1. Introduction
Environmental stresses limit plant growth and yield, leading to high losses. In particular, chilling affects the development and productivity of crops originating from subtropical regions [
1,
2], such as tomato [
3]. Tomato (
Solanum lycopersicum L.) is an economically important horticultural crop [
4,
5], cultivated worldwide over ~4.7 million ha, and with a total production of ~177 million tons [
6].
Tomato fruits are consumed fresh in salads and sandwiches or processed into products like whole peeled, diced products, juices, sauces and soups [
7]. The genotypes suitable for processing tomato production are bred for adaptation to mechanical harvesting and canning purposes [
8]. Most of the cultivated processing tomato genotypes are sensitive to low temperatures (0–12 °C) in all growth stages [
9]. The negative effects of chilling are more remarkable during germination and at the seedling stage [
10]. Chilling damages cellular membranes, generates Reactive Oxygen Species (ROS) and accumulates toxic compounds [
11,
12,
13]. In addition, the chloroplasts are the primary visible sites of chilling injury [
14] that causes a reduction of chlorophyll fluorescence and of photosynthetic activity [
15].
Unlike the fresh tomato that may also be cultivated in heated greenhouses (preserving plant to chilling occurrences), processing tomato plants are cultivated only in open fields where chilling occurrences cannot be avoided. In Mediterranean environments, such as Italy and Spain, the growing season starts in March–April, when the probability of chilling events is still high [
16]. A typical management practice applied by farmers is to transplant in the field processing tomato seedlings produced in nursery. Besides the possibility of chill spells in early spring, an increase of temperature and more frequent drought events have been reported in climatic models for the 21st century, especially in Southern European countries [
17]. Warmer temperatures can accelerate tomato phenology, resulting in lower total biomass accumulation with a negative impact on yield [
18]. It is therefore expected that the practice of early transplant will become increasingly widespread.
The use of beneficial microorganisms could be a sustainable way that allows a reduction of external inputs and improve tolerance to biotic and abiotic stresses [
19]. In fact, microorganisms, such as arbuscular mycorrhizal fungi (AMF) or/and plant growth promoting rhizobacteria (PGPR), can increase plant tolerance to abiotic stresses like drought, salinity, metal toxicity and high temperature on many crops like wheat (
Triticum aestivum L.), sunflower (
Helianthus annuus L.), pea (
Pisum sativum L.), barley (
Hordeum vulgare L), rice (
Oryza sativa L.), tomato (
Solanum lycopersicum L.) grapevine (
Vitis vinifera L.), lettuce (
Lactuca sativa L.) and corn (
Zea mays L.) [
20,
21,
22,
23]. AMF belong to phylum Glomeromycota and are the most widespread fungal symbionts of plants [
24,
25], including many agricultural crops [
26]. AMF provide several benefits to host plants, like enhanced water and nutrient uptake, increased tolerance to soil-borne pathogens and environmental stresses, reduced sensitivity to heavy metals and positive contribution to soil structure [
27,
28,
29,
30,
31,
32,
33]. These benefits could be ascribed to the influence of AMF on plant physiology and, in particular, on plastid biosynthetic pathways, Krebs cycle and secondary metabolism [
32].
PGPR include a wide range of microorganisms which positively influence growth, yield and stress tolerance of plants through several direct and indirect mechanisms of actions colonizing both the rhizosphere and the endo-rhizosphere [
34,
35,
36,
37]. PGPR can induce physical and chemical changes in the plants by producing enzymes, osmolytes, siderophores and organic acids or/and by triggering the plants to produce hormones [
21,
36,
37,
38]. Ait Barka et al. [
39] reported an increase of phenolic compounds and starch in leaves and shoots of grapevine cv. Chardonnay after treatment with the endophytic bacterium
Paraburkholderia phytofirmans. In addition, when explants of grapevine were exposed to 4 °C for 2 weeks, an increase of the content of proline was recorded compared to the un-inoculated control. Interestingly, some PGPR, such as
Agrobacterium spp.,
Azospirillum spp.,
Azotobacter spp. and
(Para)Burkholderia spp., can promote mycorrhiza colonization (in this case, the PGPR are named mycorrhiza helper bacteria–MHB) [
40].
Although some evidence of the positive influence of AMF and PGPR under sub-optimal temperatures has been reported [
39,
41,
42] on tomato and cucumber (
Cucumis sativus L.), a precise characterization of the physiological responses in terms of photochemical efficiency of photosystem II (PSII), integrity of cell membranes, recovery and regrowth capacity of inoculated tomato plants exposed to severe chilling (1 °C for 24 h) is missing. In fact, these parameters are very important because, as previously reported, the earliest visible damage caused by chilling stress is the impairment of the integrity of cell membranes and of photosystem activity. In addition, a study on four tomato recombinant inbred lines inoculated with AFM and PGPR showed that, during doughty stress, the microbial inoculant effects were depending on the recombinant inbred line considered [
23].
Hence, the objectives of this research were to investigate: (i) the efficacy of F. mosseae and P. graminis C4D1M in avoiding injuries to cell membranes and reduction of PSII efficiency after severe stress (1 °C for 24 h); (ii) the synergic effect of the two microorganisms inoculated as a consortium; (iii) if the putative microorganism effects depended on the processing tomato genotype.
3. Results
3.1. Physiological, Morphological and Agronomic Parameters Evaluated before Chilling Stress
Measurements of morpho-physiological and agronomic parameters, such as the ratio between height and diameter, dry weights, and leaf content of chlorophyll, flavonoid and anthocyanin, represent a relevant indicator of the plant status already before stress exposure [
52,
53,
54]. Accordingly, in order to evaluate plant status, some physiological and morphological parameters were assessed before chilling stress exposure (
Table 2A,B).
Considering the physiological parameters, the seedlings treated with B showed always the highest values of chlorophyll leaf content (CHL0), +33% and +14%, in the first and second experiments, respectively, in comparison with the non-inoculated control (
Table 2A,B). In the second experiment, the genotype ‘Pearson’ showed the highest leaf content of chlorophyll while ‘Everton’ showed the lowest leaf content of flavonoids and anthocyanins and the highest NBI value (
Table 2B). Regarding the morphological and agronomic parameters, in the second experiment, the seedling, generally, showed a higher development (D0, LN0, LDW0, SDW0, RDW0 and TDW0) in comparison with the seedling of the first experiment. In both experiments, the non-inoculated control showed the highest diameter and G + B showed the highest H/D0 ratio (+20.3% and +33.8%, in the first and in the second experiments, respectively, in comparison with the non-inoculated control). In the first experiment the inoculated seedlings showed the highest fraction of total dry weight to roots (G + 47.8%; B + 26.1% and G + B +68.2% in comparison with the non-inoculated treatment) (
Table 2A). On the other hand, the inoculated seedlings showed the highest total dry weight (G + 42.9%, B + 19.0% and G + B + 28.6% in comparison with the non-inoculated treatment) in the second experiment.
‘Pearson’ ranked first for many parameters (H0, D0, LN0, LA0, LDW0, SDW0, RDW0 and TDW0); while the modern genotype ‘Everton’ and ‘H3402′ showed similar morphological characteristics for LA0, LDW0, SDW0, RDW0 and TDW0 (
Table 2B).
No interaction was observed between treatments and genotypes apart for H0 and FLV0. Regarding H0, ‘H3402′ inoculated with B showed the lowest values, while ‘Pearson’ inoculated with G + B the highest ones. For FLV0 ‘Everton’ non-inoculated and ‘H3402′ inoculated with G + B showed the lowest values, while ‘H3402′ inoculated with B showed the highest ones.
In order to verify the photochemical efficiency of PSII before chilling exposure, Fv/Fm values were measured. All the seedling showed Fv/Fm values higher than 0.600 and in the second experiment G, B and G + B highlighted higher values than the non-inoculated treatment.
3.2. Effects of AMF and PGPR Inoculations on Leaf Damage and Performance after Chilling Stress
In order to verify whether AMF and PGPR inoculations help processing tomato seedlings to overcome chilling stress, the size of cell membrane damage and the photochemical efficiency of PSII were assessed (
Table 3A,B) at the end of chilling stress and after 24 h.
At the end of chilling stress, all the treatments with microorganisms reduced the EL% 24 h values and the treatment G displayed lower values of EL% 24 h in comparison with the non-inoculated seedlings (−49.21% and −65.26% in experiment 1 and 2, respectively). In addition, in the second experiment, all the treatments with microorganisms reported higher Fv/Fm 24 h ratios.
In both experiments, all the treatments with microorganisms increased the Fv/Fm 48 h ratio and the seedlings inoculated with G showed the highest values (+351.0% and +47.0%, in the first and in the second experiment, respectively, in comparison with the non-inoculated seedlings) 24 h after chilling treatment. Considering the genotype effect, ‘Everton’ and ‘Person’ recorded the higher values compared to ‘H3402′.
3.3. Effects of AMF and PGPR Inoculation after Regrowth
In order to evaluate the effects of microorganism inoculations on regrowth capacity, some parameters were also evaluated 15 days after the end of the stress (
Table 4A,B).
In both experiments, the treatment containing microorganisms increased the
Fv/
Fm 15 days ratio in comparison with the non-inoculated control. In the second experiment the highest
Fv/
Fm 15 days ratio was showed by G + B (+44.4%, in comparison with the non-inoculated seedlings). When the interaction between genotype and treatment was considered, the best
Fv/
Fm 15 days ratios were shown by ‘Everton’ inoculated with G and ‘H3402′ inoculated with G + B. For chlorophyll assessment, in the second experiment, all the treatments with microorganisms increased the values of CHL 15 days. In general, after chilling stress, in both experiments the FLAV 15 days values increased and NBI 15 days values decreased in comparison with the values measured before chilling stress (FLAV0 and NBI0). In the second experiment, treatment G showed the highest values of FLAV 15 days, while G + B recorded the lowest value of ANTH 15 days. At the end of the regrowth period, the long-term effect of treatments was evaluated also by VS 15 days (
Figure 1). In the first experiment, the seedlings inoculated with G and B showed the best regrowth capacity recording both a value of VS 15 days of 4.0. In addition, treatment G also confirmed the best regrowth capacity in the second experiment (VS 15 days = 4.4). Regarding the genotype effect, in the second experiment, ‘H3402′ showed the highest VS 15 days while ‘Pearson’ was the most damaged.
3.4. AMF Root Colonization in Tomato Seedling after Chilling Stress
Based on real-time analysis the presence of
F. mosseae DNA was confirmed in the processing tomato roots inoculated with
F. mosseae and with
F. mosseae +
P. graminis (
Figure 2). In contrast, all non-inoculated roots (controls) and the
P. graminis inoculated roots were negative for
F. mosseae DNA presence (flat lines). Significant differences were found for AMF colonization among treatments, but not among genotypes (
p = 0.586).
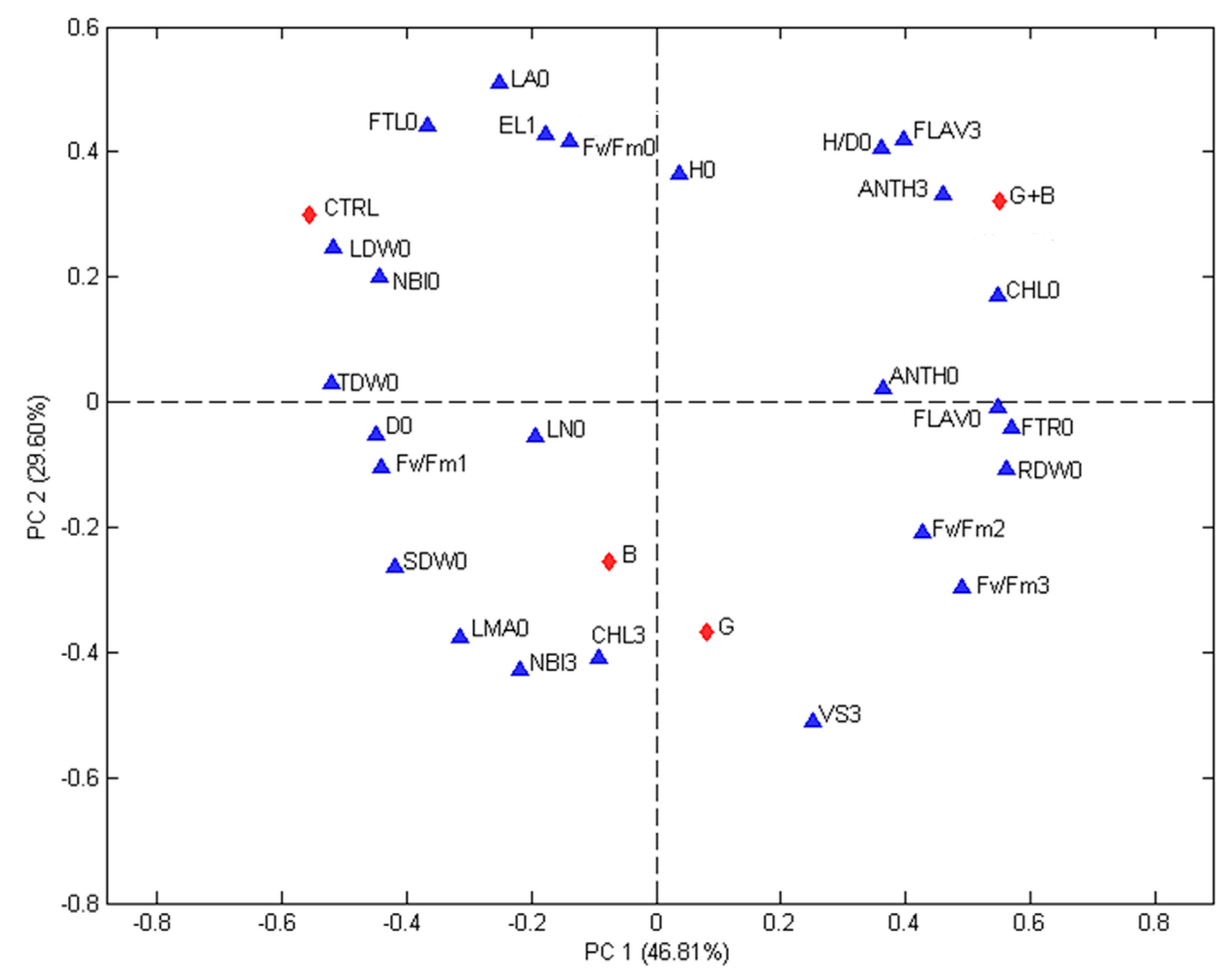
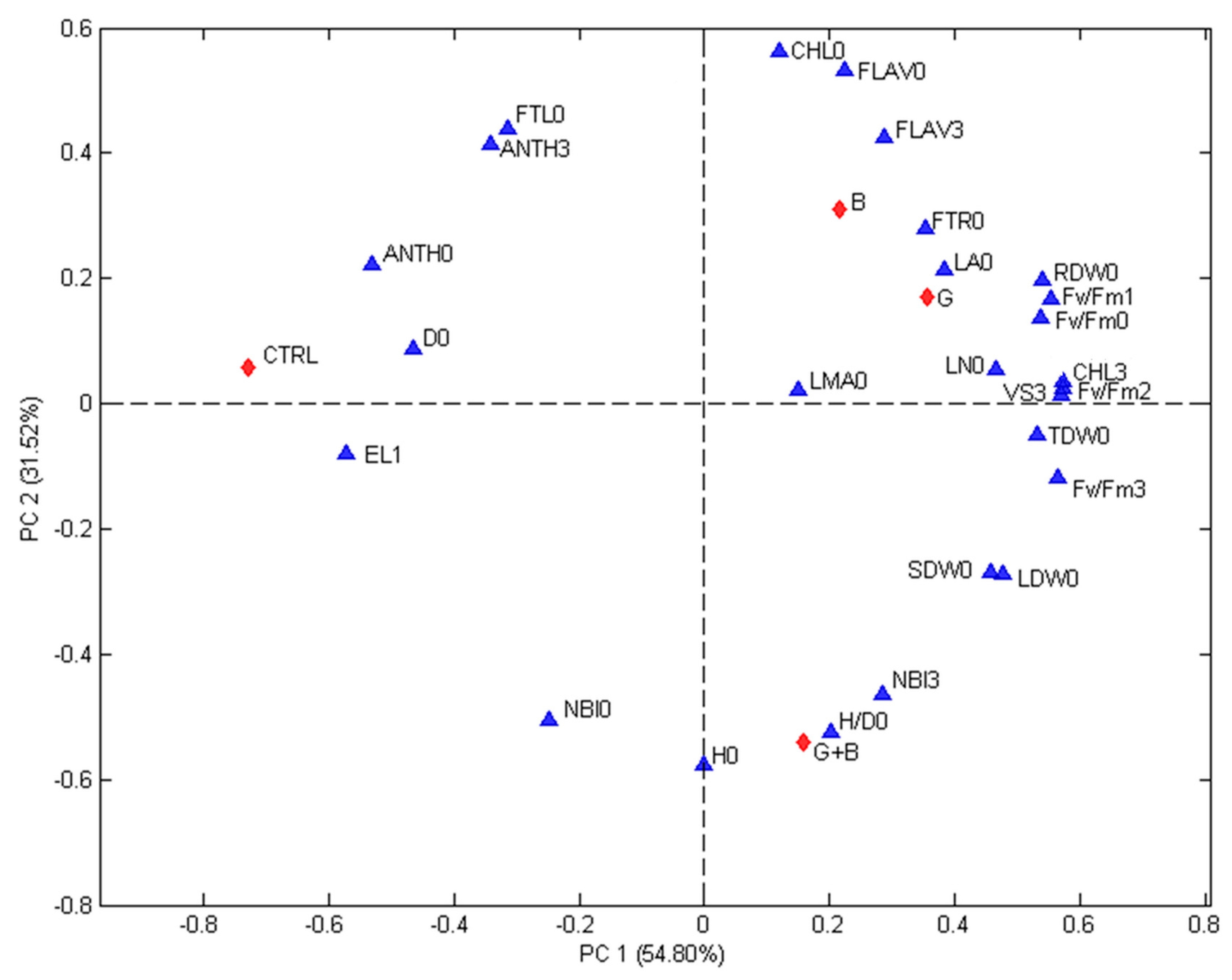
3.5. Relationships between Treatments and Evaluated Parameters
The correlations among treatments and evaluated parameters were studied using PCA.
Figure 3 (first experiment) and
Figure 4 (second experiment) show the biplots of the PCA models calculated for each experiment. The contributions of the two first principal components are 46.81% (PC1) and 29.60% (PC2) for the first experiment and 54.80% (PC1) and 31.52% (PC2) for the second one. In both Figures, differences among treatments and recorded parameters are visible and the first principal component gives an indication on the effect of treatments. The non-inoculated seedlings were always found on the top left quadrant while genotype inoculated with G and G + B were always found on the positive side. In both experiments, the seedlings inoculated with G were associated with VS15 days,
Fv/
Fm 48 h and
Fv/
Fm 15 days, while seedlings inoculated with G + B were linked to H/D0 ratio. In both experiments D0, NBI0, EL24 h, and FTL0 were associated with the control (non-inoculated seedlings).
The studied parameters (blue triangles) are: H/D = height to diameter ratio, CHL = index of the chlorophyll content in a leaf measured using a DUALX instrument, FLAV = index of the flavonoids content in leaf measured using a DUALX instrument, ANTH = index of the anthocyanins content in leaf measured using a DUALX instrument, NBI = nitrogen balance index, LN (no.) = number of leaves per seedling, LA = leaf area, LDW = leaf dry weight, SDW = stem dry weight, RDW = root dry weight, TDW = total dry weight, FTL = fraction of total dry weight to leaves, FTR = fraction of total dray weight to roots, LMA = leaf mass area, Fv/Fm = photochemical efficiency of photosystem II (PSII), EL = electrolyte leakage, VS 3 = VS 15 days = visual score recorded, 0 = measured or recorded before stress, 1 = measured or recorded immediately after the stress, 2 = measured or recorded 24 h after the end of the stress, 3 = measured or recorded at regrowth (15 days after stress). The studied treatments (red diamonds) are: CTRL = seedlings without microorganism treatment, G = seedlings inoculated with Funneliformis mosseae, B = seedlings inoculated with Paraburkholderia graminis, and G + B = seedlings inoculated with Funneliformis mosseae + Paraburkholderia graminis.
The studied parameter (blue triangles) are: H/D = height to diameter ratio, CHL = index of the chlorophyll content in leaf measured using a DUALX instrument, FLAV = index of the flavonoid content in leaf measured using a DUALX instrument, ANTH = index of the anthocyanin content in leaf measured using a DUALX instrument, NBI = nitrogen balance index, LN (no.) = number of leaves per seedling, LA = leaf area, LDW = leaf dry weight, SDW = stem dry weight, RDW = root dry weight, TDW = total dry weight, FTL = fraction of total dry weight to leaves, FTR = fraction of total dray weight to roots, LMA = leaf mass area, Fv/Fm = photochemical efficiency of photosystem II (PSII), EL = electrolyte leakage, VS 3 = VS 15 days = the visual score recorded, 0 = measured or recorded before stress, 1 = measured or recorded immediately after the stress, 2 = measured or recorded 24 h after the end of the stress, 3 = measured or recorded at regrowth (15 days after stress). The studied treatments (red diamonds) are: CTRL = seedlings without microorganism treatment, G = seedlings inoculated with Funneliformis mosseae, B = seedlings inoculated with Paraburkholderia graminis, and G + B = seedlings inoculated with Funneliformis mosseae + Paraburkholderia graminis.
4. Discussion
Processing tomato is a globally important horticultural crop [
55]. It is generally grown in high-input conventional systems [
56] and typically transplanted during spring, a period in which chill spells could still occur in Mediterranean environments. Hence, to improve agricultural sustainability, innovative strategies are required that can improve processing tomato tolerance to environmental stresses, avoiding high yield losses [
57]. The wide variety of beneficial microorganisms present in the rhizosphere could be used to help crops to overcome abiotic stresses that reduce external inputs, thereby facilitating sustainable agricultural production [
58]. In the present work, the physiological responses (efficacy of PSII, cell membrane injuries in term of EL%), recovery and regrowth capacity of processing tomato genotypes inoculated with
F. mosseae,
P. graminis and their consortium under severe chilling stress (1 °C for 24 h) were evaluated. Before chilling stress, physiological, morphological and agronomic parameters were measured to evaluate the effect of the treatments and the genotypes in the absences of stress. Leaf chlorophyll content value (CHL) is considered to be a good indicator of the status of plants, because it is the key pigment involved in photosynthesis [
53]. In the present study, the seedlings inoculated with
P. graminis showed an increase of leaf chlorophyll content (CHL0) (
Table 2A,B). These results could be due to different reasons. Some studies reported capability of bacteria of the genus
Paraburkholderia to fix nitrogen asymbiotically [
59,
60]. In addition, a recent study [
61] showed that
Paraburkholderia graminis can produce gramibactin, a siderophore that can bind iron and an essential element for chlorophyll production. Among the three genotypes, the values of leaf chlorophyll content were higher in genotype ‘Pearson’ (
Table 2B), confirming the results showed by Ronga et al. [
16].
The height to diameter ratio of seedlings is an important parameter to assess the seedling quality in nursery production [
52,
54]. In the present study, the treatment containing the consortium (G + B) increased the height/diameter ratio of seedlings (
Table 2A,B) conferring more vigor to seedlings. Seedlings grown in nursery are often sown in alveolar fixed seed trays with very small holes, allowing the growth of higher number of plants per unit area. However, the container size can affect the development of seedlings [
62,
63]. In fact, in the second experiment, where the alveolar fixed seed trays with bigger holes were used, the seedlings had higher development (D0, LN0, LDW0, SDW0, RDW0 and TDW0). These results are consistent with findings previously reported by NeSmith and Duval [
63]. In addition, in the second experiment, the total dry weight values, recorded before chilling stress, confirming the previous results showed by reference [
42] under optimal growth temperature condition. In contrast, in the first experiment, there were not differences among treatments, therefore, it possible to presume an interaction between treatments and the higher amount of soil available to seedlings in the second experiments. However, this theory should be validated by further experiments.
Low temperature stress is known to reduce the development of the plant due to leaf tissue damage. In particular, injury of the membranes, with an increase in permeability, is the main effect caused by chilling stress [
1,
62,
63]. Electrolyte leakage (EL) is a useful parameter to measure cell membrane damage [
58,
64]; however, this parameter is influenced by plant and leaf age as well as leaf position on the plant [
64,
65,
66,
67]. Hence, in both experiments the measurements were conducted at the same seedling age (40 days after sowing) using the upper fully expanded leaves. In the present study, the obtained data proved the protective activity of studied microorganisms towards cell membranes. However, only in the first experiments, significant differences among the treatments containing microorganisms were found. This could be ascribed to the different agronomic performance of the seedlings. In fact, seedlings with higher development are more tolerant to environmental stresses [
52]. Therefore, it is possible to hypothesize that the higher tolerance of seedlings could have attenuated or hidden the different effects of the treatments at recovery. In addition, this hypothesis was supported by lesser EL values shown by the non-inoculated control in the second experiment in comparison with the first one.
The chloroplast is the primary site of a chilling injury [
68]. Chilling stress affected the photosystem putatively, leading to a photoinhibition of photosynthesis due to photoinactivation of catalase and a decline of variable fluorescence [
69]. Photosynthetic efficiency is a good marker to assess the effects of treatments and genotypes, after chilling stress.
Fv/
Fm parameter gives an idea of the PSII efficiency and, consequently, the damage of photosynthetic apparatus due to chilling stress [
70] and the ability for recovery and regrowth of the seedlings to occur. Before chilling stress, all seedlings showed
Fv/
Fm0 ratios ranging from 0.73 to 0.83 (
Table 2A,B), which are the typical values (
Fv/
Fm ratio) of many higher plants [
69]. Instead, immediately after the chilling stress, the values of the
Fv/
Fm ratio were lower than the 0.73–0.83 range [
71] (
Table 2B and
Table 3B). These data confirmed the results previously displayed by Caffagni et al. [
45] and Ronga et al. [
16]). In addition, in the first experiment differences between control and treatments were not observed. By contrast, in the second experiment, the seedlings inoculated with microorganisms showed higher values of
Fv/
Fm 24 h in comparison with the control seedling. It is possible to suppose that there was an interaction between treatments and higher amount of soil available for seedlings in the second experiments. However, this theory should be validated by further experiments. On the other hand, when the PSII efficiency was evaluated 24 h after the end of chilling stress (
Fv/
Fm 48 h), independent of genotype,
F. mosseae was the treatment that mainly preserved the PSII to chilling stress. The differences in protecting the PSII highlighted by the different treatments containing microorganisms could be due to different microhabitats of the two microorganisms (
F. mosseae vs.
P. graminis). In fact,
F. mosseae is an endophyte [
24] while
P. graminis lives in the rhizosphere [
72]. Therefore, the seedling tissues could have protected
F. mosseae from chilling stress [
23] and could have influenced his efficacy. In addition, a study performed by Liu et al. [
42] reported that
F. mosseae increased content of redox compounds in the tomato roots under optimal temperature. Therefore, the presence of redox compounds before the chilling stress could also make seedlings more reactive to overcome the chilling stress.
A good regrowth capacity after stress is an important and desirable ability of crops, as it allows plants to develop, in short time, new leaves and shoots that are very important for recovering photosynthetic activity and carbon fixation. All the treatments containing microorganisms showed a high efficiency of PSII 15 days after the chilling treatment. However, when the seedling had higher development (in the second experiment), the seedlings inoculated with the consortium (G + B) showed the best efficiency of PSII and a less content of secondary metabolites in the leaf (FLAV3 and ANTH3). When the genotype was considered, the same results were achieved by using the most modern genotype “Everton”. VS 15 days is another method used to assess the ability of regrowth of the seedlings. Our results revealed that the best performances were obtained when the seedlings were inoculated with F. mosseae or the genotype “H3402” was used. The opposed results between Fv/Fm 15 days and VS 15 days could be due to different aspects that they consider: while the Fv/Fm ratio only considers the efficacy of PSII, the VS 15 days considers the regrowth capacity of the whole plant in general (i.e., appearance of new leaves).
Knowledge of the interaction between genotype and treatment could help farmers in choosing the best microorganism to help plants to overcome environmental stress. Some studies on rice and tomato revealed that different genotypes of rice and tomato responded differently to different microorganism inoculations [
73,
74]. Also in our study, the three processing tomato genotypes showed different responses to the different treatments containing microorganisms. Interestingly, the more recent genotypes “Everton” achieved a higher
Fv/
Fm 15 days result when inoculated with
F. mosseae, while “H3402” achieved the higher
Fv/
Fm 15 days result when inoculated with the consortium. When we considered the interaction between
F. mosseae and
P. graminis, no differences were observed on the AFM colonization. These results agree with some studies [
75,
76,
77], in which bacteria treatments did not influence the AFM root colonization.
Also, the analysis of biplots confirmed the ability of microorganisms to help processing tomato seedlings during chilling stress. Interestingly, F. mosseae, Fv/Fm 48 h and Fv/Fm 15 days and VS 15 days were closely associated, proving that processing tomato seedlings successfully overcome chilling stress when inoculated with F. mosseae. In addition, FTL0 was always opposed to F. mosseae and Fv/Fm 15 days, suggesting that F. mosseae induced a reduction of the biomass allocated to leaf (improving biomass allocated to roots. This lower biomass allocated to leaf may lead to lower damage during chilling stress. However, further studies are necessary to corroborate this hypothesis.