Abstract
Warm-season turfgrass species prevail in tropical and subtropical areas, but can also be grown in the transition zone. In this case, cold tolerance is a key aspect for germination and successful turfgrass establishment. The germination response to sub-optimal temperatures was investigated for Cynodon dactylon (cvs Jackpot, La Paloma, Transcontinental, Yukon, Riviera), Buchloe dactyloides (cv SWI 2000) and Paspalum vaginatum (cv Pure Dynasty). Four temperature regimes were applied, i.e., 20/30 °C, 15/25 °C, 10/20 °C and 5/15 °C, with a 12:12 h (light:dark) photoperiod. Germination assays were performed twice, with six replicates (Petri dishes) per treatment in each experiment, fifty seeds per dish. The final germinated percentages at last inspection time (FGP) were obtained for each Petri dish and processed by using a generalized linear mixed model (binomial error and logit link). Germination curves were fitted to each Petri dish by using time-to-event methods and germination rates (GR) for the 10th, 20th and 30th percentiles were derived and used to fit a linear thermal-time model. For all cultivars, FGP decreased with decreasing mean daily temperatures. Base temperatures (Tb) ranged between 11.4 °C and 17.0 °C, while the thermal time to obtain 30% germination ranged from 51.3 °C day for SWI 2000 to 144.0 °C day for Pure Dynasty. The estimated parameters were used to predict germination time in the field, considering the observed soil temperatures in Legnaro. The estimated date for the beginning of germination in the field would range from early April for SWI 2000 and Transcontinental to mid-May for Riviera. These results might be used as a practical support for planning spring sowing, which is crucial for successful turfgrass establishment, especially without irrigation.
1. Introduction
In arid regions, warm-season species are preferred over cool-season ones for lawns and other recreational landscapes due to their low water requirements [1,2]. Recently, because of global warming and the resulting climate change, the use of warm-season species for ornamental and sports turfs has also been encouraged in the Mediterranean countries of Europe. In these regions, which fall within the transition zone, cool-season species are the most common, but warm-season ones can successfully be used as, in addition to low water consumption, they have other environmental benefits: greater ability to compete with weeds, higher tolerance to biotic and abiotic agents, traffic and salinity [3,4]. Warm-season grasses are cold sensitive, and in transition zones, they can suffer from the long winters resulting in a delay in spring green-up shortening the growing period. Proper cultural practices and the selection of cultivars adapted to the specific environment are essential to enhance the growing season length [5,6,7,8,9,10].
Moreover, warm-season grasses can be seeded or propagated vegetatively as sod, plugs or sprigs [11,12] but the establishment of turfgrass from seed is preferred over sodding or sprigging as it is cheaper [13]. However, for some species such as bermudagrass (Cynodon dactylon (L.) Pers.), St. Augustinegrass (Stenotaphrum secundatum (Walt.) Kuntze), seashore paspalum (Paspalum vaginatum Swartz), and zoysiagrass (Zoysia spp.), the turfgrass industry developed vegetatively propagated cultivars that are widely used because of the high turf quality due to improved morphological uniformity [14]. Seeded-type cultivars can be a challenge to establish by seed in transition zones as they need higher temperatures to germinate and grow after emergence than cool-season species [15]. In transition zones of Europe, warm-season species are generally seeded in late spring when the temperature is sufficiently high for a rapid establishment [10,16]. Spring average monthly temperatures in European transition zones range roughly from 8–9 °C in March to 17–18 °C in May [17,18]. Warm-season grasses have cardinal germination temperatures shifted to higher levels in comparison with cool-season grasses, and tolerance to sub-optimal temperatures appears to be crucial for seeding them in transition zones.
Several studies highlighted the importance of an early seeding of bermudagrass for proper establishment [11,13,19,20], demonstrating that seeding in late spring or early summer (June or July) does not ensure proper turfgrass establishment before winter dormancy, while early spring seeding resulted in faster establishment and a reduction of the risk of winter injury [15,21]. There are a large number of bermudagrass cultivars, which give a range of adaptation to different environments [5,6,7,17]. Several studies [16,21,22] showed that vast differences in germination temperatures occur among bermudagrass cultivars. Giolo et al. [23], in a growth chamber experiment in which ten bermudagrass cultivars were tested, found an average base germination temperature of 15 °C corroborating field studies demonstrating that early spring seeding could be effectively used to successfully establish bermudagrass in transition zones [21]. Similar findings were also observed for ‘Sea Spray’ seashore paspalum [16], although Fontenot [24] found that this cultivar has a significantly higher mean germination time (MGT) when seeds are subjected to 20 °C, compared with 25 °C, 30 °C and 35 °C.
Growing degree-day (GDD) units are often used to predict turfgrass establishment, although information on germination base temperature of warm-season grasses is limited. Unruh et al. [25] found a base temperature of 5 °C for vegetative bermudagrasses, later also widely used for germination of seeded-type cultivars. More recent studies indicated a base germination temperature for seeded-type cultivars close to 15 °C [23]. Knowledge of base temperature is fundamental to the choice of seeding dates for these species, but it has scarcely been studied. Cardinal temperatures are considered in thermal time (TT) models, developed to describe germination patterns [26]. The ordinal range of cardinal temperatures for seed germination includes a minimum or base temperature (Tb), optimum temperature (To) and maximum or ceiling temperature (Tc). Temperatures between Tb and To represent suboptimal temperatures. In TT models, the timing of germination can be described for suboptimal temperatures introducing the concept of thermal time (θT), which is considered constant for a given percentile (g) [27]. Differently from hydro time and hydrothermal time models, TT models do not account for water potential, which is assumed to be 0. This study aimed to reduce the lack of information related to base germination temperatures in warm-season species, which has been little studied [28]. A growth chamber experiment was conducted in 2018 to investigate the germination response to sub-optimal temperatures of some cultivars currently available in the European turfgrass markets of bermudagrass seashore paspalum, and one cultivar of buffalograss (Buchloe dactyloydes (Nutt.) Engelm). Buffalograss is a warm-season species not yet widespread in Europe, but that may have high potential for low-maintenance lawns and other non-trafficked areas [29,30].
2. Materials and Methods
2.1. Germination Assay: Experimental Setting
The study was conducted in 2018 at the laboratory of CREA DC – Research Centre for Plant Protection and Certification of Lonigo (Vicenza, Italy). Seven turf-type cultivars of warm-season species were tested in a germination chamber under specific controlled conditions. They included five cultivars of bermudagrass: ’Jackpot’, ‘La Paloma’, ‘Transcontinental’, ‘Yukon’, and ‘Riviera’, ‘SWI 2000’ buffalograss, and ‘Pure Dynasty’ seashore paspalum. Four temperature regimes were applied consisting of two alternating temperatures each: 20/30 °C, as optimal temperature treatment according to ISTA methods [31], and 15/25 °C, 10/20 °C, 5/15 °C as suboptimal temperature treatments. The sub-temperatures chosen for the experiment represent a possible temperature range, that takes into account the temperatures in early sin the transition zone and studies and observations on base temperature of warm-season turfgrass species [21,23]. For each treatment (i.e., each cultivar combined with each temperature treatment), six replicates (Petri dishes of 140 mm diameter) of fifty seeds were laid out in a randomized blocks design. All the seeds used, except for ‘Jackpot’, were unhulled, whereas for ‘SWI 2000’ buffalograss the bur was used, which includes 2–3 caryopses [32], and was considered as a seed unit. Before the test, seeds and burs were stored at a temperature of 5 °C and 50% relative humidity into a storage chamber for six months to break possible seed dormancy. The filter paper in Petri dishes was moistened with 6 mL solution 0.2% of KNO3 [31] and then kept moist with deionized water. Petri dishes were incubated at 12:12 h (light:dark) photoperiod. Germinated seeds were counted five times a week for 20 days. Seed germination was assayed by monitoring primary root elongation. A bur was considered germinated when at least one seed was germinated. Germinated seeds and burs were removed at the time of counting. The experiment was run twice using the same germination chambers. In total, we had 336 Petri dishes (2 runs × 6 blocks × 7 cultivars × 4 temperatures).
2.2. Soil Temperature Data
In order to predict germinations in field conditions (see later), soil temperatures were recorded by a weather station on the Experimental Farm of Padova University in Legnaro [33]. The area is a typical transition zone with a sandy loam soil [6,7,23]. Seven-year temperatures (2008–2014) recorded by ground surface temperature sensors TSS of MTX srl were considered. Temperatures were recorded near to the soil surface, that is where the small seeds of these species are usually sown.
2.3. Data Analyses
The final germinated proportions (FGPs) obtained for each Petri dish at the final inspection time were processed by using a Generalized Linear Mixed Model (binomial error and logit link), where the temperature and cultivar were included as fixed effects, while the run and block within the run were included as random effects. Back-transformed proportions were derived from model parameters. Standard errors on the link scale were derived by the Hessian and were back-transformed by using the delta-method [34].
Pairwise comparisons were performed by using the generalised hypothesis testing procedure devised by [27].
In order to evaluate the effect of temperature on germination rates, the time-course of germination was determined for each of the 336 treatment combinations (4 temperature levels × 7 cvs × 6 replicates × 2 runs). Model fitting was accomplished by using a time-to-event model, considering a Weibull distribution of germination times [35,36]. The cumulative probability function was:
where: g is the proportion of germinated seeds at time t, d is the higher asymptote, b is the shape parameter and e is the location parameter.
The fitted models were used to derive the germination rates (GRg) for the 10th, 20th and 30th percentiles (GR10, GR20 and GR30) for each Petri dish as the inverse of the germination times (t10, t20 and t30). The choice of using only up to GR30 instead of GR50, as would be usual, was made in agreement with Bewley et al. [26], based on the fact that, in our study, the FGP differed widely among cultivars and in some cases (‘Yukon’ and ‘Pure Dynasty’) was even lower than 50%.
The derived GRg values were used to fit the thermal-time model of Garcia-Huidobro [27]:
where: GRg is the germination rate for the g percentile (i.e., the 10th, 20th and 30th percentile), T is the average daily temperature, Tb is the base temperature (in °C and common for all percentiles) and θT (g) is the thermal time for the germination of the g percentile (in °C d). By fitting this model, we could derive the main thermal-time parameters for all the species/cultivars. Nonlinear least squares were used to fit the model and the average daily temperature was used as explanatory variable [37]. A transform-both-sides technique was used to account for heteroscedasticity, with λ = 0 [38].
The estimated values for thermal time (θT (30)) and base temperature were used to predict the germination in field conditions, by using soil temperature data. The 10-day means for March, April and May (Table 1) were then calculated and used to determine germination degree days using the following equation [39,40]:
where if < , then = and if < , then = .

Table 1.
Ten-day average of maximum, minimum, and mean daily temperatures (°C) in Legnaro (Padova, Italy) for the period 2008–2014, and averages and standard deviations over the seven years.
For each cultivar, the time (days) required to reach the value of θT (30), as determined from Equation (1), which is the time needed to reach 30% germination, was derived.
Data analyses were performed by using the R statistical environment [41], together with the “drc” [42] and “emmeans” [43] packages.
3. Results
The FGP (Table 2) varied with cultivar and temperature regime: Wald chi square statistics showed that all the effects were significant (chi square values equal to 1002.16, 244.37 and 355.69, respectively for cultivar, temperature and ‘cultivar × temperature’ interaction. The degrees of freedom were, respectively, 6, 3 and 18, corresponding to p-levels always lower than 2.2 × 10−16).

Table 2.
Germination percentage (FGP) at the final inspection time of five cultivars of warm-season turfgrass species: bermudagrass (Cynodon dactylon (L.) Pers.); buffalograss (Buchloe dactyloides (Nutt.) Englem), and seashore paspalum (Paspalum vaginatum Swartz.), subjected to four temperature regimes (5/15 °C, 10/20 °C, 15/25 °C, 20/30 °C) (The values represent the means of two experiment runs and six replications within each run; standard errors are in parentheses).
In all cultivars, as expected, the highest FGP was achieved at the highest temperature regime (20/30 °C) [31]. In four cultivars of bermudagrass (’Jackpot’, ‘La Paloma’, ‘Riviera’, ‘Transcontinental’) FGP was close to or higher than 70. For all the cultivars tested, the FGP decreased with decreasing temperature. At 10/20 °C, FGP dropped to almost nil for ‘Pure Dynasty’, ‘Riviera’ and ‘Yukon’; it ranged from 10% to 20% for ‘La Paloma’ and ‘Jackpot’, while it was between 20% and 40% for ‘Transcontinental’ and ‘SWI 2000’. At 5/15 °C, only ‘SWI 2000’ buffalograss maintained a little germination (FGP = 8.7%).
Considering the germination time-course, the fit of Weibull cumulative probability functions was always good, although, in some cases, no germinations were observed and, therefore, no time-to-event model could be fit. In other instances, the higher asymptote could not be estimated with precision and, therefore, it was constrained to one, considering that our aim was to achieve the best fit within the observed time lapse. Figure 1 shows the observed data (as averages of two runs and six replicates per run) and the fitted models, as obtained by averaging model parameters across runs and replicates. The estimated parameters for the different cultivars and temperature regimes, as averages across runs and replicates, are reported as supplemental data (Table S1).
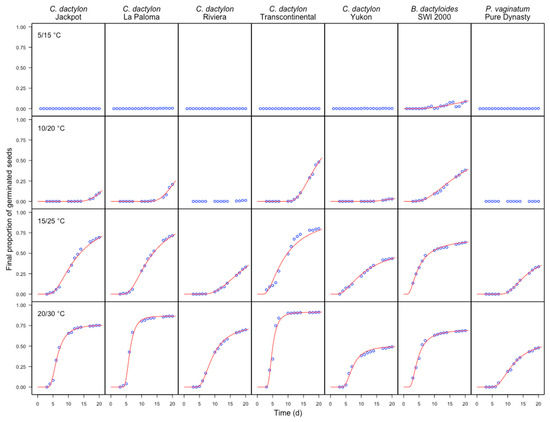
Figure 1.
Time course of germination for seven cultivars of warm-season turfgrass species (Jackpot, La Paloma, Riviera, Transcontinental, Yukon; SWI 2000, Pure Dynasty; each variety is shown in a different column of the panel graph) subjected to four temperature regimes (5/15 °C, 10/20 °C, 15/25 °C, 20/30 °C; each temperature regime is shown in a different row of the panel graph). Symbols represent observed data and solid lines show the fitted models (Equation (1), together with the parameters in Table S1). The observed data represent the means of six blocks and two runs.
Fitted curves were used to derive the germination rates for the 10th, 20th, and 30th percentiles (GR10, GR20, GR30), which were used to parameterise Equation (2). The fit was always good; in agreement with Bradford [44], the three linear relationships of GR10, GR20 and GR30 merged at the intercept of the x-axis, confirming that Tb was equal for all seeds of the lot (Figure 2, Table 3) [44].
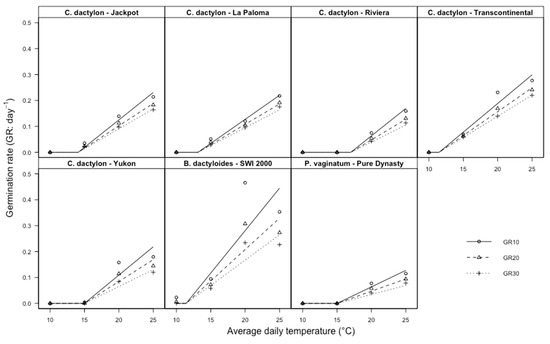
Figure 2.
Germination rates of the 10th, 20th and 30th percentiles for seven cultivars of warm-season turfgrass species (Jackpot, La Paloma, Transcontinental, Yukon, SWI 2000, Pure Dynasty) subjected to four temperature regimes (20/30 °C, 15/25 °C, 10/20 °C, 5/15 °C, corresponding, respectively, to average daily temperatures of 25 °C, 20 °C, 15 °C and 10 °C) [27].

Table 3.
Thermal-time parameters of seven cultivars of warm-season turfgrass species as obtained by nonlinear regression (Equation (1); [27]) (standard errors are in parentheses).
For all cultivars, except for ‘SWI 2000’, the fitted lines are very close (Figure 2) and, therefore, the thermal times required to reach 10, 20 and 30% germination (θT(10), θT(20), and θT(30)) were very similar (Table 3).
The five bermudagrass cultivars showed very different Tb varying from 11.5 for ‘Transcontinental’ to 17.0 °C for ‘Riviera’. The cultivar SWI 2000 also showed a very low Tb (11.4 °C), while that of ‘Pure Dynasty’ was intermediate (15.0 °C).
Based on the estimated values for thermal time (θT(30)), base temperature (Tb) and considering the observed temperature levels (see Table 1), we calculated that, to reach 30% germination (Table 4), seeding time would range from early April for ‘SWI 2000’ and ‘Transcontinental’ to mid-May for ‘Riviera’ and ‘Pure Dynasty’.

Table 4.
Cumulated degree days per 10-days for seven cultivars of warm-season turfgrass species in March, April and May. These values were calculated by using weather data and the estimated parameters in Table 3. The time intervals with degree days highlighted in bold are those necessary to reach the thermal time for the 30th percentile (θT(30)). The cultivars are listed by increasing Tb value.
4. Discussion
In four cultivars of bermudagrass (’Jackpot’, ‘La Paloma’, ‘Riviera’, ‘Transcontinental’), FGP was close to or higher than 70%, which is the minimum threshold for the seed market of this species in the EU [45]. The low FGP of ‘Yukon’ (49%) was probably due to the low vigor of the seed lot. Low germination of ‘Pure Dynasty’ seashore paspalum corroborated previous results obtained by [46].
For ‘SWI 2000’, the germination rates of the three percentiles were different; this result could be related to the fact that this species is dioecious and cultivars include many genotypes [47]. In agreement with Bradford [44], our result show that base temperature should be equal for all seeds of the lot (Figure 2, Table 3) [44], even though some exceptions to this rule can be found in literature [48].
The low Tb of ‘Transcontinental’ bermudagrass and ‘SWI 2000’ buffalograss may allow early spring sowings, thus ensuring quick establishment reducing the risk of winter injuries [13,20]. In this regard, however, ‘SWI 2000’ should be taken into consideration in virtue of the lower thermal time of the 30th percentile (51.3), both at 15/25 °C and 10/20 °C (Table 3, Figure 2). The Tb of ‘Riviera’ (17.0 °C) is in line with that observed in previous studies [22,23]. The wide range of Tb observed in the bermudagrass cultivars is of great interest for turf specialists who may select the most suitable cultivar for a specific location. Thirty varieties of bermudagrass are listed in the OECD catalogue [49], as a result of the considerable breeding activity on this species. Instead, buffalograss has only four varieties, and seashore paspalum has none currently listed.
In the transition zone, the recommended sowing period is between late spring and early summer [16]. Sub-optimal temperatures considered in this study generally occur in the earlier spring months (March and April).
The low germination temperature of ‘Transcontinental’ makes this cultivar very suitable for transition zones where early spring seeding is required [13,20]. Among the three species studied, buffalograss is the least known in Europe; however, our results demonstrated that ‘SWI 2000’ may have a good possibility to be successfully seeded in European transition zones under early spring conditions. Furthermore, ‘SWI 2000’, despite having a similar Tb to ‘Transcontinental’, showed a lower thermal time (Table 3, Figure 2). Instead, ‘Riviera’ bermudagrass and ‘Pure Dynasty’ seashore paspalum appear to be suitable only for late spring sowing. It is worth noting that ‘Pure Dynasty’ showed an intermediate Tb of 15 °C but a very high thermal time (144 °C) in comparison with ‘Yukon’ that showed the same Tb with a thermal time of 76.8 °C. Consequently, the turfgrass establishment with this species would be very slow and late, with related drawbacks in terms of irrigation requirements and weed competition and reduced cold tolerance during the first winter [9].
5. Conclusions
The results indicate that there is a wide variability among warm-season turfgrasses in the temperature requirements for germination. However, none of the species or cultivars was able to germinate below 11.4 °C and the earliest date for achieving 30% of seed germination in the Northern Italian plain is early April for the cultivars with the lowest base temperature, i.e., ‘SWI 2000’ buffalograss and ‘Transcontinental’ bermudagrass. This information is useful to identify the earliest sowing date in spring for each of the species and cultivars used in this experiment and to predict the time necessary for their germination. This approach might represent a practical tool for planning spring sowing, which is crucial for successful turfgrass establishment, especially without irrigation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/9/8/421/s1, Table S1. Estimated parameters for Equation (1).
Author Contributions
M.G. planned the experiment and drafted the manuscript; P.B. verified and discussed the results; G.A. performed the experiment; S.M. conceived and supervised the study; A.O. processed the data and performed the analysis. All Authors contributed to the final version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The research was supported by University Funds and is part of the PhD thesis of Maurizio Giolo at the University of Padova.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Green, R.L.; Beard, J.B.; Casnoff, D.M. Leaf Blade Stomatal Characterizations and Evapotranspiration Rates of 12 Cool-season Perennial Grasses. HortScience 1990, 25, 760–761. [Google Scholar]
- Huang, B. Turfgrass Water Requirements and Factors Affecting Water Usage; Beard, J.B., Kenna, M.P., Eds.; CAST Special Publication; Water Quality and Quantity Issues for Turfgrass in Urban Landscapes; PSI Printing Services, Inc.: Belmond, IWA, USA.
- Minelli, A.; De Luca, A.; Croce, P.; Cevenini, L.; Zuffa, D. Transition from cool-season to warm-season grass: Environmental effects in a golf course in the North of Italy. In Proceedings of the 4th European Turfgrass Society Conference, Osnabrueck, Germany, 6–9 July 2014; pp. 1–4. [Google Scholar]
- Puhalla, J.; Krans, J.; Goatley, M. Sports Fields: A Manual for Design, Construction and Maintenance; John Wiley & Sons, Inc.: Hoboken, NJ, USA; ISBN 978-1-57504-070-7.
- Sever Mutlu, S.; Mutlu, N.; Shearman, R.; Gurbuz, E.; Gulsen, O.; Hocagil, M.M.; Karaguzel, O.; Heng-Moss, T.; Riordan, T.; Gaussoin, R. Establishment and Turf Qualities of Warm-season Turfgrasses in the Mediterranean Region. HortTechnology 2011, 21, 67–81. [Google Scholar] [CrossRef]
- Rimi, F.; Macolino, S.; Richardson, M.D.; Karcher, D.E.; Leinauer, B. Influence of Three Nitrogen Fertilization Schedules on Bermudagrass and Seashore Paspalum: I. Spring Green-up and Fall Color Retention. Crop Sci. 2013, 53, 1161–1167. [Google Scholar] [CrossRef]
- Rimi, F.; Macolino, S.; Richardson, M.D.; Karcher, D.E.; Leinauer, B. Influence of Three Nitrogen Fertilization Schedules on Bermudagrass and Seashore Paspalum: II. Carbohydrates and Crude Protein in Stolons. Crop Sci. 2013, 53, 1168–1178. [Google Scholar] [CrossRef]
- Magni, S.; Gaetani, M.E.; Grossi, N.; Caturegli, L.; Bella, S.L.; Leto, C.; Virga, G.; Tuttolomondo, T.; Lulli, F. Bermudagrass adaptation in the Mediterranean climate: Phenotypic traits of 44 accessions. Adv. Hortic. Sci. 2014, 28, 29–34. [Google Scholar]
- Seppoloni, I.; Staglianò, N.; Cecchi, S.; Argenti, G. Performance of warm-season turfgrasses in an area of central Italy. Adv. Hortic. Sci. 2015, 29, 53–58. [Google Scholar]
- Schiavon, M.; Macolino, S.; Leinauer, B.; Ziliotto, U. Seasonal Changes in Carbohydrate and Protein Content of Seeded Bermudagrasses and Their Effect on Spring Green-Up. J. Agron. Crop Sci. 2016, 202, 151–160. [Google Scholar] [CrossRef]
- Munshaw, G.C.; Williams, D.W.; Powell, A.J.; Dougherty, C.T. Growth and development of seeded versus vegetative bermudagrass varieties. Agron. Abstr. 1998, 136. [Google Scholar]
- Pessarakli, A.J. Handbook of Turfgrass Management and Physiology; CRC Press, Taylor & Francis Publishing Company: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-0648-3. [Google Scholar]
- Patton, A.J.; Hardebeck, G.A.; Williams, D.W.; Reicher, Z.J. Establishment of Bermudagrass and Zoysiagrass by Seed. Crop Sci. 2004, 44, 2160–2167. [Google Scholar] [CrossRef]
- Hanna, W.W.; Anderson, W.F. Development and Impact of Vegetative Propagation in Forage and Turf Bermudagrasses. Agron. J. 2008, 100, S-103–S-107. [Google Scholar] [CrossRef]
- Patton, A.J.; Richardson, M.D.; Karcher, D.E.; Boyd, J.W.; Reicher, Z.J.; Fry, J.D.; McElroy, J.S.; Munshaw, G.C. A Guide to Establishing Seeded Bermudagrass in the Transition Zone. Ats 2008, 5, 19. [Google Scholar] [CrossRef]
- Pornaro, C.; Macolino, S.; Leinauer, B. Seeding time affects establishment of warm-season turfgrasses. Acta Hortic. 2016, 1122, 27–34. [Google Scholar] [CrossRef]
- Rimi, F.; Macolino, S.; Leinauer, B.; Ziliotto, U. Green-up of Seeded Bermudagrass Cultivars as Influenced by Spring Scalping. HortTechnology 2011, 21, 230–235. [Google Scholar] [CrossRef]
- HNMS Hellenic National Meteorological Service. Available online: http://www.emy.gr/emy/en/ (accessed on 6 May 2019).
- Musser, H.B.; Perkins, A.T. Guide to Seedbed Preparation. In Hanson, A.A., and Juska, F.V. Turfgrass Science; American Society of Agronomy: Madison, WI, USA, 1969; pp. 462–473. [Google Scholar]
- Richardson, M.D.; Karcher, D.E.; Berger, P.; Boyd, J.W. Utilizing improved seeded bermudagrasses on transition-zone sports fields. Acta Hortic. 2004, 661, 369–374. [Google Scholar] [CrossRef]
- Shaver, B.R.; Richardson, M.D.; McCalla, J.H.; Karcher, D.E.; Berger, P.J. Dormant Seeding Bermudagrass Cultivars in a Transition-Zone Environment. Crop Sci. 2006, 46, 1787–1792. [Google Scholar] [CrossRef]
- Deaton, M.T.; Williams, D.W. Temperature Effects on the Speed and Completion of Germination of 19 Commercially Available Seeded Bermudagrass Cultivars. HortTechnology 2013, 23, 82–85. [Google Scholar] [CrossRef]
- Giolo, M.; Ferrari, F.; Macolino, S. Estimation of Base Germination Temperature of Ten Seeded-Type Bermudagrass Cultivars. Eur. J. Hortic. Sci. 2014, 79, 129–134. [Google Scholar]
- Fontenot, D.P. Evaluating Seashore Paspalum Seed Germination and Enhancement, Erosion Abatement and Potential Use as a Vegetative Landfarm Cap. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2007. [Google Scholar]
- Unruh, J.B.; Gaussoin, R.E.; Wiest, S.C. Basal Growth Temperatures and Growth Rate Constants of Warm-Season Turfgrass Species. Crop Sci. 1996, 36, 997–999. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds:Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4693-4. [Google Scholar]
- Garcia-Huidobro, J.; Monteith, J.L.; Squire, G.R. Time, Temperature and Germination of Pearl Millet (Pennisetum typhoides S. & H.): I. constant temperature. J. Exp. Bot. 1982, 33, 288–296. [Google Scholar]
- Sandlin, T.N.; Munshaw, G.; Philley, H.W.; Baldwin, B.S.; Steward, B.S. Temperature Affects Germination of Seeded Bermudagrasses. In Proceedings of the ASA-CSSA-SSSA International Annual Meeting, Indianapolis, IN, USA, 12–16 November 2006. [Google Scholar]
- Wu, L.; Huff, D.; Harivandi, M. Buffalograss as a low-maintenance turf. Calif. Agric. 1989, 43, 23–25. [Google Scholar]
- Johnson, P.G.; Riordan, T.P.; Johnson-Cicalese, J. Low-Mowing Tolerance in Buffalograss. Crop Sci. 2000, 40, 1339–1343. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). International Rules for Seed Testing, 2013 ed.; ISTA: Zurich, Switzerland, 2013; ISBN 13 978-3-906549-72-9. [Google Scholar]
- Svoboda, J.F. Seedling Germination and Establishment of Buffalograss Caryopses vs. Burs. Master’s Thesis, Faculty of The Graduate College of the University of Nebraska, Lincoln, NE, USA, 1991. [Google Scholar]
- Regional Agency for Environmental Protection of Veneto Region (ARPAV). Dipartimento per la Sicurezza del Territorio. Centro Meterologico: Teolo, Padova, Italy, 2019. Available online: http://www.arpa.veneto.it/temi-ambientali/climatologia/dati (accessed on 11 April 2019).
- Weisberg, S. Applied Linear Regression|Wiley Series in Probability and Statistics, 3rd ed.; John Wiley & Sons Inc. Books: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of germination data from agricultural experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Onofri, A.; Benincasa, P.; Mesgaran, M.B.; Ritz, C. Hydrothermal-time-to-event models for seed germination. Eur. J. Agron. 2018, 101, 129–139. [Google Scholar] [CrossRef]
- Masin, R.; Onofri, A.; Gasparini, V.; Zanin, G. Can alternating temperatures be used to estimate base temperature for seed germination? Weed Res. 2017, 57, 390–398. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar] [CrossRef]
- McMaster, G. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Moore, J.L.; Remais, J.V. Developmental models for estimating ecological responses to environmental variability: Structural, parametric, and experimental issues. Acta Biotheor. 2014, 62, 69–90. [Google Scholar] [CrossRef]
- R Core Team. R 3.5.0 R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Ritz, C.; Strebig, J.C. Analysis of Dose-Response Curves, “drc”, R package version 3.0-1; 2016. Available online: https://rdrr.io/cran/drc/ (accessed on 15 May 2019).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means. 2018. Available online: https://rdrr.io/cran/emmeans/man/emmeans.html (accessed on 30 May 2019).
- Bradford, K.J. Applications of hydrothermal time to quantifying and modeling seed germination and dormancy. Weed Sci. 2002, 50, 248–260. [Google Scholar] [CrossRef]
- The Council of the European Economic Community, Council Directive of 14 June 1966 on the Marketing of Fodder Plant Seed. Conditions to be Satisfied by the Seed; EU Publications: Luxembourg, Luxembourg, 1966. [Google Scholar]
- Shin, J.S.; Raymer, P.; Kim, W. Environmental factors influencing germination in seeded seashore paspalum. Hortscience 2006, 41, 1330–1331. [Google Scholar] [CrossRef]
- Budak, H.; Shearman, R.C.; Parmaksiz, I.; Dweikat, I. Comparative analysis of seeded and vegetative biotype buffalograsses based on phylogenetic relationship using ISSRs, SSRs, RAPDs, and SRAPs. Theor. Appl. Genet. 2004, 109, 280–288. [Google Scholar] [CrossRef]
- Larsen, S.; Martin Bibby, B. Differences in Thermal Time Requirement for Germination of Three Turfgrass Species. Crop Sci. 2005, 45, 2030–2037. [Google Scholar] [CrossRef]
- OECD. The OECD List of Varieties Eligible for Seed Certification, 2019th Edition. Available online: http://www.oecd.org/agriculture/seeds/documents/codes-and-schemes-list-of-varieties-eligible-for-seed-certification.pdf (accessed on 5 May 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).