1. Introduction
Barley has demonstrated a capacity to thrive in arid and semi-arid climates where drought is a determinant of crop productivity. The projected increase in incidence and severity of drought conditions combined with the predicted decline in water availability has sparked interest in crop production that is less water dependent [
1,
2]. As a result of the increasing demand for cereal grains, attributable to human and animal consumption, there is an emerging consensus that demand could be better met through the development, dissemination, and adoption of drought tolerant genotypes [
3,
4].
Major drought tolerance traits assessed in this study relate to crop growth, water use, and yield. Crop growth as measured by biomass is indicative of a crop’s ability to retain function in a dehydrated state [
5,
6,
7,
8]. Greater early-season leaf or shoot biomass, known as early vigor, is another drought adaptive trait. Early season canopy cover reduces evaporation from the soil surface leaving more water available for crop growth [
9,
10,
11]. Plant height under drought stress compared to under non-stress conditions is also an indicator of crop drought tolerance as is the allocation of carbohydrate reserves to grain filling, expressed by harvest index [
12,
13,
14].
Harvest index, the ratio of grain to total above-ground biomass, and grain yield in cereals is most affected by terminal drought stress, i.e., stress during the critical reproductive stage of anthesis. Therefore, traits associated with increased water access during grain fill, or changes in crop duration are associated with greater harvest index and grain yield [
15,
16]. A primary way to increase crop water access is through deeper roots, if subsurface water is available. Water use during this period is critical as photosynthate is allocated to grain growth [
17,
18]. In association with rooting depth, penetration ability of roots is of interest, as soil hardness generally increases as the soil dries [
19,
20].
Another means to improve water access is by adapting crop phenology to seasonal moisture availability. Considered one of the most impactful breeding strategies for low-water environments, faster phenological development has led to improved yield of cereals in Mediterranean-type environments characterized by winter precipitation and terminal drought stress [
21,
22,
23]. Shorter crop duration maximizes growth when temperatures and vapor pressure deficit are lower.
The development and release of one-irrigation barley cultivars marks a significant achievement in breeding efforts for drought tolerance; however, the specific physiological and phenological traits associated with their drought tolerance remain unclear. In an effort to elucidate these traits, two years of replicated field trials were conducted in Tucson, Arizona comparing low-input varieties “Solar” and “Solum” to high-input, conventional varieties “Kopious” and “Cochise”. We hypothesize that the low-input varieties will perform better under low water conditions and vice versa at high water conditions due to differences in yield components, phenology, water use patterns, and rooting characteristics.
2. Materials and Methods
2.1. Varietal Information
Development of barley cultivars adapted to water-limited environments was the motivation for a breeding program headed by R.T. Ramage and R. K. Thompson with the Agriculture Experiment Station (AES) of the University of Arizona and the Agriculture Research Service (ARS), USDA. In 1981, Composite Cross XXXIX was released, a population adapted to a single irrigation of 15 cm to 20 cm applied at or before planting (enough to wet the soil profile to a depth of 1.5 m to 1.8 m), with normal seasonal rainfall around 7 cm to 11 cm in Southwest Arizona. Selection criteria included turgid plants at flowering, plump seed, and ability to outcross (male sterility) [
24].
Continued breeding efforts using the Composite Cross XXXIX germplasm resulted in the release of six-row “one-irrigation barley” varieties: Solum in 1991 (by the ARS, USDA and Arizona AES) and Solar (by Arizona AES) in 2006. Originating as F6 selections, these varieties were released as spring barleys for winter crop production in low-water-use environments in the Southwestern United States where only one or two irrigations were applied. Variety trials by Arizona AES (2002 to 2004) have confirmed “Solar” has significantly higher yield, test weight, and lodging resistance than its predecessor “Solum” [
25].
The conventional varieties included in this study were “Kopious” and “Cochise”, derived from Composite Cross XXXII, a short-straw high-input population developed by Ramage et al. [
26]. These varieties normally receive 5 to 7 flood irrigations of around 150 mm per irrigation each season.
2.2. Site Characteristics
Field studies were conducted to study barley performance under high- and low-irrigation conditions. Trials were conducted in the years of 2015 and 2018 at the University of Arizona, Campus Agricultural Station in Tucson, AZ located at 32°9′36″ N, and 110°33′36″ W. Soil type was a Gila very fine sandy loam (coarse-loamy, mixed, superactive, calcareous, thermic Typic Torrifluvent). The field was fallow prior to the 2015 and 2018 planting.
2.3. Cultural Methods
The cultural methods were similar each year, with slight variations. For the 2015 trial, planting occurred on 3 December 2014 and was established with sprinkler irrigation from 12 December to 16 December (33 mm). For the 2018 trial, planting occurred on 7 December 2017 and was established with sprinkler irrigation from 12 December to 1 January (51 mm). In both years, the seed was planted into dry, flat soil with a cone planter. The low-input varieties were planted at a seeding rate of 78 kg ha−1 (1.24 million seeds ha−1) and the high-input varieties were planted at a seeding rate of 157 kg ha−1 (2.4 million seeds ha−1) to reflect the optimum seeding rate for each variety type.
Nitrogen was applied in the form of urea (46–0–0) at a rate of 56 kg N ha−1 at planting. During the growing season, an additional 112 kg N ha−1 was applied around the tillering stage to both irrigation treatments. Weeds and insects were controlled as needed. Each plot was 7 rows wide with a row spacing of 15 cm and a plot length of 9 m. The experimental design was Latin square with each variety having four reps per treatment.
2.4. Soil Water Measurements
Neutron probe access tubes were installed to a depth of 1.8 m to measure volumetric water content at depth increments of 0.3 m. Soil water was measured approximately once a week and relative to irrigations (0–1 day before, and 3–4 days after irrigation) with a neutron probe (CPN Model 503DR, Campbell Pacific Nuclear International Inc., Concord, CA, USA). The neutron probe was calibrated by regressing volumetric water content against standardized neutron count ratios using multiple paired measurements when soil was wet and dry. Volumetric soil moisture content for the neutron probe calibration was determined from gravimetric water content and bulk density.
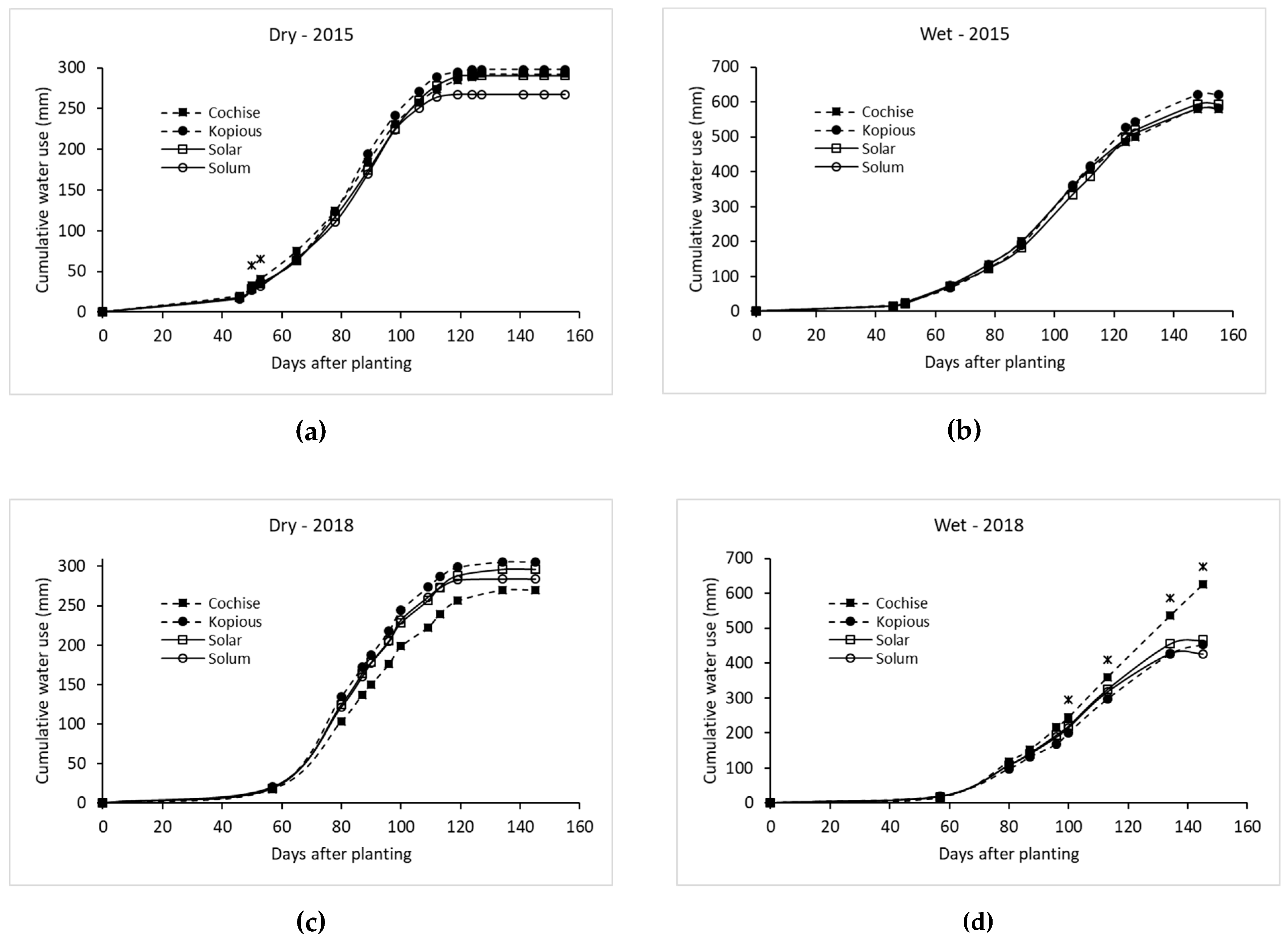
Neutron probe counts from 0 m to 1.8 m were used to calculate period water use for each variety by the water balance method, with precipitation and irrigation included and runoff assumed to be negligible. The period water use values were summed at the end of the season for total water use of each variety.
Water use (ET) was estimated from the differences in soil water content between specific time periods plus rainfall. Deep percolation was considered negligible as soil water content was taken at least 3 days after an irrigation when field capacity had been reached and most deep percolation had already occurred. Furthermore, soil water content was measured at 1.8 m, a depth beyond which not much water would percolate. The time periods between soil water measurements were at least 3 days after an irrigation and 0–1 day before the next irrigation, as mentioned above. Water use between soil water measurements before and after an irrigation were estimated by averaging the estimated daily water use before and after the soil water measurements. This daily water use was calculated from the crop coefficient (Kc) multiplied by reference evapotransiration (ETo). The Kc was calculated from ET/ETo. Water use between planting and the first soil water measurement was estimated using a Kc of 0.25 multiplied by ETo from the nearby weather station.
2.5. Soil Water Retention Characterization
Soil water characteristics previously reported by Miller and Ottman were used [
27]. Soil bulk density was 1.48 g cm
−3. Volumetric soil water content was 0.24 m
3 m
−3 at field capacity (θvFC), 0.085 m
3 m
−3 at permanent wilting point (θvPWP), and plant-available water (θvPAW) was 0.155 m
3 m
−3. The differences in soil water characteristics among depths were small and less than the standard error of these measurements, so values were averaged across depths.
2.6. Irrigation Treatments
The experiment included two irrigation treatments: high and low, hereon referred to as HI and LI respectively (
Table 1). In both years, sprinkler irrigation was applied to both treatments until seedling emergence. Sprinkler irrigation was used to germinate the seed as it results in better stand establishment than flooding, where a soil crust often forms inhibiting seedling emergence. Flood irrigation was used during the season after emergence since the soil profile could be saturated with a single flood irrigation, whereas several sprinkle irrigation events would be required to do so to prevent surface runoff. The frequency of flood irrigations in the HI treatment was based on levels of soil water depletion (SWD), with irrigations occurring around 50% depletion or approximately every two weeks during mid-season, totaling 6 irrigations per season. In 2015, the LI treatment only received sprinkler irrigation and no subsequent flood irrigations due to high precipitation in December and January. In 2018, the LI treatment received one flood irrigation following emergence, and an additional flood irrigation at the five-leaf stage. The source of irrigation water was tertiary-treated municipal effluent.
2.7. Plant Measurements
Measurements of plant biomass and interception of photo synthetically active radiation (PAR) were taken throughout the season around five leaf, first node, boot, and flowering stages. Date of heading, flowering, and physiological maturity were recorded. Biomass samples were obtained from an area of two rows by a 45.7 cm cut at the base. Dry weight was assessed after samples had dried at 60 °C for 2 to 10 days depending on growth stage. Intercepted PAR was measured with a Sunfleck Ceptometer (Decagon Devices, Pullman, WA, USA). Measurements were taken within one hour of solar noon on clear days. Three averaged readings were taken at the soil surface within the canopy, along with companion measurements of incident PAR outside the influence of the canopy.
Heading date was recorded when 50% of the heads were past the leaf collar. Flowering date was recorded when 50% of heads had anthers extruded. Maturity date was noted when 50% of heads and peduncle had changed to tan color.
At harvest, biomass yield, height, and lodging were assessed as well as grain yield, test weight, and seed weight. Plant height was averaged from 10 heads. For final biomass yield, 1 m2 of whole barley plants were hand-harvested and dried at 60 °C. Grain weight was assessed from the 1 m2 sample. For final grain yield, 5 rows by 1.5 m were hand-harvested and threshed. Clean seed was assessed for test weight and seed weight.
2.8. Method of Root Analysis
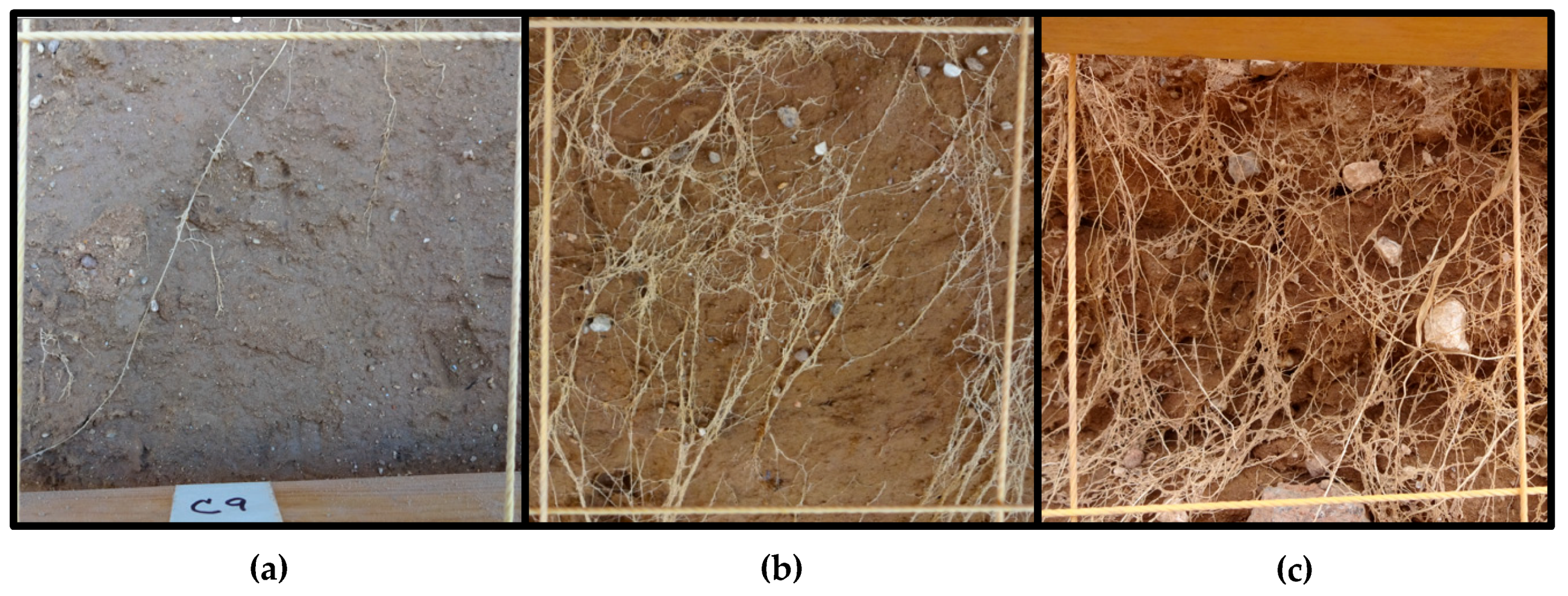
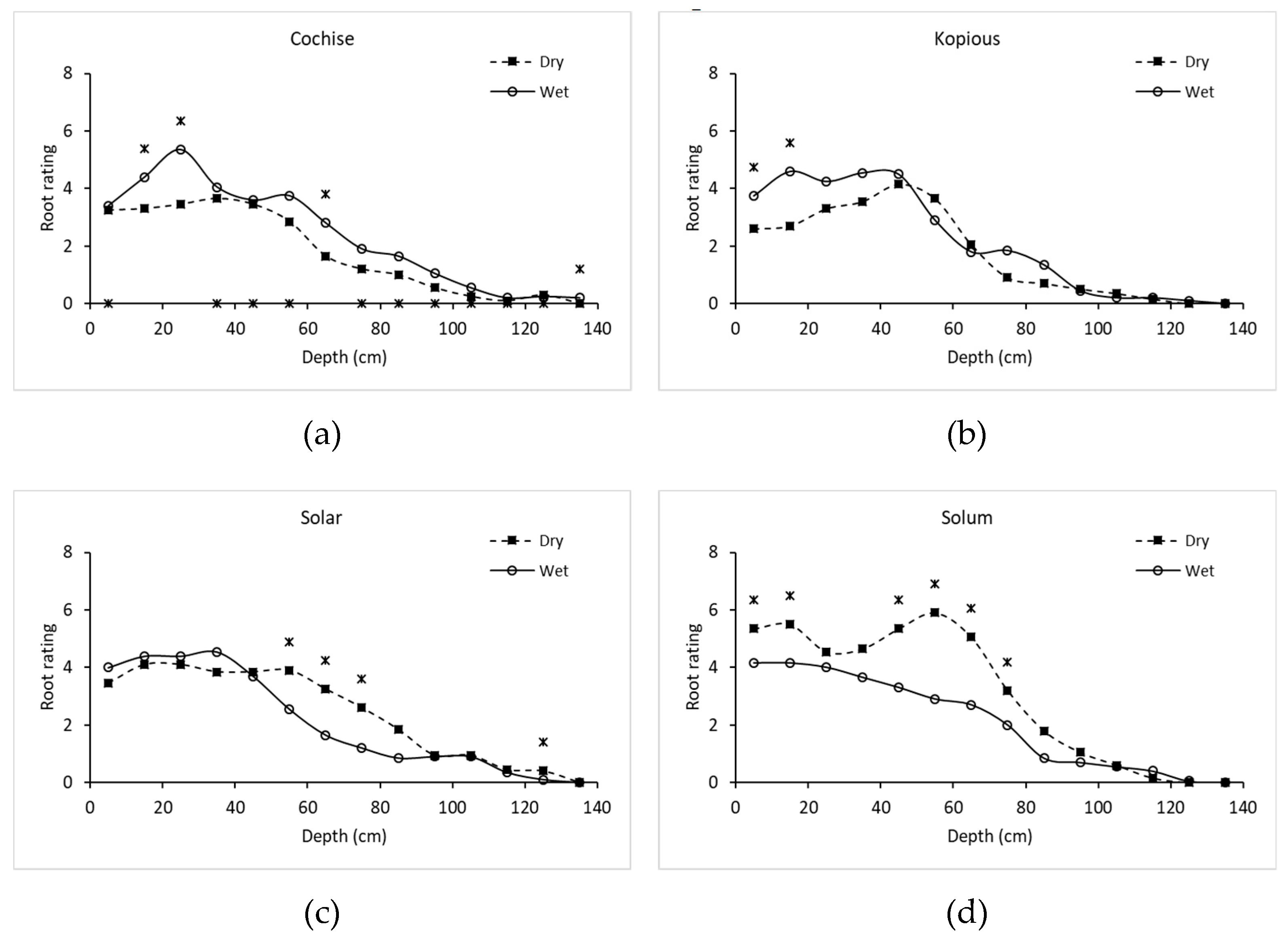
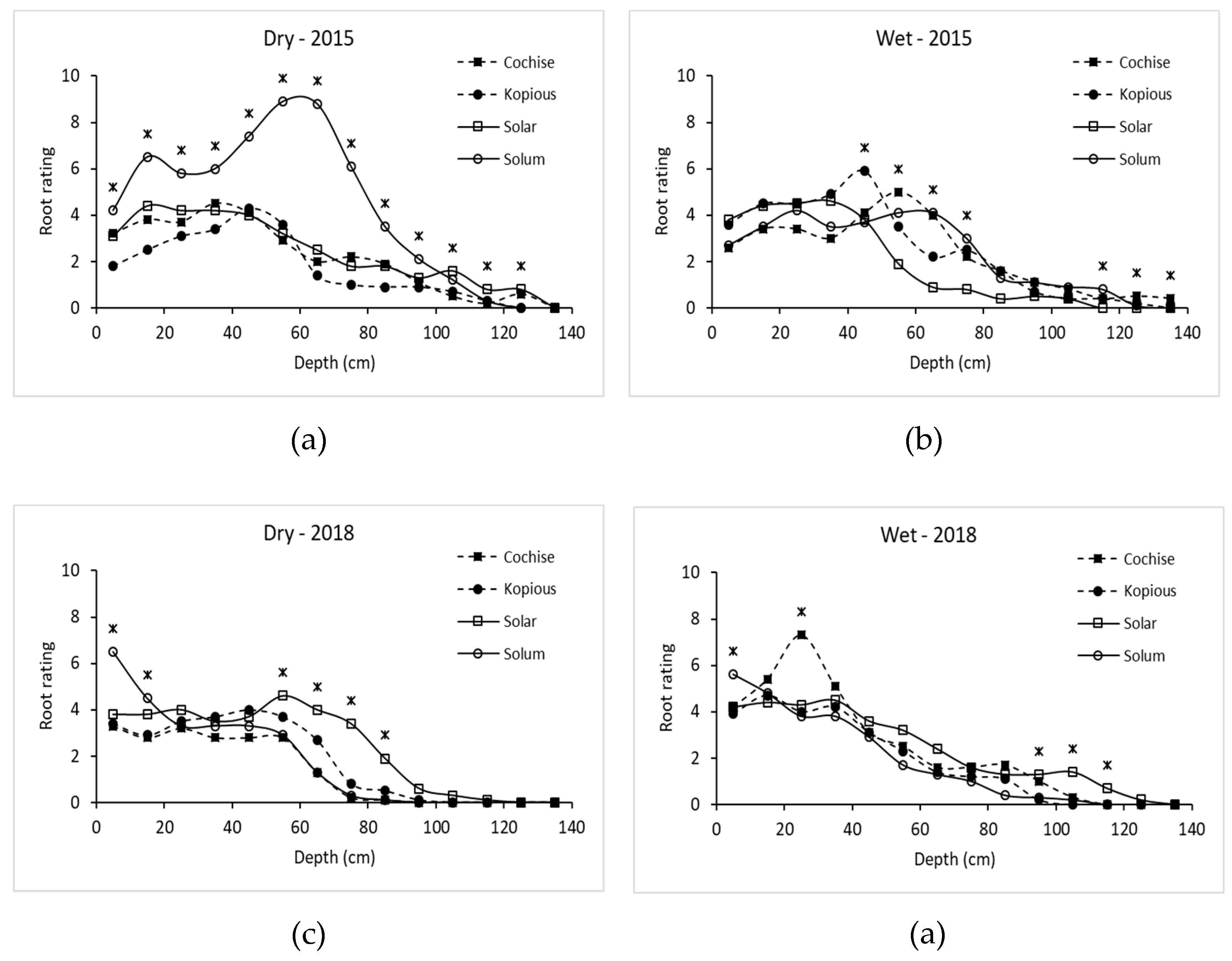
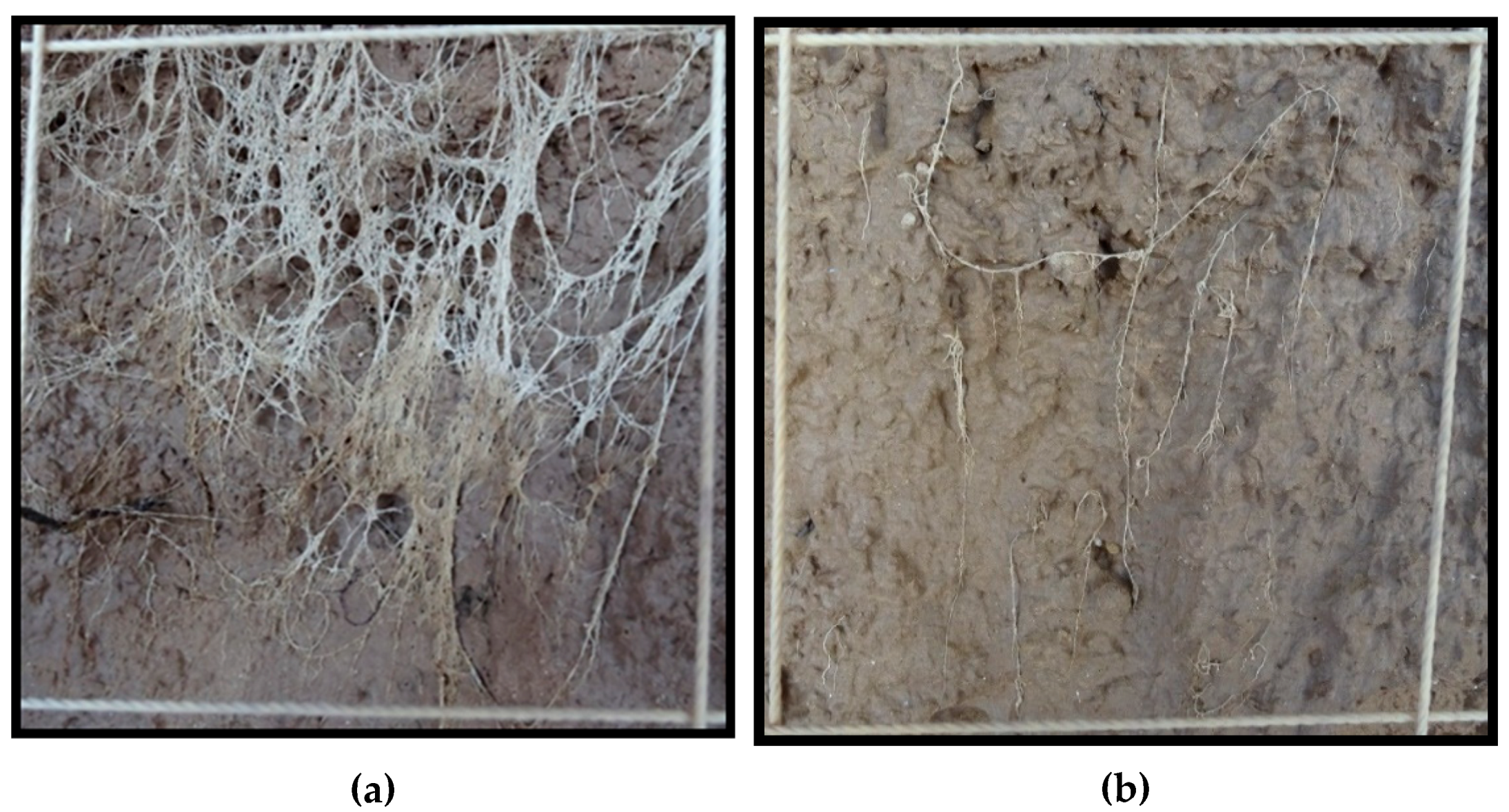
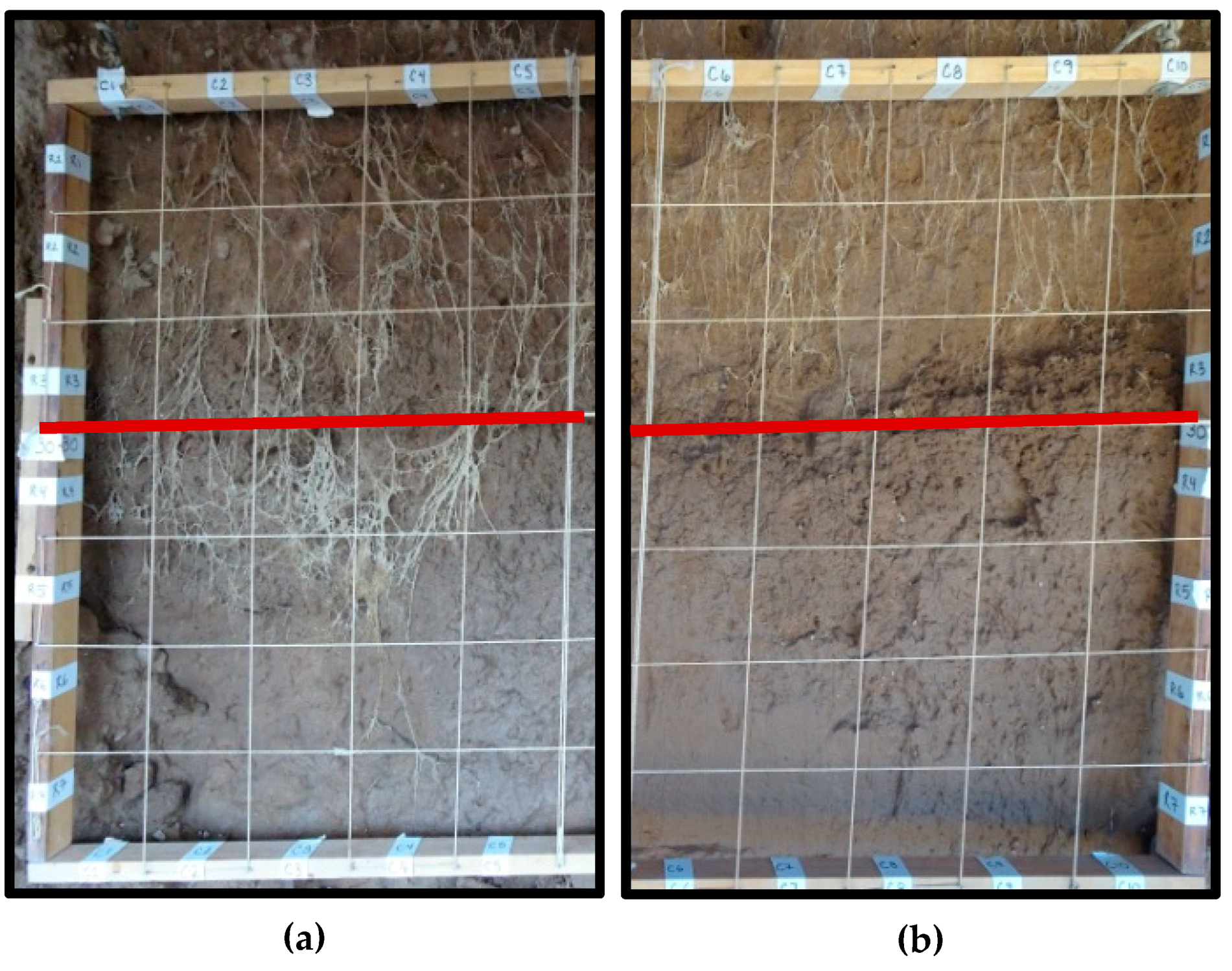
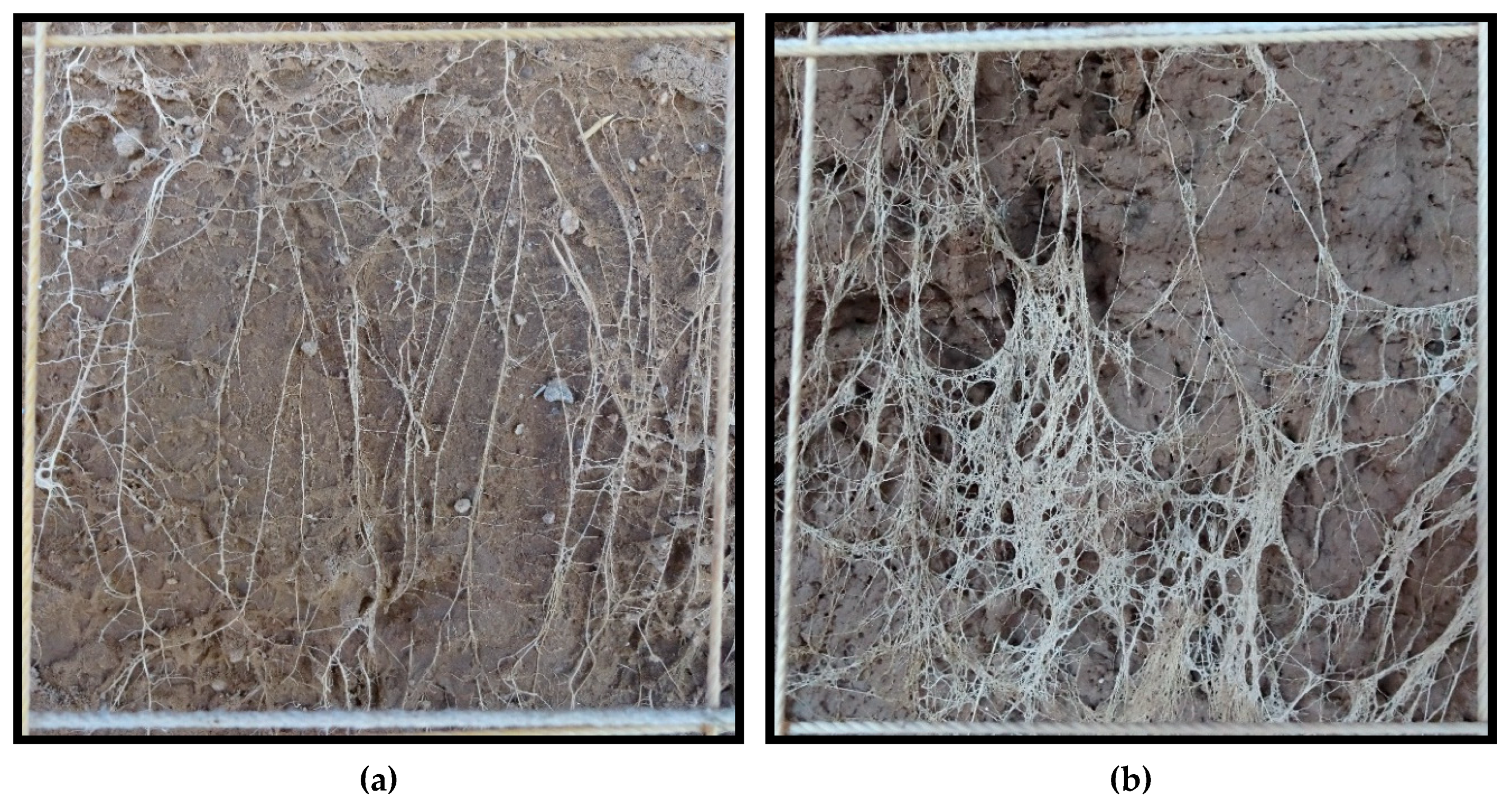
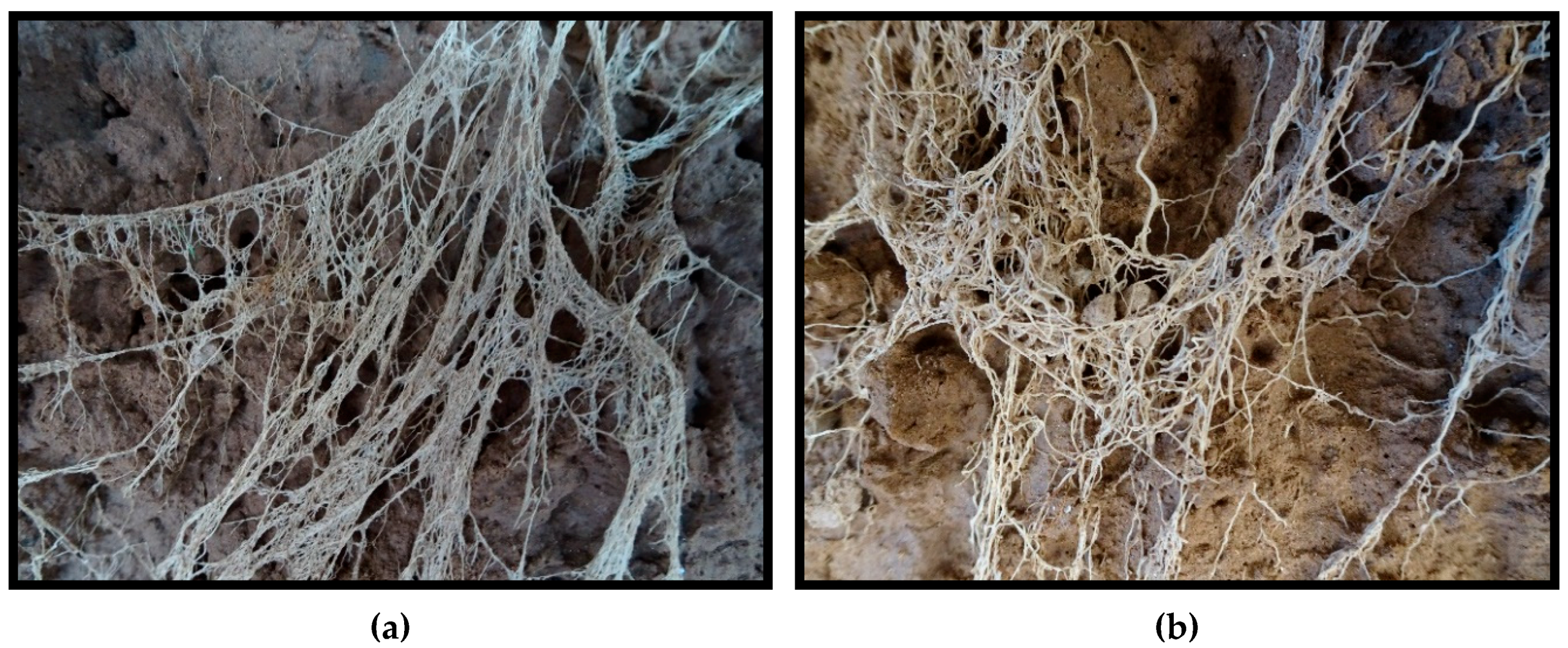
Root density at physiological maturity was assessed using the profile wall method. A 1.8-m deep trench traversing the plots was dug with a back-hoe, taking care to create a vertical, flat profile, that was later smoothed with a shovel. The profile wall was washed slowly with water using a fanned spray nozzle to expose the roots. For each variety under both irrigation treatments, the density of roots within 10 cm × 10 cm grids were rated on a scale of 1 to 10 along the total profile (100-cm wide by 180-cm deep). For example, a rating of 1 was given when 1 to 2 roots were present in the grid square, a rating of 5 was given when approximately 50 percent of the grid square was filled with roots, and a rating of 10 was scored when roots filled the majority of the grid square. See
Figure 1 for examples of root ratings.
In 2018, a 6-mm thick plexiglass sheet measuring 1.2 m × 0.85 m was placed flush against each profile wall and buried early in the season. The intention was to have the roots grow up to the plexiglass and be observable without washing, however, washing was still required. Placement of the plexiglass did create a flat soil surface for when the root profile wall was dug and greatly facilitated the process of washing away the soil.
2.9. Statistical Analysis
The data was analyzed by SAS version 9.4 using the PROC GLM procedure, except for water use which was analyzed using the PROC MIXED procedure, and the root profile wall data which was analyzed using SPSS version 25 with the GLM procedure.
2.10. Weather
Weather data was recorded by the Arizona Meteorological Network (AZMET) Campus Agricultural Center weather station, located about 0.5 km from the experimental plots. See
Table 2 for growing season temperature, precipitation, and ET
o.
5. Conclusions
Though debate exists regarding whether selection under optimal or stress conditions is preferable when breeding for drought tolerance, the results of this study show the varieties bred for low-water conditions had greater grain yield and test weight under LI than varieties bred for high-water-use conditions. Advantageous traits under HI conditions were associated with poorer performance under drought stress, specifically later maturity, shallow root systems, and less water-use post-anthesis. Observed strategies of drought tolerance in this study included early vigor, early flowering, greater root density at depth, and greater water extraction post-anthesis. The root profile pit addressed the need for root studies conducted in realistic environments and revealed insights difficult to capture with other field-based methods.