Theoretical and Experimental Analysis of Nutrient Variations in Electrical Conductivity-Based Closed-Loop Soilless Culture Systems by Nutrient Replenishment Method
Abstract
1. Introduction
2. Materials and Methods
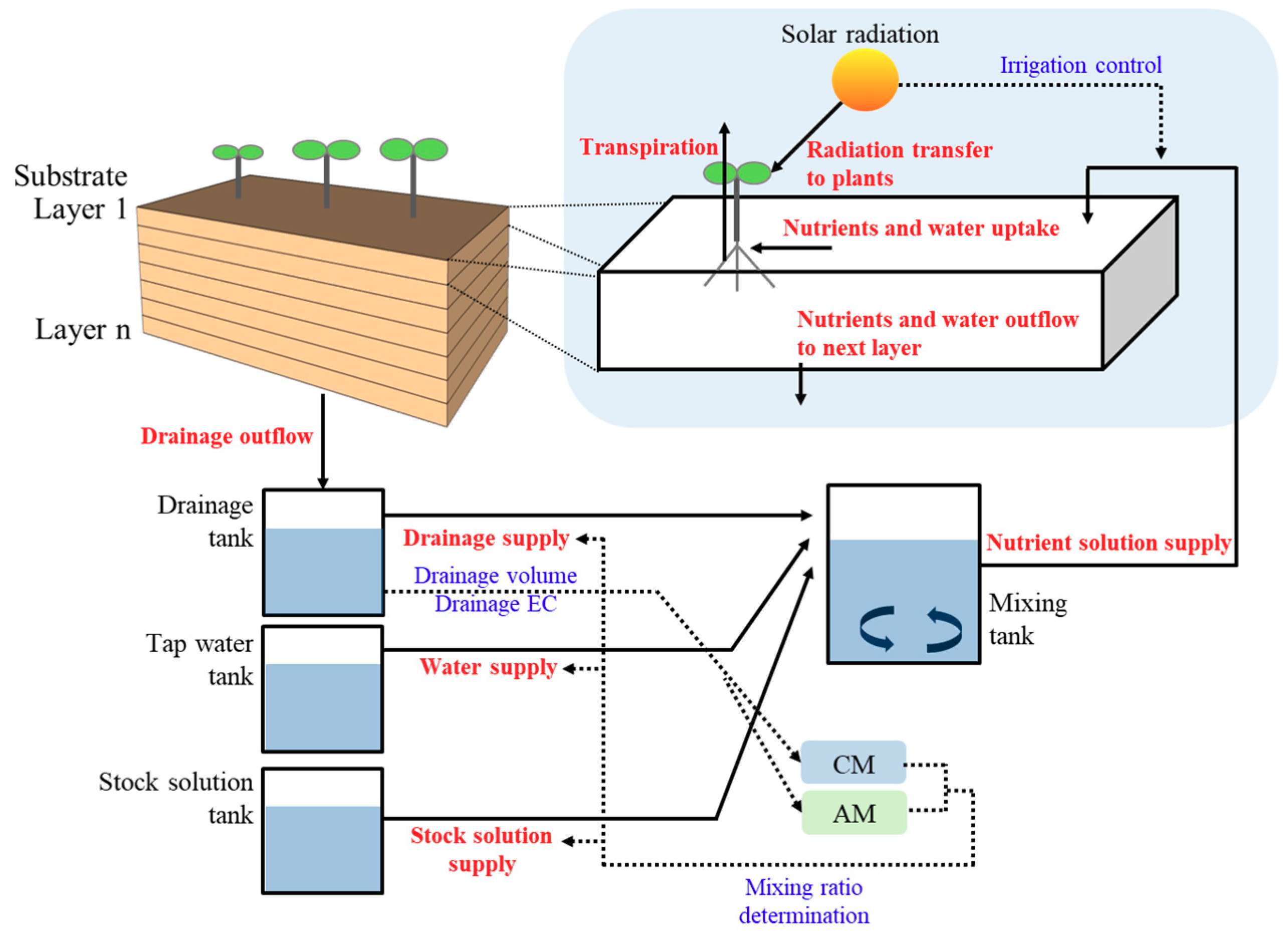
2.1. Soilless Culture System Model
2.2. Water and Nutrient Transport in a Substrate
2.3. Plant Variables and Growth Parameters
2.3.1. Leaf Area Index
2.3.2. Evapotranspiration
2.3.3. Root Length Density and Specific Root Surface Area
2.3.4. Nutrient Uptake
2.4. Mixing of Nutrient Solutions
2.5. Experimental Analysis
2.5.1. Cultivation Conditions
2.5.2. Measurement of Fruit Yield and Analyses of Nutrient Content in Leaves and Substrate
2.5.3. Nutrient-Replenishment Method
2.5.4. Nutrient Solution Mixing Module and Data Collection
3. Results and Discussion
3.1. Theoretical Analysis: Reconsideration of Problem and Derivation of Possible Solution
3.2. Experimental Analysis: Demonstration Experiment for the Theoretical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Bouchaaba, Z.; Santamaria, P.; Choukr-Allah, R.; Lamaddalena, N.; Montesano, F.F. Open-cycle drip vs. closed-cycle subirrigation: Effects on growth and yield of greenhouse soilless green bean. Sci. Hortic. 2015, 182, 77–85. [Google Scholar] [CrossRef]
- Olympios, C.M. Overview of soilless culture: Advantages, constraints and perspectives for its use in Mediterranean countries. Cah. Options Méditerr. 1999, 31, 307–324. [Google Scholar]
- Beerling, E.A.M.; Blok, C.; van der Maas, A.A.; van Os, E.A. Closing the water and nutrient cycles in soilless cultivation systems. Acta Hortic. 2014, 1034, 49–55. [Google Scholar] [CrossRef]
- Van Noordwijk, M. Synchronisation of Supply and Demand is Necessary to Increase Efficiency of Nutrient Use in Soilless Horticulture; Plant Nutrition-Physiology and Applications; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Raviv, M.; Lieth, J.H. Soilless Culture: Theory and Practice, 1st ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2008; ISBN 978-0-444-52975-6. [Google Scholar]
- Kläring, H.-P. Strategies to control water and nutrient supplies to greenhouse crops. A review. Agronomie 2001, 21, 311–321. [Google Scholar] [CrossRef]
- Moon, T.; Ahn, T.I.; Son, J.E. Forecasting root-zone electrical conductivity of nutrient solutions in closed-loop soilless cultures via a recurrent neural network using environmental and cultivation Information. Front. Plant Sci. 2018, 9, 859. [Google Scholar] [CrossRef]
- Zekki, H.; Gauthier, L.; Gosselin, A. Growth, productivity, and mineral composition of hydroponically cultivated greenhouse tomatoes, with or without nutrient solution recycling. J. Am. Soc. Hortic. Sci. 1996, 121, 1082–1088. [Google Scholar] [CrossRef]
- Eggert, R.J. Engineering Design; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2005; ISBN 978-0-13-143358-8. [Google Scholar]
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Savvas, D.; Manos, G. Automated composition control of nutrient solution in closed soilless culture systems. J. Agric. Eng. Res. 1999, 73, 29–33. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Bibbiani, C.; Carmassi, G.; Malorgio, F.; Pardossi, A. Simulation of crop water and mineral relations in greenhouse soilless culture. Environ. Modell. Softw. 2011, 26, 711–722. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A Targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M.; Fukuda, H.; Wada, T. Plant Factory Using Artificial Light: Adapting to Environmental Disruption and Clues to Agricultural Innovation, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-813974-5. [Google Scholar]
- Hao, X.; Papadopoulos, A.P. Growth, photosynthesis and productivity of greenhouse tomato cultivated in open or closed rockwool systems. Can. J. Plant Sci. 2002, 82, 771–780. [Google Scholar] [CrossRef]
- Incrocci, L.; Malorgio, F.; Della Bartola, A.; Pardossi, A. The influence of drip irrigation or subirrigation on tomato grown in closed-loop substrate culture with saline water. Sci. Hortic. 2006, 107, 365–372. [Google Scholar] [CrossRef]
- Rouphael, Y.; Raimondi, G.; Caputo, R.; De Pascale, S. Fertigation strategies for improving water use efficiency and limiting nutrient loss in soilless Hippeastrum Production. Hortscience 2016, 51, 684–689. [Google Scholar] [CrossRef]
- Silberbush, M.; Ben-Asher, J.; Ephrath, J.E. A model for nutrient and water flow and their uptake by plants grown in a soilless culture. Plant Soil 2005, 271, 309–319. [Google Scholar] [CrossRef]
- Silberbush, M.; Ben-Asher, J. Simulation study of nutrient uptake by plants from soilless cultures as affected by salinity buildup and transpiration. Plant Soil 2001, 233, 59–69. [Google Scholar] [CrossRef]
- Snape, J.B.; Dunn, I.J.; Ingham, J.; Prenosil, J.E. Dynamics of Environmental Bioprocesses: Modelling and Simulation; VCH: New York, NY, USA, 1995; ISBN 978-3-527-61538-4. [Google Scholar]
- Ta, T.H.; Shin, J.H.; Ahn, T.I.; Son, J.E. Modeling of transpiration of paprika (Capsicum annuum L.) plants based on radiation and leaf area index in soilless culture. Hortic. Environ. Biotechnol. 2011, 52, 265–269. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.S.; Son, J.E. Estimating the actual transpiration rate with compensated levels of accumulated radiation for the efficient irrigation of soilless cultures of paprika plants. Agric. Water Manag. 2014, 135, 9–18. [Google Scholar] [CrossRef]
- Baille, M.; Baille, A.; Laury, J.C. A Simplified model for predicting evapotranspiration rate of nine ornamental species vs. climate factors and leaf-area. Sci. Hortic. 1994, 59, 217–232. [Google Scholar] [CrossRef]
- Bassirirad, H. Kinetics of nutrient uptake by roots: Responses to global change. New Phytol. 2000, 147, 155–169. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley and Sons: New York, NY, USA, 1995; ISBN 0-471-58747-8. [Google Scholar]
- Savvas, D.; Adamidis, K. Automated management of nutrient solutions based on target electrical conductivity, pH, and nutrient concentration ratios. J. Plant Nutr. 1999, 22, 1415–1432. [Google Scholar] [CrossRef]
- Shin, J.H.; Son, J.E. Application of a modified irrigation method using compensated radiation integral, substrate moisture content, and electrical conductivity for soilless cultures of paprika. Sci. Hortic. 2016, 198, 170–175. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rahman, A.; Azam, H.; Kim, H.S.; Kwon, M.J. Characterizing nutrient uptake kinetics for efficient crop production during Solanum lycopersicum var. cerasiforme Alef. growth in a closed indoor hydroponic system. PLoS ONE 2017, 12, e0177041. [Google Scholar] [CrossRef] [PubMed]
- Carmassi, G.; Incrocci, L.; Maggini, R.; Malorgio, F.; Tognoni, F.; Pardossi, A. An aggregated model for water requirements of greenhouse tomato grown in closed rockwool culture with saline water. Agric. Water Manag. 2007, 88, 73–82. [Google Scholar] [CrossRef]
- Carmassi, G.; Incrocci, L.; Maggini, R.; Malorgio, F.; Tognoni, F.; Pardossi, A. Modeling salinity build-up in recirculating nutrient solution culture. J. Plant Nutr. 2005, 28, 431–445. [Google Scholar] [CrossRef]
- Savvas, D. Automated replenishment of recycled greenhouse effluents with individual nutrients in hydroponics by means of two alternative models. Biosyst. Eng. 2002, 83, 225–236. [Google Scholar] [CrossRef]
- Savvas, D.; Stamati, E.; Tsirogiannis, I.L.; Mantzos, N.; Barouchas, P.E.; Katsoulas, N.; Kittas, C. Interactions between salinity and irrigation frequency in greenhouse pepper grown in closed-cycle hydroponic systems. Agric. Water Manag. 2007, 91, 102–111. [Google Scholar] [CrossRef]
- Ta, T.H.; Shin, J.H.; Noh, E.H.; Son, J.E. Transpiration, growth, and water use efficiency of paprika plants (Capsicum annuum L.) as affected by irrigation frequency. Hortic. Environ. Biotechnol. 2012, 53, 129–134. [Google Scholar] [CrossRef]
- Xu, G.; Levkovitch, I.; Soriano, S.; Wallach, R.; Silber, A. Integrated effect of irrigation frequency and phosphorus level on lettuce: P uptake, root growth and yield. Plant Soil 2004, 263, 297–309. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables—A review. Eur. J. Hortic. Sci. 2018, 83, 11. [Google Scholar] [CrossRef]
- Tadesse, T.; Nichols, M.A.; Fisher, K.J. Nutrient conductivity effects on sweet pepper plants grown using a nutrient film technique. N. Z. J. Crop Hortic. 1999, 27, 239–247. [Google Scholar] [CrossRef]
- Steiner, A.A. The selective capacity of plants for ions and its importance for the composition and treatment of the nutrient solution. Acta Hortic. 1980, 98, 87–97. [Google Scholar] [CrossRef]
- Ingestad, T. Relative addition rate and external concentration; Driving variables used in plant nutrition research. Plant Cell Environ. 1982, 5, 443–453. [Google Scholar] [CrossRef]
- Ko, M.T.; Ahn, T.I.; Shin, J.H.; Son, J.E. Effects of renewal pattern of recycled nutrient solution on the ion balance in nutrient solutions and root media and the growth and ion uptake of paprika (Capsicum annuum L.) in closed soilless cultures. Korean J. Hortic. Sci. Technol. 2014, 32, 463–472. [Google Scholar] [CrossRef]
- Ehret, D.L.; Menzies, J.G.; Helmer, T. Production and quality of greenhouse roses in recirculating nutrient systems. Sci. Hortic. 2005, 106, 103–113. [Google Scholar] [CrossRef]










| Symbol | Description | Value | Reference Number |
|---|---|---|---|
| Leaf area index parameter | 3.5 | [22] | |
| Leaf area index parameter | 13.2 | [22] | |
| Leaf area index parameter | 37.2 | [22] | |
| Evapotranspiration parameter | 0.98 | [22] | |
| Evapotranspiration parameter | 2.08 × 10−4 | [22] | |
| Latent heat of vaporization | 2.45 | [22] | |
| Light extinction coefficient | 0.84 | [22] | |
| Maximal root length density | 50,000 m m−3 | [20] | |
| Coefficient of the root growth function | 770 | [20] | |
| Coefficient of the root growth function | 500 | [20] | |
| Maximum absorption rate | 2.89 × 10−3 | [20] | |
| Maximum absorption rate | 3.54 × 10−4 | [20] | |
| Maximum absorption rate | 4.20 × 10−4 | [20] | |
| Michaelis-Menten constant | 0.0127 | [20] | |
| Michaelis-Menten constant | 0.039 | [20] | |
| Michaelis-Menten constant | 0.015 | [20] | |
| Minimal concentration for uptake | 0.002 | [19] | |
| Minimal concentration for uptake | 0.002 | [19] | |
| Minimal concentration for uptake | 0.002 | [19] | |
| Target total equivalent concentration | 15 | ||
| Total equivalent concentration in tap water | 1 | ||
| Field capacity | 0.74 | [4] | |
| Difficult available water | 0.0068 | [4] | |
| Volume of substrate layer | 1.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, T.I.; Son, J.E. Theoretical and Experimental Analysis of Nutrient Variations in Electrical Conductivity-Based Closed-Loop Soilless Culture Systems by Nutrient Replenishment Method. Agronomy 2019, 9, 649. https://doi.org/10.3390/agronomy9100649
Ahn TI, Son JE. Theoretical and Experimental Analysis of Nutrient Variations in Electrical Conductivity-Based Closed-Loop Soilless Culture Systems by Nutrient Replenishment Method. Agronomy. 2019; 9(10):649. https://doi.org/10.3390/agronomy9100649
Chicago/Turabian StyleAhn, Tae In, and Jung Eek Son. 2019. "Theoretical and Experimental Analysis of Nutrient Variations in Electrical Conductivity-Based Closed-Loop Soilless Culture Systems by Nutrient Replenishment Method" Agronomy 9, no. 10: 649. https://doi.org/10.3390/agronomy9100649
APA StyleAhn, T. I., & Son, J. E. (2019). Theoretical and Experimental Analysis of Nutrient Variations in Electrical Conductivity-Based Closed-Loop Soilless Culture Systems by Nutrient Replenishment Method. Agronomy, 9(10), 649. https://doi.org/10.3390/agronomy9100649






