Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture
2.2. Supplemental Ultraviolet B (UV-B) Radiation and Photosynthetic Photon Flux Density (PPFD) Treatments
2.3. Measurements
2.3.1. Growth Parameters
2.3.2. Gas-Exchange Rate, Relative Chlorophyll Concentration, and Chlorophyll Fluorescence
2.3.3. Secondary Plant Metabolites
2.4. Statistical Analyses
3. Results
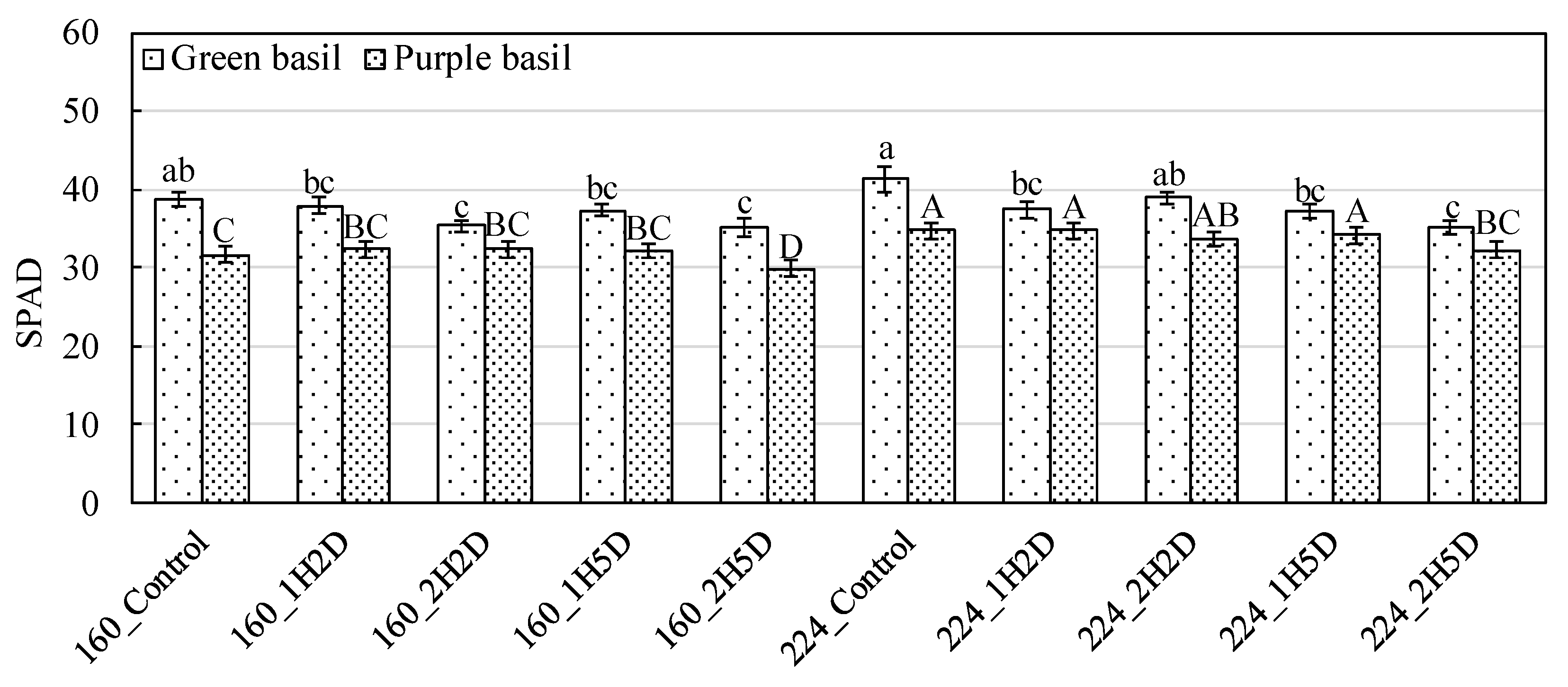
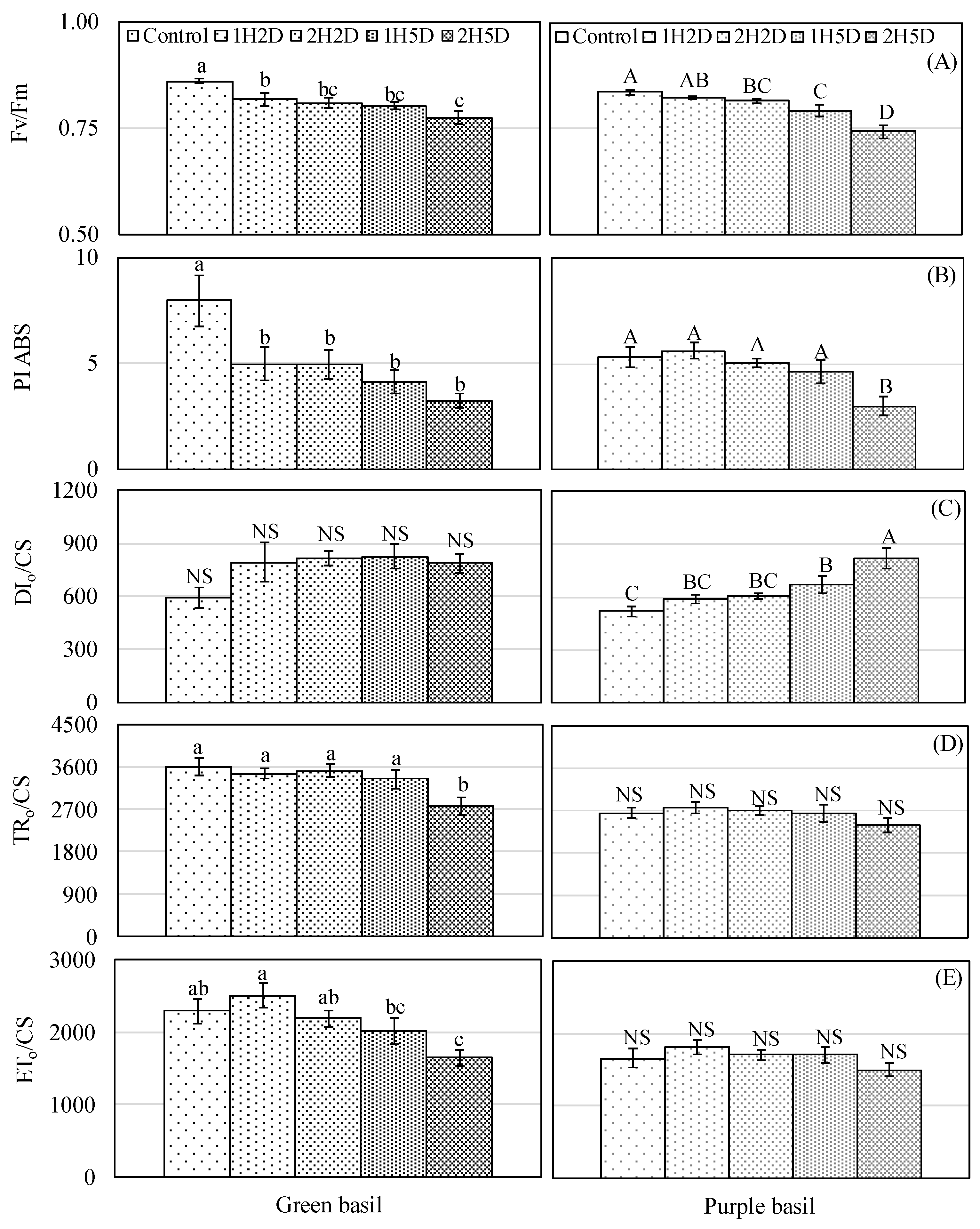
3.1. Gas Exchange Rate, Relative Chlorophyll Concentration, and Chlorophyll Fluorescence
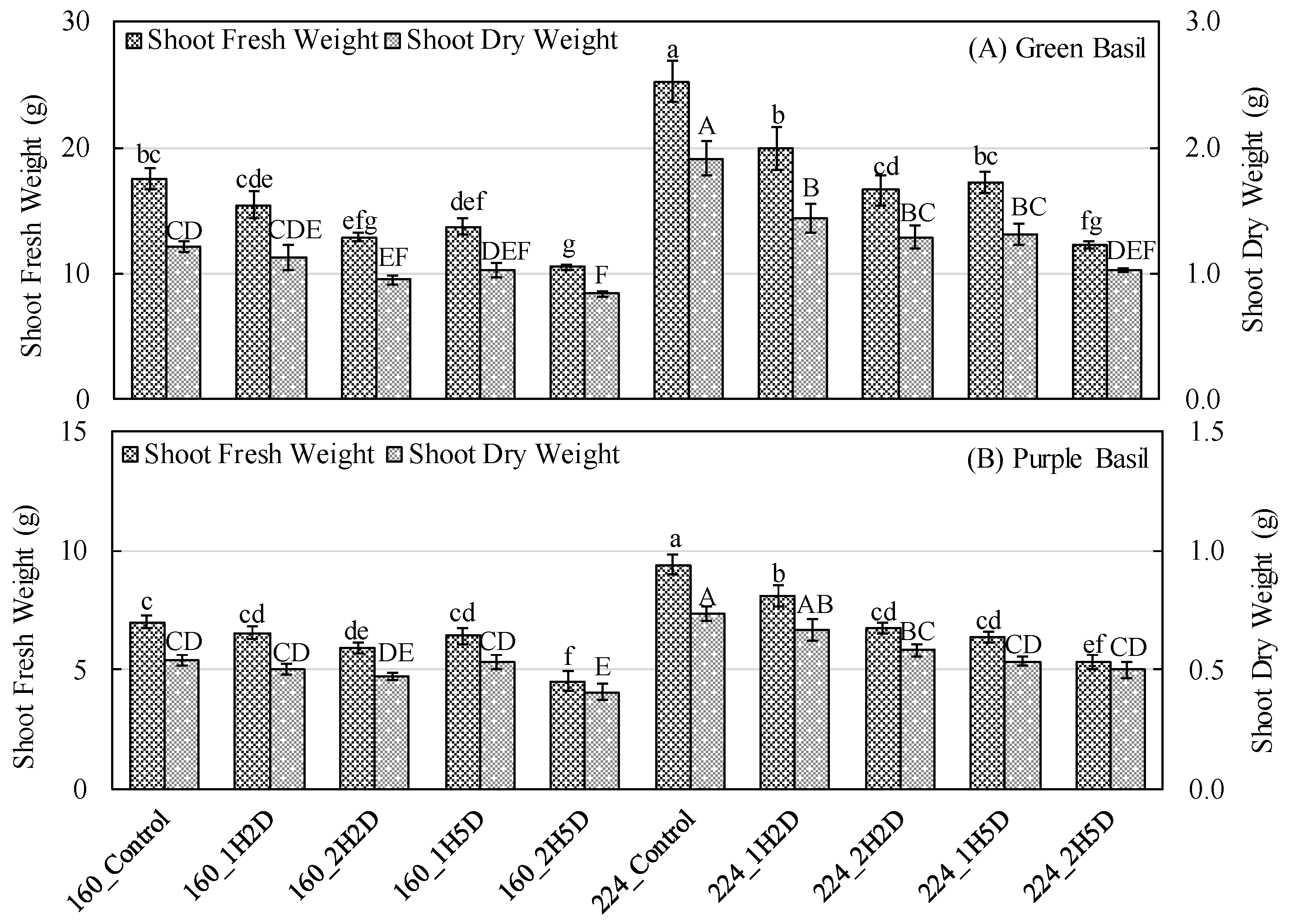
3.2. Growth Parameters and Crop Yield
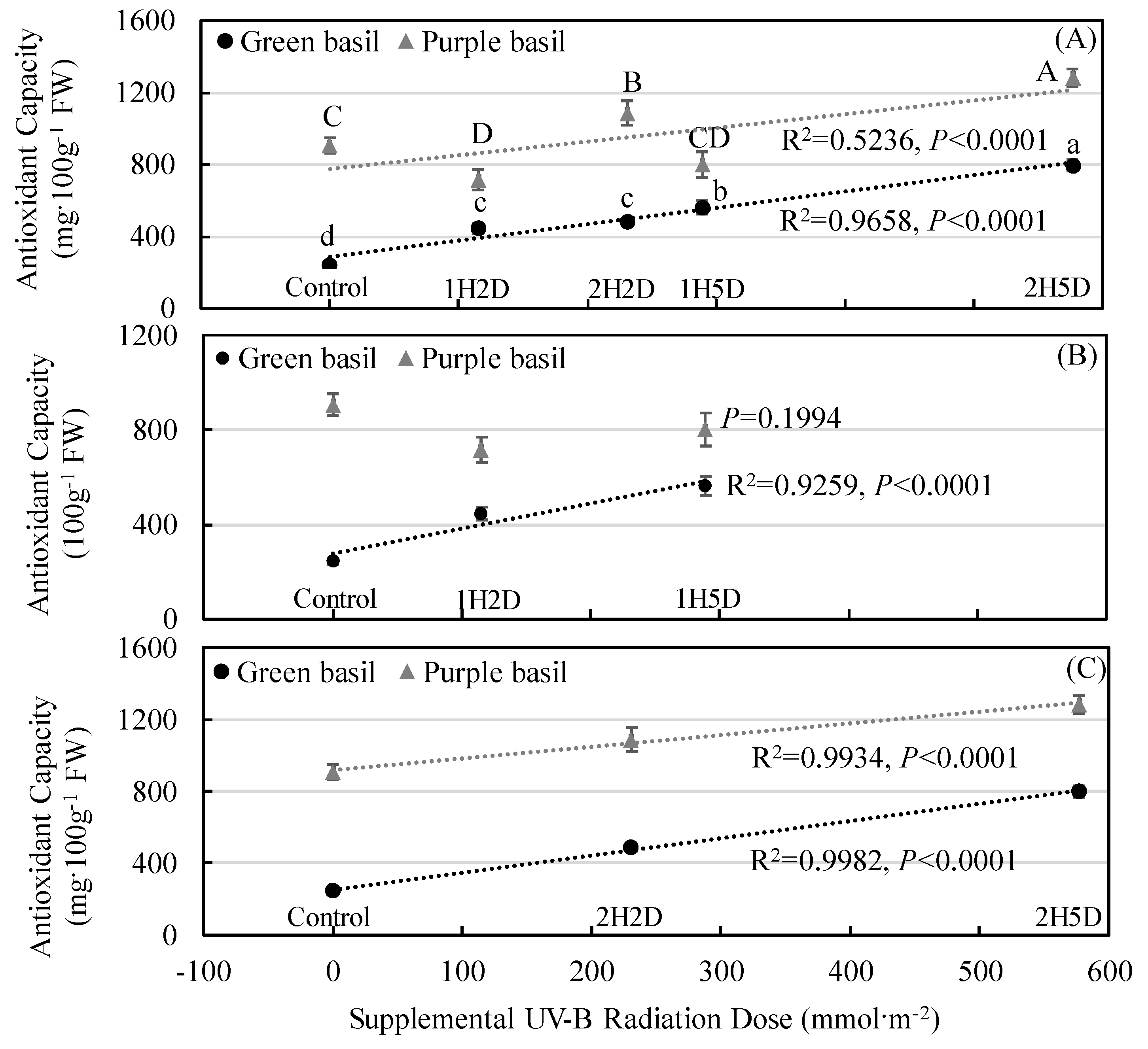
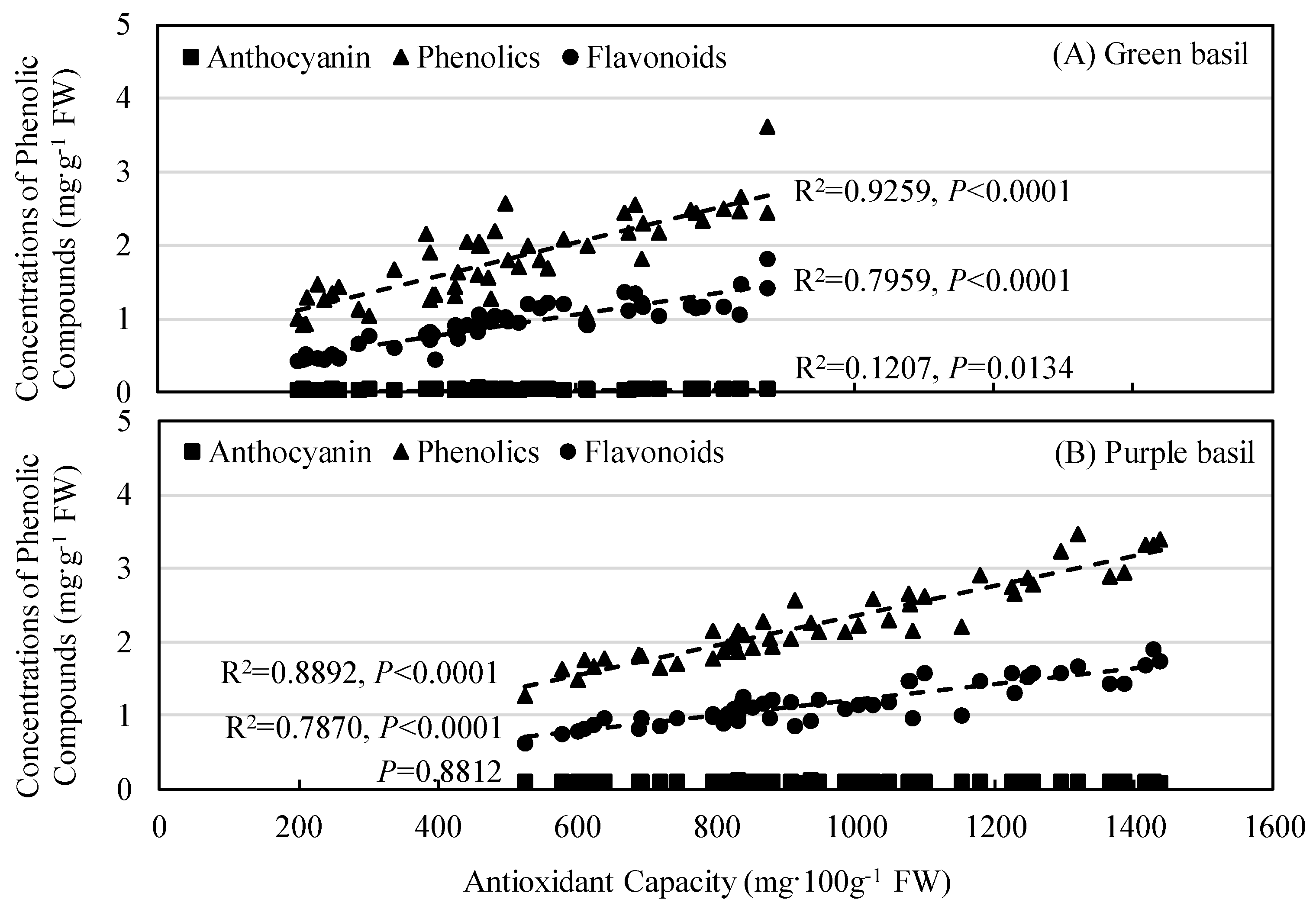
3.3. Secondary Plant Metabolites Accumulation and Antioxidant Capacity
4. Discussion
4.1. Impacts of UV-B and PPFD on Photosynthesis, Relative Chlorophyll Concentration, and Chlorophyll Fluorescence
4.2. Impacts of UV-B and PPFD on Growth and Yield
4.3. Impacts of UV-B and PPFD on Phytochemical Accumulation and Antioxidant Capacity
4.4. Impacts of UV-B Radiation Doses and Radiation Patterns on Phytochemical Accumulation and Antioxidant Capacity
4.5. Implications of Study Findings
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory—An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Academic Press, Elsevier: New York, NY, USA, 2019; in press. [Google Scholar]
- Goto, E.; Hayashi, K.; Furuyama, S.; Hikosaka, S.; Ishigami, Y. Effect of UV Light on Phytochemical Accumulation and Expression of Anthocyanin Biosynthesis Genes in Red Leaf Lettuce. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016; pp. 179–186. [Google Scholar]
- Wargent, J.J. UV LEDs in Horticulture: From Biology to Application. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016; pp. 25–32. [Google Scholar]
- Caldwell, M.; Flint, S.; Searles, P. Spectral Balance and UV-B Sensitivity of Soybean: A Field Experiment. Plant. Cell Environ. 1994, 17, 267–276. [Google Scholar] [CrossRef]
- Wargent, J.J.; Moore, J.P.; Roland Ennos, A.; Paul, N.D. Ultraviolet Radiation as a Limiting Factor in Leaf Expansion and Development. Photochem. Photobiol. 2009, 85, 279–286. [Google Scholar] [CrossRef]
- Sun, R.; Hikosaka, S.; Goto, E.; Sawada, H.; Saito, T.; Kudo, T.; Ohno, T.; Shibata, T.; Yoshimatsu, K. Effects of UV Irradiation on Plant Growth and Concentrations of Four Medicinal Ingredients in Chinese Licorice (Glycyrrhiza uralensis). In Proceedings of the VII International Symposium on Light in Horticultural Systems, Wageningen, The Netherlands, 15–18 October 2012; pp. 643–648. [Google Scholar]
- Castagna, A.; Dall’Asta, C.; Chiavaro, E.; Galaverna, G.; Ranieri, A. Effect of Post-harvest UV-B Irradiation on Polyphenol Profile and Antioxidant Activity in Flesh and Peel of Tomato Fruits. Food Bioprocess. Technol. 2014, 7, 2241–2250. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; de Pascale, S.; Colla, G. Improving Vegetable Quality in Controlled Environments. Sci. Hort. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-induced Secondary Plant Metabolites—Potential Benefits for Plant and Human Health. Crit. Rev. Plant. Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental Reprogramming by UV-B Radiation in Plants. Plant. Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV Light Regulates Flavonoid Metabolism in Apple (Malus x domestica). Plant. Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef]
- Jansen, M.A.; Bornman, J.F. UV-B Radiation: From Generic Stressor to Specific Regulator. Physiol. Plant. 2012, 145, 501–504. [Google Scholar] [CrossRef]
- Höll, J.; Lindner, S.; Walter, H.; Joshi, D.; Poschet, G.; Pfleger, S.; Ziegler, T.; Hell, R.; Bogs, J.; Rausch, T. Impact of Pulsed UV-B Stress Exposure on Plant Performance: How Recovery Periods Stimulate Secondary Metabolism While Reducing Adaptive Growth Attenuation. Plant. Cell Environ. 2019, 42, 801–814. [Google Scholar] [CrossRef]
- Brown, B.A.; Jenkins, G.I. UV-B Signaling Pathways with Different Fluence-rate Response Profiles Are Distinguished in Mature Arabidopsis Leaf Tissue by Requirement for UVR8, HY5, and HYH. Plant. Physiol. 2008, 146, 576–588. [Google Scholar] [CrossRef]
- Favory, J.J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J. Interaction of COP1 and UVR8 Regulates UV-B-induced Photomorphogenesis and Stress Acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef]
- Alexandru Suchar, V.; Robberecht, R. Integration and Scaling of UV-B Radiation Effects on Plants: The Relative Sensitivity of Growth Forms and Interspecies Interactions. J. Plant. Ecol. 2017, 11, 656–670. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exper. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Liaros, S.; Botsis, K.; Xydis, G. Technoeconomic Evaluation of Urban Plant Factories: The Case of Basil (Ocimum basilicum). Sci. Total Environ. 2016, 554, 218–227. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Meinen, E.; van Ieperen, W. Finding the Optimal Growth-Light Spectrum for Greenhouse Crops. In Proceedings of the the VII International Symposium on Light in Horticultural Systems, Wageningen, The Netherlands, 15–18 October 2012; pp. 357–363. [Google Scholar]
- Stutte, G.W. Controlled Environment Production of Medicinal and Aromatic Plants. In Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization; Jeliazkov, V.D., Cantrell, C.L., Eds.; ACS Publications: Washington, DC, USA, 2016; pp. 49–63. [Google Scholar]
- Johnson, C.B.; Kirby, J.; Naxakis, G.; Pearson, S. Substantial UV-B-mediated Induction of Essential Oils in Sweet Basil (Ocimum basilicum L.). Phytochemistry 1999, 51, 507–510. [Google Scholar] [CrossRef]
- Sakalauskaite, J.; Viskelis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A.; Duchovskis, P.; Urbonaviciene, D. The Effects of Different UV-B Radiation Intensities on Morphological and Biochemical Characteristics in Ocimum basilicum L. J. Sci. Food Agr. 2013, 93, 1266–1271. [Google Scholar] [CrossRef]
- Sakalauskaite, J.; Viskelis, P.; Duchovskis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A. Supplementary UV-B Irradiation Effects on Basil (Ocimum basilicum L.) Growth and Phytochemical Properties. J. Food Agr. Environ. 2012, 10, 342–346. [Google Scholar]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B Lighting to Enhance Phenolic Accumulation of Sweet Basil. Sci. Hort. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Xu, C.P.; Mou, B.Q. Responses of Spinach to Salinity and Nutrient Deficiency in Growth, Physiology, and Nutritional Value. J. Amer. Soc. Hort. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B. Variability in Antioxidant Activity in Blueberry and Correlations Among Different Antioxidant Activity Assays. J. Am. Soc. Hort. Sci. 2002, 127, 238–244. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Teramura, A.H. Field Study of the Interaction Between Solar Ultraviolet-B Radiation and Drought on Photosynthesis and Growth in Soybean. Plant. Physiol. 1990, 92, 141–146. [Google Scholar] [CrossRef]
- Lidon, F.J.; Reboredo, F.H.; Silva, M.M.A.; Duarte, M.P.; Ramalho, J.C. Impact of UV-B Radiation on Photosynthesis—An Overview. Emir. J. Food Agr. 2012, 24, 546–556. [Google Scholar] [CrossRef]
- Yadav, S.; Shrivastava, A.K.; Agrawal, C.; Sen, S.; Chatterjee, A.; Rai, S.; Rai, R.; Singh, S.; Rai, L. Impact of UV-B Exposure on Phytochrome and Photosynthetic Machinery: From Cyanobacteria to Plants. In UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth; Singh, S., Prasad, S.M., Parhar, P., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2017; pp. 259–277. [Google Scholar]
- Searles, P.S.; Flint, S.D.; Caldwell, M.M. A Meta-analysis of Plant Field Studies Simulating Stratospheric Ozone Depletion. Oecologia 2001, 127, 1–10. [Google Scholar] [CrossRef]
- Kappes, U.P.; Luo, D.; Potter, M.; Schulmeister, K.; Rünger, T.M. Short- and Long-wave UV light (UVB and UVA) Induce Similar Mutations in Human Skin Cells. J. Investig. Dermatol. 2006, 126, 667–675. [Google Scholar] [CrossRef]
- Ikehata, H.; Ono, T. The Mechanisms of UV Mutagenesis. J. Radiat. Res. 2011, 52, 115–125. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis Inc.: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Rai, K.; Agrawal, S. Effects of UV-B Radiation on Morphological, Physiological and Biochemical Aspects of Plants: An Overview. J. Sci. Res. 2017, 61, 87–113. [Google Scholar]
- Takahashi, S.; Badger, M.R. Photoprotection in Plants: A New Light on Photosystem II Damage. Trends Plant. Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green Light Drives CO2 Fixation Deep Within Leaves. Plant. Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Cen, Y.P.; Bornman, J.F. The Effect of Exposure to Enhanced UV-B Radiation on the Penetration of Monochromatic and Polychromatic UV-B Radiation in Leaves of Brassica napus. Physiol. Plant. 1993, 87, 249–255. [Google Scholar] [CrossRef]
- Wargent, J.J.; Jordan, B.R. From Ozone Depletion to Agriculture: Understanding the Role of UV Radiation in Sustainable Crop Production. New Phytol. 2013, 197, 1058–1076. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.; Kakani, V.; Read, J.; Sullivan, J. Growth and Physiological Responses of Cotton (Gossypium hirsutum L.) to Elevated Carbon Dioxide and Ultraviolet-B Radiation under Controlled Environmental Conditions. Plant. Cell Environ. 2003, 26, 771–782. [Google Scholar] [CrossRef]
- Kaiserli, E. Ultraviolet Rays Light Up Transcriptional Networks Regulating Plant Growth. Dev. Cell 2018, 44, 409–411. [Google Scholar] [CrossRef]
- Fraser, D.P.; Sharma, A.; Fletcher, T.; Budge, S.; Moncrieff, C.; Dodd, A.N.; Franklin, K.A. UV-B Antagonises Shade Avoidance and Increases Levels of the Flavonoid Quercetin in Coriander (Coriandrum sativum). Sci. Rep. 2017, 7, 17758. [Google Scholar] [CrossRef]
- Teklemariam, T.; Blake, T.J. Effects of UVB Preconditioning on Heat Tolerance of Cucumber (Cucumis sativus L.). Environ. Exp. Bot. 2003, 50, 169–182. [Google Scholar] [CrossRef]
- Kakani, V.; Reddy, K.; Zhao, D.; Mohammed, A. Effects of Ultraviolet-B Radiation on Cotton (Gossypium hirsutum L.) Morphology and Anatomy. Ann. Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef]
- Logan, B.A.; Stafstrom, W.C.; Walsh, M.J.; Reblin, J.S.; Gould, K.S. Examining the Photoprotection Hypothesis for Adaxial Foliar Anthocyanin Accumulation by Revisiting Comparisons of Green- and Red-leafed Varieties of Coleus (Solenostemon scutellarioides). Photosyn. Res. 2015, 124, 267–274. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Clearwater, M.J.; Gould, K.S. The Functional Significance of Black-pigmented Leaves: Photosynthesis, Photoprotection and Productivity in Ophiopogon planiscapus ‘Nigrescens’. PLoS ONE 2013, 8, e67850. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef]
- Rodríguez-Calzada, T.; Qian, M.; Strid, A.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B Radiation on Morphology, Phenolic Compound Production, Gene Expression, and Subsequent Drought Stress Responses in Chili Pepper (Capsicum annuum L.). Plant. Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.K.; Strid, A. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associations? Trends Plant. Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Csepregi, K.; Coffey, A.; Cunningham, N.; Prinsen, E.; Hideg, É.; Jansen, M.A. Developmental Age and UV-B Exposure Co-determine Antioxidant Capacity and Flavonol Accumulation in Arabidopsis Leaves. Environ. Exp. Bot. 2017, 140, 19–25. [Google Scholar] [CrossRef]
- Behn, H.; Albert, A.; Marx, F.; Noga, G.; Ulbrich, A. Ultraviolet-B and Photosynthetically Active Radiation Interactively Affect Yield and Pattern of Monoterpenes in Leaves of Peppermint (Mentha × piperita L.). J. Agr. Food Chem. 2010, 58, 7361–7367. [Google Scholar] [CrossRef]
- Tattini, M.; Landi, M.; Brunetti, C.; Giordano, C.; Remorini, D.; Gould, K.S.; Guidi, L. Epidermal Coumaroyl Anthocyanins Protect Sweet Basil Against Excess Light Stress: Multiple Consequences of Light Attenuation. Physiol. Plant. 2014, 152, 585–598. [Google Scholar] [CrossRef]
- Li, F.R.; Peng, S.L.; Chen, B.M.; Hou, Y.P. A Meta-analysis of the Responses of Woody and Herbaceous Plants to Elevated Ultraviolet-B Radiation. Acta Oecologica 2010, 36, 1–9. [Google Scholar] [CrossRef]
- Wargent, J.J.; Elfadly, E.M.; Moore, J.P.; Paul, N.D. Increased Exposure to UV-B Radiation During Early Development Leads to Enhanced Photoprotection and Improved Long-term Performance in Lactuca sativa. Plant. Cell Environ. 2011, 34, 1401–1413. [Google Scholar] [CrossRef]
- Yin, R.; Ulm, R. How Plants Cope with UV-B: From Perception to Response. Cur. Opin. Plant. Biol. 2017, 37, 42–48. [Google Scholar] [CrossRef]
- Schultze, M.; Bilger, W. Acclimation of Arabidopsis thaliana to Low Temperature Protects Against Damage of Photosystem II Caused by Exposure to UV-B Radiation at 9 °C. Plant. Physiol. Biochem. 2018, 134, 73–80. [Google Scholar] [CrossRef]

| Cultivar | Treatment | Pn (μmol·m−2·s−1) | E (mmol·m−2·s−1) | Gs (mmol·m−2·s−1) | |||
|---|---|---|---|---|---|---|---|
| Green Basil | Control | 13.2 | a z | 2.76 | a | 130 | A |
| 1H2D | 7.8 | B | 1.74 | bc | 79 | B | |
| 2H2D | 8.5 | B | 1.93 | b | 93 | ab | |
| 1H5D | 7.4 | B | 1.82 | b | 71 | B | |
| 2H5D | 4.2 | C | 1.24 | c | 46 | C | |
| Purple Basil | Control | 7.4 | A | 2.73 | A | 131 | A |
| 1H2D | 4.3 | B | 1.49 | B | 60 | B | |
| 2H2D | 3.1 | C | 1.20 | B | 42 | CD | |
| 1H5D | 3.8 | BC | 1.33 | B | 49 | BC | |
| 2H5D | 2.2 | D | 0.86 | C | 31 | D | |
| Cultivar. | Treatment | Height (cm) | Width (cm) | Leaf Area (cm2) | Specific Leaf Area (cm2·g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Green Basil | 160_Control | 18.3 | bc z | 11.9 | ab | 520 | bc | 531 | a |
| 160_1H2D | 17.8 | Cd | 12.1 | ab | 453 | cdef | 497 | abc | |
| 160_2H2D | 16.7 | D | 11.6 | bc | 420 | ef | 528 | a | |
| 160_1H5D | 17.2 | Cd | 11.7 | b | 421 | def | 502 | ab | |
| 160_2H5D | 14.4 | E | 10.6 | d | 315 | g | 446 | d | |
| 224_Control | 21.7 | A | 12.1 | ab | 687 | a | 454 | d | |
| 224_1H2D | 21.3 | A | 12.3 | a | 591 | b | 513 | a | |
| 224_2H2D | 19.6 | B | 12.3 | a | 497 | cd | 477 | bcd | |
| 224_1H5D | 19.6 | B | 12.0 | ab | 494 | cde | 466 | cd | |
| 224_2H5D | 16.7 | d | 11.0 | cd | 387 | fg | 450 | d | |
| PPFD | *** | ** | *** | *** | |||||
| UV-B | *** | *** | *** | *** | |||||
| PPFD × UV-B | NS | NS | NS | ** | |||||
| Purple Basil | 160_Control | 15.6 | BC | 16.0 | A | 261 | BC | 610 | A |
| 160_1H2D | 15.4 | C | 15.5 | A | 217 | DE | 553 | BC | |
| 160_2H2D | 15.1 | C | 15.4 | A | 212 | DE | 575 | AB | |
| 160_1H5D | 14.6 | C | 15.2 | A | 221 | D | 545 | BC | |
| 160_2H5D | 12.9 | D | 13.8 | B | 176 | F | 530 | C | |
| 224_Control | 17.7 | A | 16.0 | A | 332 | A | 558 | BC | |
| 224_1H2D | 17.2 | A | 16.1 | A | 274 | B | 542 | BC | |
| 224_2H2D | 16.8 | AB | 15.7 | A | 233 | CD | 531 | CD | |
| 224_1H5D | 15.5 | BC | 16.0 | A | 219 | DE | 534 | C | |
| 224_2H5D | 14.6 | C | 14.0 | B | 187 | EF | 490 | D | |
| PPFD | *** | NS | *** | *** | |||||
| UV-B | *** | *** | *** | *** | |||||
| PPFD × UV-B | NS | NS | * | NS | |||||
| Cultivar | Treatment | Anthocyanin Conc. (mg·100g−1 FW) | Phenolics Conc. (mg·g−1 FW) | Flavonoids Conc. (mg·g−1 FW) | |||
|---|---|---|---|---|---|---|---|
| Green Basil | 160_Control | 3.19 | d z | 1.10 | E | 0.45 | e |
| 160_1H2D | 3.68 | Abcd | 1.41 | De | 0.92 | cd | |
| 160_2H2D | 3.92 | A | 1.48 | D | 0.81 | d | |
| 160_1H5D | 3.49 | Abcd | 1.68 | Cd | 1.00 | abcd | |
| 160_2H5D | 3.87 | Ab | 2.49 | A | 1.21 | a | |
| 224_Control | 3.29 | Cd | 1.38 | De | 0.54 | e | |
| 224_1H2D | 3.39 | Bcd | 2.06 | B | 0.97 | bcd | |
| 224_2H2D | 3.78 | abc | 1.95 | Bc | 0.99 | abcd | |
| 224_1H5D | 3.35 | bcd | 2.13 | Ab | 1.15 | abc | |
| 224_2H5D | 3.89 | ab | 2.34 | Ab | 1.19 | ab | |
| PPFD | NS | *** | NS | ||||
| UV-B | ** | *** | *** | ||||
| PPFD × UV-B | NS | NS | NS | ||||
| Purple Basil | 160_Control | 10.63 | A | 2.06 | CD | 0.94 | CD |
| 160_1H2D | 11.02 | A | 1.63 | E | 0.82 | D | |
| 160_2H2D | 10.84 | A | 2.66 | B | 1.41 | B | |
| 160_1H5D | 10.74 | A | 2.18 | C | 1.14 | C | |
| 160_2H5D | 10.75 | A | 3.35 | A | 1.68 | A | |
| 224_Control | 10.97 | A | 2.03 | CD | 1.04 | C | |
| 224_1H2D | 11.43 | A | 1.93 | CD | 1.09 | C | |
| 224_2H2D | 10.97 | A | 2.62 | B | 1.49 | B | |
| 224_1H5D | 10.85 | A | 1.85 | DE | 1.03 | C | |
| 224_2H5D | 11.07 | A | 2.85 | B | 1.42 | B | |
| PPFD | * | * | NS | ||||
| UV-B | NS | *** | *** | ||||
| PPFD × UV-B | NS | *** | ** | ||||
| Cultivar. | Treatment | Total Amount of Anthocyanin (mg·plant−1) | Total Amount of Phenolics (mg·plant−1) | Total Amount of Flavonoids (mg·plant−1) | |||
|---|---|---|---|---|---|---|---|
| Green Basil | 160_Control | 0.47 | cde z | 16.0 | d | 6.6 | d |
| 160_1H2D | 0.47 | Cde | 18.0 | d | 11.8 | b | |
| 160_2H2D | 0.42 | Def | 16.0 | d | 8.8 | cd | |
| 160_1H5D | 0.40 | Ef | 19.2 | cd | 11.4 | bc | |
| 160_2H5D | 0.36 | F | 23.8 | bc | 11.6 | bc | |
| 224_Control | 0.67 | A | 28.4 | ab | 10.8 | bc | |
| 224_1H2D | 0.55 | Bc | 33.2 | a | 15.4 | a | |
| 224_2H2D | 0.59 | Ab | 25.6 | b | 12.8 | ab | |
| 224_1H5D | 0.52 | Bcd | 31.0 | a | 15.6 | a | |
| 224_2H5D | 0.41 | Ef | 24.0 | bc | 12.2 | b | |
| Purple Basil | 160_Control | 0.63 | C | 12.0 | BC | 5.6 | DE |
| 160_1H2D | 0.58 | D | 8.6 | E | 4.2 | F | |
| 160_2H2D | 0.51 | E | 12.6 | BC | 6.0 | CDE | |
| 160_1H5D | 0.57 | D | 11.0 | CD | 5.2 | EF | |
| 160_2H5D | 0.38 | G | 11.4 | BC | 5.6 | DE | |
| 224_Control | 0.83 | A | 15.4 | A | 8.0 | A | |
| 224_1H2D | 0.72 | B | 12.2 | BC | 7.0 | ABC | |
| 224_2H2D | 0.57 | D | 13.0 | B | 7.2 | AB | |
| 224_1H5D | 0.54 | D | 9.4 | DE | 5.4 | DE | |
| 224_2H5D | 0.47 | F | 12.2 | BC | 6.4 | BCD | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy 2019, 9, 434. https://doi.org/10.3390/agronomy9080434
Dou H, Niu G, Gu M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy. 2019; 9(8):434. https://doi.org/10.3390/agronomy9080434
Chicago/Turabian StyleDou, Haijie, Genhua Niu, and Mengmeng Gu. 2019. "Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants" Agronomy 9, no. 8: 434. https://doi.org/10.3390/agronomy9080434
APA StyleDou, H., Niu, G., & Gu, M. (2019). Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy, 9(8), 434. https://doi.org/10.3390/agronomy9080434







