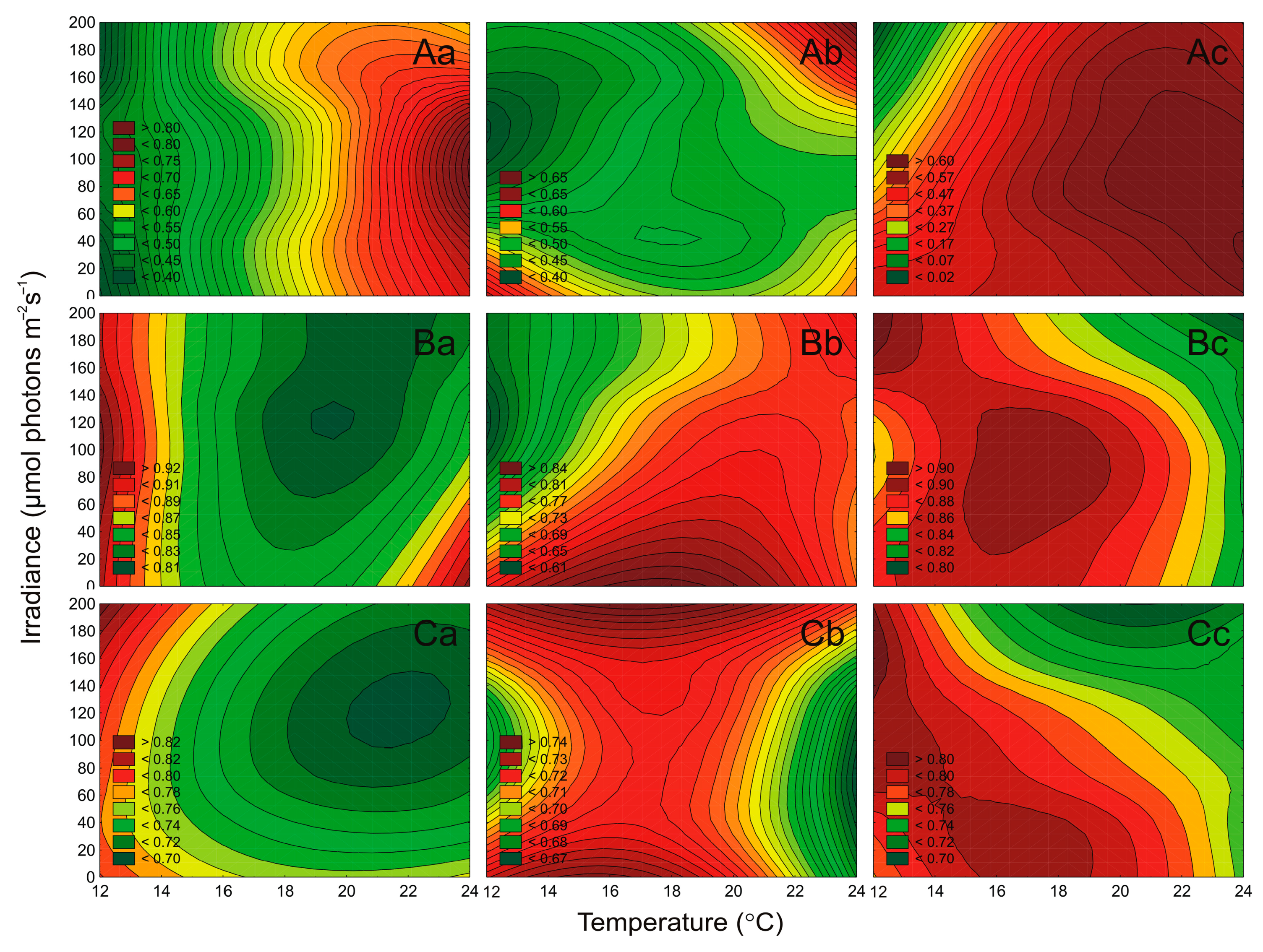
2.4. Effect of Irradiance and Temperature on the Maximum PSII Quantum Efficiency
3. Materials and Methods
3.1. Culture Conditions
3.2. Calculation of Cell Density
3.3. Air Masses Trajectories
3.4. Determination of the Chlorophyll, Carotenoids, and Phycobiliproteins Content
3.5. Determination of the Chlorophyll a Fluorescence
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Wozniczka, D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the southern Baltic Sea. Mar. Pollut. Bull. 2017, 125, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment–Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef]
- Després, V.R.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich- Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus Ser. B Chem. Phys. Meteorol. 2012, 64, 15598–15656. [Google Scholar] [CrossRef]
- Tesson, S.V.M.; Skjøth, C.A.; Santl-Temkiv, T.; Londahl, J. Airborne microalgae: Insights, opportunities, and challenges. Appl. Environ. Microbiol. 2016, 82, 1978–1991. [Google Scholar] [CrossRef]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef]
- Tesson, S.V.M.; Šantl-Temkiv, T. Ice nucleation activity and Aeolian dispersal success in airborne and aquatic microalgae. Front. Microbiol. 2018, 9, 2681. [Google Scholar] [CrossRef]
- Franck, U.; Herbarth, O.; Manjarrez, M.; Wiedensohler, A.; Tuch, T.; Holstein, P. Indoor and outdoor fine particles: Exposure and possible health impact. In Proceedings of the Abstracts of the European Aerosol Conference 2003, Madrid, Spain, 31 August–5 September 2003; pp. S1357–S1358. [Google Scholar]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef]
- May, N.W.; Olson, N.E.; Panas, M.; Axson, J.L.; Tirella, P.S.; Kirpes, R.M.; Craig, R.L.; Gunsch, M.J.; China, S.; Laskin, A.; et al. Aerosol emissions from great lakes harmful algal blooms. Environ. Sci. Technol. 2018, 52, 397–405. [Google Scholar] [CrossRef]
- Facciponte, D.N.; Bough, M.W.; Seidler, D.; Carroll, J.L.; Ashare, A.; Andrew, A.S.; Tsongalis, G.J.; Vaickus, L.J.; Henegan, P.L.; Butt, T.H.; et al. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K.; Singh, S.; Brown, R.M., Jr. Airborne algae: Their present status and relevance. J. Phycol. 2007, 43, 615–627. [Google Scholar] [CrossRef]
- Sahu, N.; Tangutur, A.D. Airborne algae: Overview of the current status and its implications on the environment. Aerobiologia 2014, 31, 89–97. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, S.C. Survival of blue-green and green algae under stress conditions. Folia. Microbiol. 2006, 51, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska-Wilczewska, S.; Cieszyńska, A.; Konik, M.; Maculewicz, J.; Latała, A. Environmental drivers of bloom-forming cyanobacteria in the Baltic Sea: Effects of salinity, temperature, and irradiance. Estuar. Coast. Shelf Sci. 2019, 219, 139–150. [Google Scholar] [CrossRef]
- Jewson, D.H.; Lowry, S.F.; Bowen, R. Co-existence and survival of diatoms on sand grains. Eur. J. Phycol. 2006, 41, 131–146. [Google Scholar] [CrossRef]
- Ojaveer, H.; Jaanus, A.; Mackenzie, B.R.; Martin, G.; Olenin, S.; Radziejewska, T.; Telesh, I.; Zettler, M.L.; Zaiko, A. Status of biodiversity in the Baltic sea. PLoS ONE 2010, 5, e12467. [Google Scholar] [CrossRef]
- Brown, R.M.; Larson, D.A.; Bold, H.C. Airborne algae: Their abundance and heterogeneity. Science 1964, 143, 583–585. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2020; Available online: http://www.algaebase.org (accessed on 4 November 2020).
- Roy, S.; Llewellyn, C.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in OceanOgraphy; Cambridge University Press: Cambridge, UK, 2011; p. 784. [Google Scholar]
- Latała, A.; Jodłowska, S.; Pniewski, F. Culture Collection of Baltic Algae (CCBA) and characteristic of some strains by factorial experiment approach. Algol. Stud. 2006, 122, 137–154. [Google Scholar] [CrossRef]
- Norton, T.A.; Melkonian, M.; Andersen, R.A. Algal biodiversity. Phycologia 1996, 35, 308–326. [Google Scholar] [CrossRef]
- Chiu, C.-S.; Chiu, P.-H.; Yong, T.C.; Tsai, H.-P.; Soong, K.; Huang, H.-E.; Chen, C.-N.N. Mechanisms protect airborne green microalgae during long distance dispersal. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Cieszyńska, A.; Maculewicz, J.; Latała, A. Ecophysiological characteristics of red, green and brown strains of the Baltic picocyanobacterium Synechococcus sp.—A laboratory study. Biogeosciences 2018, 15, 6257–6276. [Google Scholar] [CrossRef]
- Sharma, K.; Rai, K.A.; Singh, S.A. Meteorological factors affecting the diversity of airborne algae in an urban atmosphere. Ecography 2006, 29, 766–772. [Google Scholar] [CrossRef]
- Singh, H.W.; Wade, R.M.; Sherwood, A.R. Diurnal patterns of airborne algae in the Hawaiian Islands: A preliminary study. Aerobiologia 2018, 34, 363–373. [Google Scholar] [CrossRef]
- Woelfel, J.; Schoknecht, A.; Schaub, I.; Enke, N.; Schumann, R.; Karsten, U. Growth and photosynthesis characteristics of three benthic diatoms from the brackish southern Baltic Sea in relation to varying environmental conditions. Phycologia 2014, 53, 639–651. [Google Scholar] [CrossRef]
- HELCOM. The Baltic Marine Environment 1999–2002. Baltic Sea Environ. Proc. 2003, 87, 1–48. [Google Scholar]
- Neumann, T. Climate-change effects on the Baltic Sea ecosystem: A model study. J. Mar. Sys. 2010, 81, 213–224. [Google Scholar] [CrossRef]
- Walday, M.; Kroglund, T. The Baltic Sea—The Largest Brackish Sea in the World; European Environment Agency: Copenhagen, Denmark, 2003; Available online: https://www.eea.europa.eu/publications/report_2002_0524_154909/regional-seas-around-europe/page141.html (accessed on 1 August 2020).
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincón, J.; Zabala, L.L.; Jiao, N.; Vera, C.S. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Konarzewska, Z.; Wiśniewska, K.; Konik, M. Photosynthetic pigments changes of three phenotypes of picocyanobacteria Synechococcus sp. under different light and temperature conditions. Cells 2020, 9, 2030. [Google Scholar] [CrossRef]
- Jodłowska, S.; Śliwińska, S. Effects of light intensity and temperature on the photosynthetic irradiance response curves and chlorophyll fluorescence in three picocyanobacterial strains of Synechococcus. Photosynthetica 2014, 52, 223–232. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Huss, V.A.R.; Montero, Z.; Torronteras, R.; Cuaresma, M.; Garbayo, I.; Vílchez, C. Phylogenetic characterization and morphological and physiological aspects of a novel acidotolerant and halotolerant microalga Coccomyxa onubensis sp. nov. (Chlorophyta, Trebouxiophyceae). J. Appl. Phycol. 2016, 28, 3269–3279. [Google Scholar] [CrossRef]
- Prelle, L.R.; Graiff, A.; Gründling-Pfaff, S.; Sommer, V.; Kuriyama, K.; Karsten, U. Photosynthesis and Respiration of Baltic Sea Benthic Diatoms to Changing Environmental Conditions and Growth Responses of Selected Species as Affected by an Adjacent Peatland (Hütelmoor). Front. Microbiol. 2019, 10, 1500. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. The ecology of Nostoc. J. Phycol. 1995, 31, 2–18. [Google Scholar] [CrossRef]
- Ploutno, A.; Carmeli, S. Modified peptides from a water bloom of the cyanobacterium Nostoc sp. Tetrahedron 2002, 58, 9949–9957. [Google Scholar] [CrossRef]
- Yu, Z.M.; Zou, J.Z.; Ma, X.N. Application of clays to removal of red tide organisms II. Coagulation of different species of red tide organisms with montmorillonite and effect of clay pretreatment. ChJOL 1994, 12, 316–324. [Google Scholar]
- Potapova, M. Patterns of Diatom Distribution In Relation to Salinity. In The Diatom World. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Kociolek, P., Eds.; Springer: Dordrecht, The Nederlands, 2011; pp. 315–332. [Google Scholar]
- Draxler, R.R.; Rolph, G.D. HYSPLIT (Hybrid Single-Particle Lagrangian Integrated Trajectory) Model Access via NOAA ARL READY Website; NOAA Air Resources Laboratory: Silver Spring, MD, USA, 2003.
- Rolph, G.D. Real-Time Environmental Applications and Display System (READY) Website; NOAA Air Resources Laboratory: Silver Spring, MD, USA, 2003. Available online: www.arl.noaa.gov/ready/hysplit4.html (accessed on 5 September 2020).
- Jasser, I.; Callieri, C. Picocyanobacteria: The Smallest Cell-Size Cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 19–27. [Google Scholar]
- Dembowska, E. Cyanobacterial blooms in shallow lakes of the Iławskie Lake District. Limnol. Rev. 2011, 11, 69–79. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Murphy, L.S.; Foss, P.; Liaaen-Jensen, S. Synechococcus spp. as likely zeaxanthin-dominant ultraphytoplankton in the North Atlantic. Limnol. Oceanogr. 1985, 30, 412–414. [Google Scholar] [CrossRef]
- Barlow, R.G.; Alberte, R.S. Photosynthetic characteristic of phycoerythrin-containing marine Synechococcus spp. I. Responses to growth photon flux density. Mar. Biol. 1985, 86, 63–74. [Google Scholar] [CrossRef]
- Stal, L.J.; Albertano, P.; Bergman, B.; Bröckel, K.; Gallon, J.R.; Hayes, P.K.; Sivonen, K.; Walsby, A.E. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea―Responses to a changing environment. Cont. Shelf Res. 2003, 23, 1695–1714. [Google Scholar]
- Darley, W.M. Phytoplankton: Environmental factorsaffecting growth. In Algal Biology: Aphysiological Approach; Darley, W.M., Ed.; Blackwell Scientific Publications: Belgium, Spain, 1982; pp. 921–952. [Google Scholar]
- Kana, T.M.; Glibert, P.M. Effect of irradiances up to 2000 μmol E·m−2·s−1 on marine Synechococcus WH7803-I. Growth, pigmentation, and cell composition. Deep-Sea Res. 1987, 34, 479–495. [Google Scholar] [CrossRef]
- Kana, T.M.; Glibert, P.M. Effect of irradiances up to 2000 μmol E·m−2·s−1 on marine Synechococcus WH7803-II. Photosynthetic responses and mechanisms. Deep-Sea Res. 1987, 34, 497–516. [Google Scholar] [CrossRef]
- Foy, R.H.; Gibson, C.E. Photosynthetic characteristics of planktonic blue-green algae: Changes in photosynthetic capacity and pigmentation of Oscillatoria redekei Van Goor under high and low light. Br. Phycol. J. 1982, 17, 183–193. [Google Scholar] [CrossRef]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Öquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Huber-Pestalozzi, G. Das Phytoplankton des Süsswassers, 3 Teil. Cryoptophyceen, Chloromonadien, Peridineen. In Die Binnengewasser; Thienemann, A., Ed.; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1950; p. 310. [Google Scholar]
- Lind, M.E.; Brook, A.J. A key to the Commoner Desmids of the English Lake District; Freswater Biol. Assoc.: Cracow, UK, 1980; p. 123. [Google Scholar]
- Komarek, J.; Fott, B. Chlorococcales, 7. Teil. Halfte. In Das Phytoplankton des Süsswassers; Elster, J., Ohle, W., Eds.; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1983; p. 1043. [Google Scholar]
- Popovski, J.; Pfiester, L.A. Dinophyceae (Dinoflagellida). In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fishre Verlag: Jena, Germany, 1990; p. 243. [Google Scholar]
- Cox, E.J. Identification of Freshwater Diatoms from Live Material; Chapman and Hall: London, UK, 1996; p. 158. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süsswasserflora von Mitteleuropa, Spektrum Akademischer Verlag; Ettl, H., Gartner, G., Heynig, H., Mollenhauer, D., Eds.; Springer: Heidelberg, Germany, 1999; p. 548. [Google Scholar]
- Hindák, F. Fotograficky Atlas Mcrsocopickych Sinic; VEDA, Vydavatelstvo Slovenskey Akademie Vied: Bratislava, Slovakia, 2001; p. 45. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanle, M.N., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Śliwińska-Wilczewska, S.; Barreiro Felpeto, A.; Maculewicz, J.; Sobczyk, A.; Vasconcelos, V.; Latała, A. Allelopathic activity of the picocyanobacterium Synechococcus sp. on unicellular eukaryote planktonic microalgae. Mar. Freshw. Res. 2018, 69, 1472–1479. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Maculewicz, J.; Barreiro Felpeto, A.; Vasconcelos, V.; Latała, A. Allelopathic activity of the picocyanobacterium Synechococcus sp. on filamentous cyanobacteria. J. Exp. Mar. Biol. Ecol. 2017, 496, 16–21. [Google Scholar] [CrossRef]
- Jeffrey, S.T.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Strickland, I.D.H.; Parsons, T.R. A practical handbook of seawater analysis. Bull. Fish. Res. Board Can. 1972, 167, 1–310. [Google Scholar]
- Stewart, D.E.; Farmer, F.H. Extraction, identification, and quantification of phycobiliprotein pigments from phototrophic plankton. Limnol. Oceanogr. 1984, 29, 392–397. [Google Scholar] [CrossRef]
- Tandeau de Marsac, N.; Houmard, J. Complementary chromatic adaptation: Physiological conditions and action spectra. In Methods in Enzymology; Packer, L., Glazer, A.N., Eds.; Academic Press: New York, NY, USA, 1988; pp. 318–328. [Google Scholar]
- Mayol, E.; Jimenez, M.A.; Herndl, G.J.; Duarte, C.M.; Arrieta, J.M. Resolving the abundance and air-sea fluxes of airborne microorganisms in the North Atlantic Ocean. Front. Microbiol. 2014, 5, 557. [Google Scholar] [CrossRef]
- Hallegraeff, G. Transport of harmful marine microalgae via ship’s ballast water: Management and mitigation with special reference to the Arabian Gulf region. Aquat. Ecosyst. Health Manag. 2015, 18, 290–298. [Google Scholar] [CrossRef]
- Finlay, B.J. Global Dispersal of Free-Living Microbial Eukaryote Species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, K.; Śliwińska-Wilczewska, S.; Lewandowska, A.; Konik, M. The Effect of Abiotic Factors on Abundance and Photosynthetic Performance of Airborne Cyanobacteria and Microalgae Isolated from the Southern Baltic Sea Region. Cells 2021, 10, 103. https://doi.org/10.3390/cells10010103
Wiśniewska K, Śliwińska-Wilczewska S, Lewandowska A, Konik M. The Effect of Abiotic Factors on Abundance and Photosynthetic Performance of Airborne Cyanobacteria and Microalgae Isolated from the Southern Baltic Sea Region. Cells. 2021; 10(1):103. https://doi.org/10.3390/cells10010103
Chicago/Turabian StyleWiśniewska, Kinga, Sylwia Śliwińska-Wilczewska, Anita Lewandowska, and Marta Konik. 2021. "The Effect of Abiotic Factors on Abundance and Photosynthetic Performance of Airborne Cyanobacteria and Microalgae Isolated from the Southern Baltic Sea Region" Cells 10, no. 1: 103. https://doi.org/10.3390/cells10010103
APA StyleWiśniewska, K., Śliwińska-Wilczewska, S., Lewandowska, A., & Konik, M. (2021). The Effect of Abiotic Factors on Abundance and Photosynthetic Performance of Airborne Cyanobacteria and Microalgae Isolated from the Southern Baltic Sea Region. Cells, 10(1), 103. https://doi.org/10.3390/cells10010103