UV-B Exposure of Black Carrot (Daucus carota ssp. sativus var. atrorubens) Plants Promotes Growth, Accumulation of Anthocyanin, and Phenolic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation
2.2. UV-B Light Treatment
2.3. Harvest, Sample Collection, and Processing
2.4. Determination of Total Monomeric Anthocyanin Content (TMC) and Total Phenolic Content (TPC)
2.5. Phytohormones Determination
2.6. Statistical Analyses
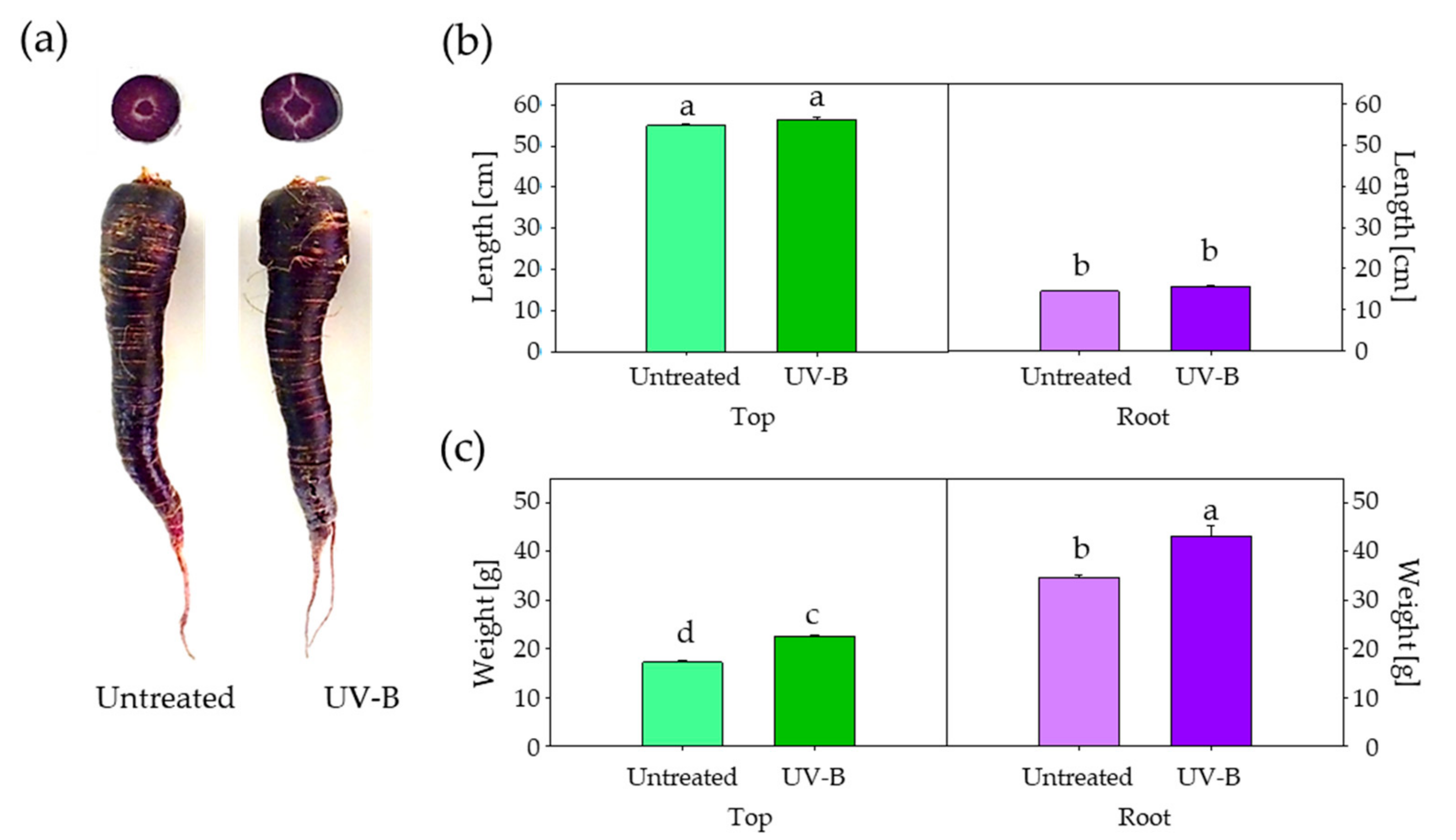
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barba-Espín, G.; Glied, S.; Crocoll, C.; Dzhanfezova, T.; Joernsgaard, B.; Okkels, F.; Lütken, H.; Müller, R. Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. sativus var. atrorubens Alef.). BMC Plant Biol. 2017, 17, 70. [Google Scholar] [CrossRef]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; Barreca, D.; D.’Angelo, V.; Germanò, M.P.; Trombetta, D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia 2018, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Burri, S.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Huda-Faujan, N.; Noriham, A.; Abdullah Sani, N.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009, 8, 484–489. [Google Scholar]
- Horvath, D.M.; Chua, N.H. The role of salicylic acid in systemic acquired resistance. Curr. Opin. Biotechnol. 1994, 5, 131–136. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros-Zevallos, L. Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar] [CrossRef]
- Heisler, G.M.; Grant, R.H.; Gao, W.; Slusser, J.R. Ultraviolet radiation and its impacts on agriculture and forests. Agric. For. Meteorol. 2003, 120, 3–7. [Google Scholar] [CrossRef]
- Jenkins, G.I. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Teramura, A.H.; Tevini, M.; Bornman, J.F.; Björn, L.O.; Kulandaivelu, G. Effects of increased solar ultraviolet radiation on terrestrial plants. In United Nations Environment Programme. Environmental Effects of Ozone Depletion: 1994 Assessment; United Nations Environment Program: Nairobi, Kenya, 1995; pp. 166–173. [Google Scholar]
- Searles, P.S.; Flint, S.D.; Caldwell, M.M. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 2001, 127, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K. Ultraviolet-B radiation effects on plants: Induction of morphogenic responses. Physiol. Plant. 2002, 116, 423–429. [Google Scholar] [CrossRef]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.R. The effects of ultraviolet-B radiation on plants: A molecular perspective. Adv. Bot. Res. 1996, 22, 97–162. [Google Scholar]
- Jansen, M.; Bornman, J.F. UV-B radiation: From generic stressor to specific regulator. Physiol. Plant. 2012, 145, 501–504. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. The interplay between light and jasmonate signalling during defence and development. J. Exp. Bot. 2011, 62, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Mao, J.; Yang, S.J.; Guo, Z.G.; Ma, Z.H.; Dawuda, M.M.; Zuo, C.W.; Chu, M.Y.; Chen, B.H. Anthocyanin accumulation correlates with hormones in the fruit skin of ‘Red Delicious’ and its four generation bud sport mutants. BMC Plant Biol. 2018, 18, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Duchovskis, P.; Urbonavičiūtė, A. Phytohormones dynamics during flowering initiation in carrots. Acta Biol. Szeged. 2005, 49, 33–37. [Google Scholar]
- Kobayashi, M.A.Y.; Mondal, M.F.; Ban, T.; Matsubara, H.; Adachi, F.; Asao, T. Growing carrots hydroponically using perlite substrates. Sci. Hortic. 2013, 159, 113–121. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Großkinsky, D.K.; Albacete, A.; Jammer, A.; Krbez, P.; van der Graaff, E.; Pfeifhofer, H.; Roitsch, T. A rapid phytohormone and phytoalexin screening method for physiological phenotyping. Mol. Plant 2014, 7, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.J.; Sørensen, S.; Burow, M.; Veierskov, B. Characterization of Top Leader Elongation in Nordmann Fir (Abies nordmanniana). J. Plant Growth Regul. 2019, 1–8. [Google Scholar] [CrossRef]
- Robson, T.M.; Aphalo, P.J.; Banaś, A.K.; Barnes, P.W.; Brelsford, C.C.; Jenkins, G.I.; Kotilainen, T.K.; Łabuz, J.; Martínez-Abaigar, J.; Morales, L.O.; et al. A perspective on ecologically relevant plant-UV research and its practical application. Photochem. Photobiol. Sci. 2019, 18, 970–988. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A. Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L. The Use of Controlled Postharvest Abiotic Stresses as a Tool for Enhancing the Nutraceutical Content and Adding-Value of Fresh Fruits and Vegetables. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Solovchenko, A.; Merzlyak, M.N. Screening of Visible and UV Radiation as a Photoprotective Mechanism in Plants. Russ. J. Plant Physiol. 2008, 55, 719–737. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Krumbein, A.; Neugart, S.; Li, L.; Schreiner, M. Mixed cropping with maize combined with moderate UV-B radiations lead to enhanced flavonoid production and root growth in faba bean. J. Plant Interact. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrich, C.; Huyskens-Keil, S. Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innov. Food Sci. Emerg. Technol. 2009, 10, 93–96. [Google Scholar] [CrossRef]
- Neugart, S.; Zietz, M.; Schreiner, M.; Rohn, S.; Kroh, L.W.; Krumbein, A. Structurally different flavonol glycosides and hydroxycinnamic acid derivatives respond differently to moderate UV-B radiation exposure. Physiol Plant. 2012, 145, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Villar, R. Fate of acquired carbon in plants: Chemical composition and construction costs. In Plant Resource Allocation; Bazzaz, F.A., Grace, J., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 39–72. [Google Scholar]
- Nithia, S.M.J.; Shanthi, N.; Kulandaivelu, G. Different responses to UV-B enhanced solar radiation in radish and carrot. Photosynthetica 2005, 43, 307–311. [Google Scholar] [CrossRef]
- Tong, H.; Leasure, C.D.; Hou, X.; Yuen, G.; Briggs, W.; He, Z.H. Role of root UV-B sensing in Arabidopsis early seedling development. Proc. Natl. Acad. Sci. USA 2008, 105, 21039–21044. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Köllmer, I.; Novak, O.; Strnad, M.; Schmülling, T.; Werner, T. Overexpression of the cytosolic cytokinin oxidase/dehydrogenase (CKX7) from Arabidopsis causes specific changes in root growth and xylem differentiation. Plant J. 2014, 78, 359–371. [Google Scholar] [CrossRef]
- Kudo, T.; Makita, N.; Kojima, M.; Tokunaga, H.; Sakakibara, H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012, 160, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Velanis, C.N.; Jenkins, G.I.; Franklin, K.A. UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. PNAS 2014, 111, 11894–11899. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, V.Y.; Karyagin, V.V.; Rakitina, T.Y.; Prudnikova, O.N.; Vlasov, P.V. UV-B stress-induced ABA production in Arabidopsis thaliana mutants defective in ethylene signal transduction pathway. Russ. J. Plant Physiol. 2008, 55, 854–856. [Google Scholar] [CrossRef]
- Gajdosová, S.; Spíchal, L.; Kamínek, M.; Hoyerová, K.; Novák, O.; Dobrev, P.I.; Galuszka, P.; Klíma, P.; Gaudinová, A.; Zizková, E.; et al. Distribution, biological activities, metabolism, and theconceivable function of cis-zeatin-type cytokinins in plants. J. Exp. Bot. 2011, 62, 2827–2840. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Escobar Bravo, R.; Chen, G.; Grosser, K.; Van Dam, N.M.; Leiss, K.A.; Klinkhamer, P.G.L. Ultraviolet radiation enhances salicylic acid-mediated defense signaling and resistance to Pseudomonas syringae DC3000 in a jasmonic acid-deficient tomato mutant. Plant Signal. Behav. 2019, 14, e1581560. [Google Scholar] [CrossRef] [PubMed]



| ABA | IAA | JA | JA-Ile | SA | cZ | t-ZROG | t-ZR | Weight | Length | TMC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IAA | −0.77 *** | ||||||||||
| JA | −0.84 *** | 0.71 * | |||||||||
| JA-Ile | −0.66 ** | 0.84 *** | 0.83 *** | ||||||||
| SA | −0.34 | 0.24 | 0.20 | 0.37 | |||||||
| cZ | 0.72 * | −0.21 | 0.04 | −0.04 | −0.75 * | ||||||
| t-ZROG | −0.84 *** | 0.81 *** | 0.89 *** | 0.77 *** | 0.37 | −0.33 | |||||
| t-ZR | 0.32 | −0.18 | −0.51 | −0.41 | 0.08 | 0.01 | −0.31 | ||||
| Weight | 0.86 *** | −0.73 *** | −0.87 *** | −0.69 ** | −0.27 | 0.26 | −0.87 *** | 0.31 | |||
| Length | −0.97 *** | 0.81 *** | 0.88 *** | 0.68 ** | 0.24 | −0.03 | 0.83 *** | −0.44 | −0.85 *** | ||
| TMC | 0.98 *** | −0.78 *** | −0.88 *** | −0.68 ** | −0.24 | −0.00 | −0.85 *** | 0.38 | 0.89 *** | −0.98 *** | |
| TPC | 0.98 *** | −0.79 *** | −0.88 *** | −0.70 ** | −0.28 | 0.39 | −0.83 *** | 0.51 | 0.88 *** | −0.98 *** | 0.97 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, R.; Acosta-Motos, J.R.; Großkinsky, D.K.; Hernández, J.A.; Lütken, H.; Barba-Espin, G. UV-B Exposure of Black Carrot (Daucus carota ssp. sativus var. atrorubens) Plants Promotes Growth, Accumulation of Anthocyanin, and Phenolic Compounds. Agronomy 2019, 9, 323. https://doi.org/10.3390/agronomy9060323
Müller R, Acosta-Motos JR, Großkinsky DK, Hernández JA, Lütken H, Barba-Espin G. UV-B Exposure of Black Carrot (Daucus carota ssp. sativus var. atrorubens) Plants Promotes Growth, Accumulation of Anthocyanin, and Phenolic Compounds. Agronomy. 2019; 9(6):323. https://doi.org/10.3390/agronomy9060323
Chicago/Turabian StyleMüller, Renate, José R. Acosta-Motos, Dominik K. Großkinsky, José A. Hernández, Henrik Lütken, and Gregorio Barba-Espin. 2019. "UV-B Exposure of Black Carrot (Daucus carota ssp. sativus var. atrorubens) Plants Promotes Growth, Accumulation of Anthocyanin, and Phenolic Compounds" Agronomy 9, no. 6: 323. https://doi.org/10.3390/agronomy9060323
APA StyleMüller, R., Acosta-Motos, J. R., Großkinsky, D. K., Hernández, J. A., Lütken, H., & Barba-Espin, G. (2019). UV-B Exposure of Black Carrot (Daucus carota ssp. sativus var. atrorubens) Plants Promotes Growth, Accumulation of Anthocyanin, and Phenolic Compounds. Agronomy, 9(6), 323. https://doi.org/10.3390/agronomy9060323








