The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
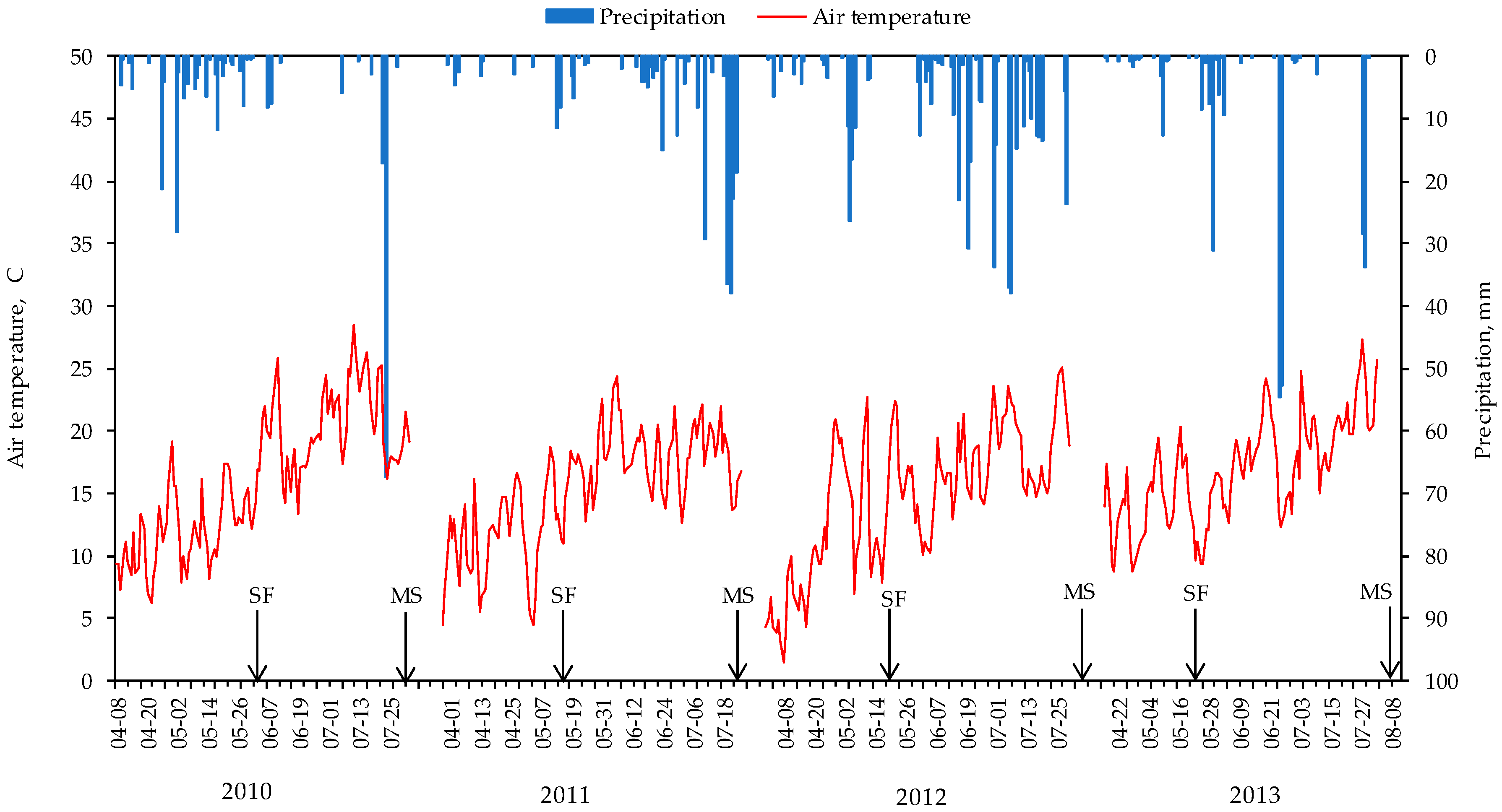
2.3. Environmental Conditions
2.4. Experimental Material
2.5. Experimental Measurements
2.6. Chemical Analysis
2.7. Chemical Score and Essential Amino Acid Index
2.8. Statistical Analysis
3. Results
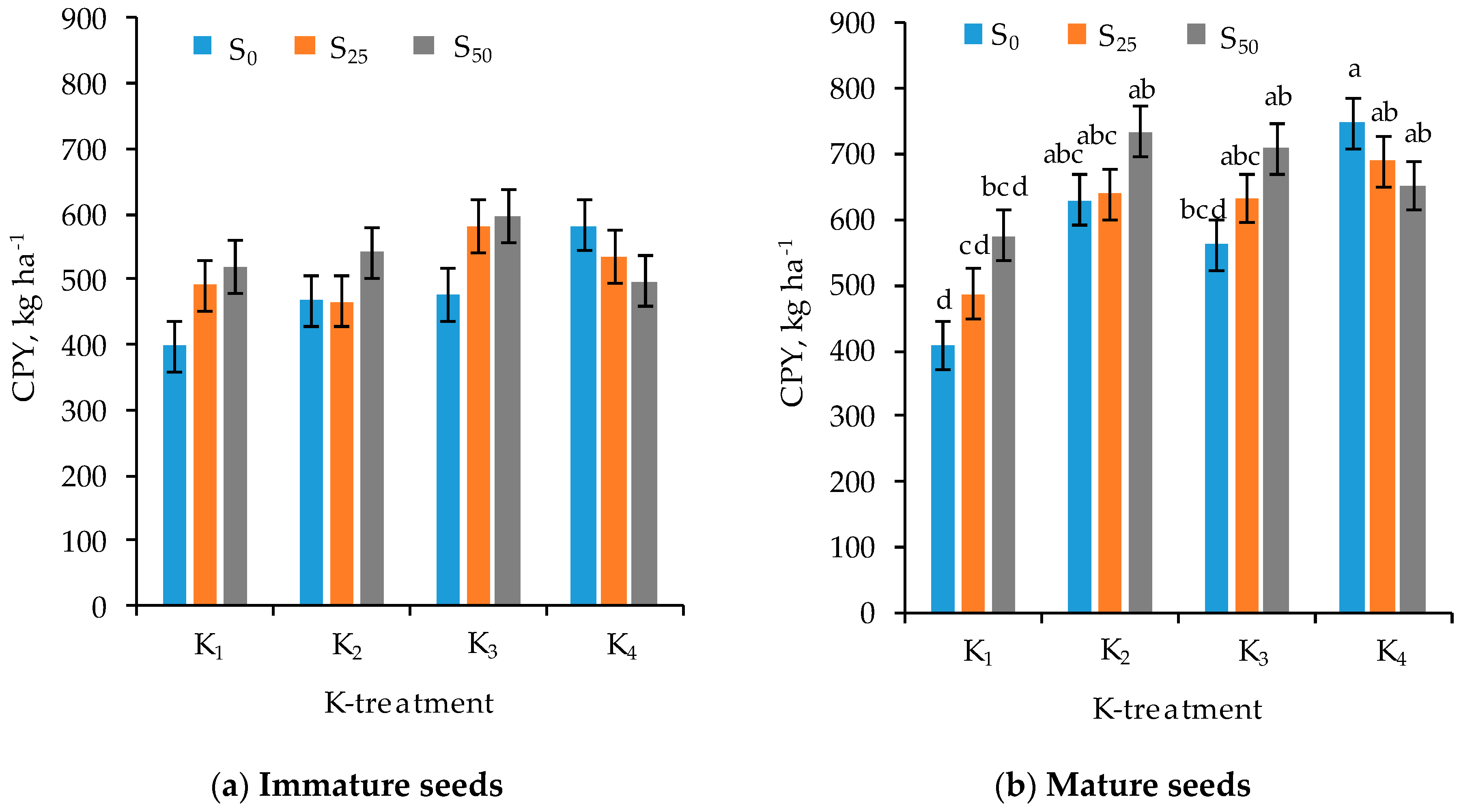
3.1. Protein Content and Yield
3.2. Tannin Content
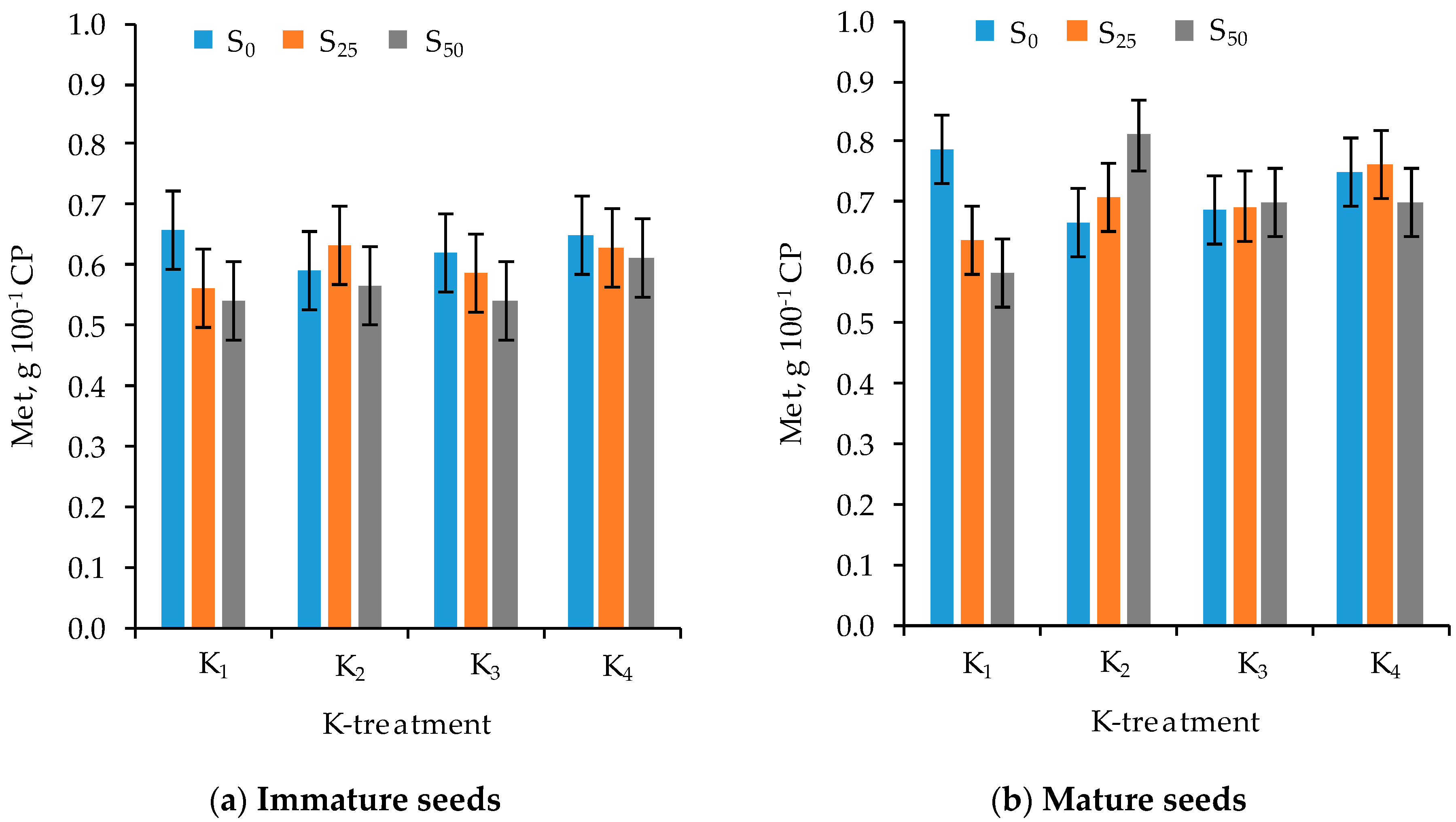
3.3. Amino Acid Composition
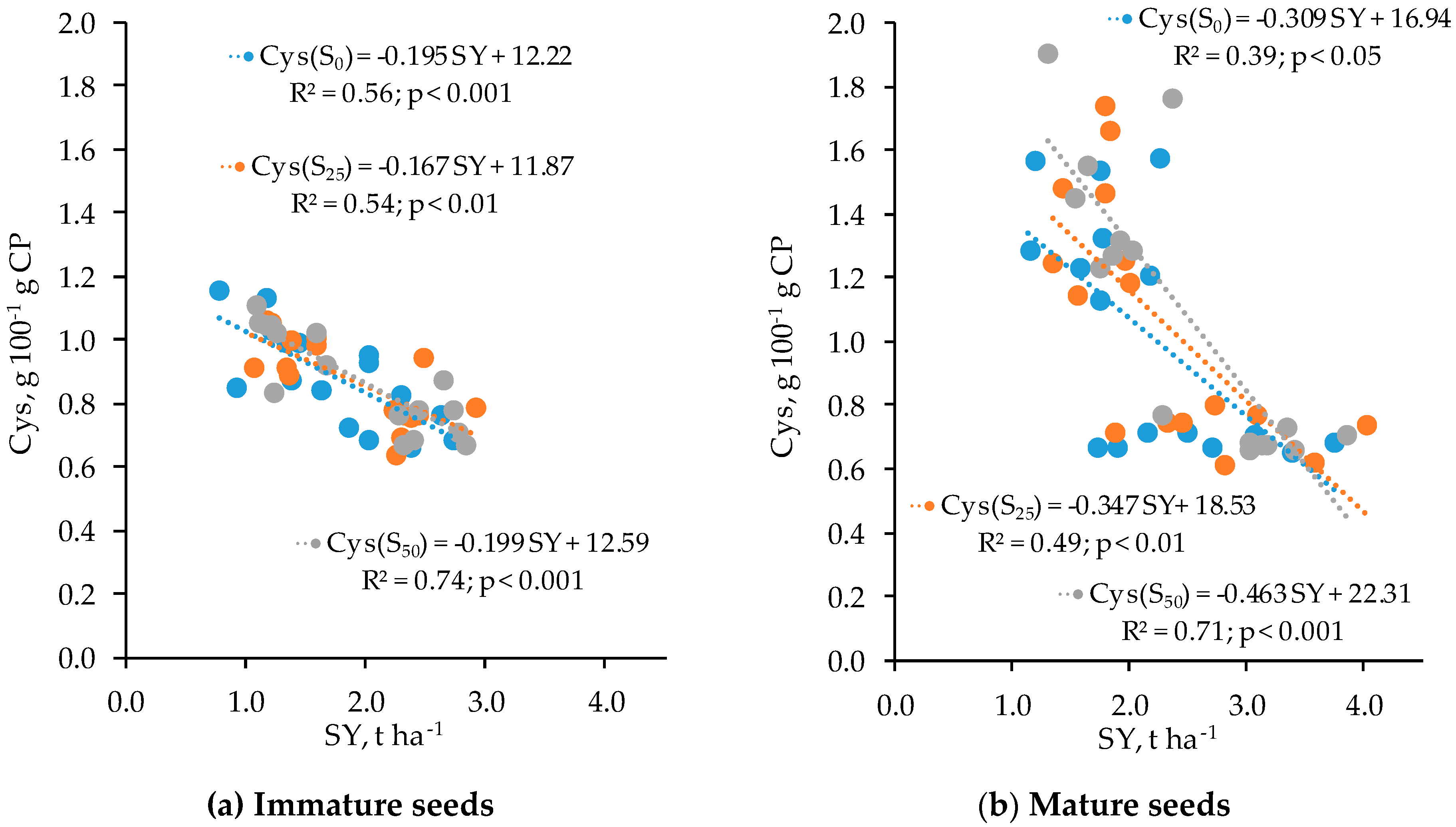
3.4. Relationships Between Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Singh, A.K.; Bharati, R.C.; Manibhushan, N.C.; Pedpati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar] [CrossRef]
- Ofuya, Z.M.; Akhidue, V. The role of pulses in human nutrition: A review. J. Appl. Sci. Environ. Manag. 2005, 9, 99–104. [Google Scholar] [CrossRef]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Rabey, J.M.; Vered, Y.; Shabtai, H.; Graff, E.; Korczyn, A.D. Improvement of Parkinsonian features correlate with high plasma levodopa values after broad bean (Vicia faba) consumption. J. Neurol. Neurosurg. Psychiatry 1992, 55, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.S.; Peples, M.B.; Hauggaard-Nielson, H. Faba bean in cropping systems. Field Crop Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Alhajj Ali, S.; Tedone, L.; Verdini, L.; Cazzato, E.; De Mastro, G. Wheat Response to No-Tillage and Nitrogen Fertilization in a Long-Term Faba Bean-Based Rotation. Agronomy 2019, 9, 50. [Google Scholar] [CrossRef]
- Duc, G. Faba bean (Vicia faba L.). Field Crop Res. 1997, 53, 99–109. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Ghanehpoor, S.; Dabbagh Mohammadi-Nasab, A. Effects of water limitation on growth and grain filling of faba bean cultivars. J. Food Agric. Environ. 2009, 7, 442–447. [Google Scholar]
- Khan, H.R.; Paull, J.G.; Siddique, K.H.M.; Stoddard, F.L. Faba bean breeding for drought-affected environments: Aphysiological and agronomic perspective. Field Crop Res. 2010, 115, 279–286. [Google Scholar] [CrossRef]
- López-Bellido, F.J.; López-Bellido, L.; López-Bellido, R.J. Competition, growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 2005, 23, 359–378. [Google Scholar] [CrossRef]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The effects of potassium fertilization on water-use efficiency in crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Pettigrew, W.W. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Kirkby, E.A.; Cakmak, J. Effect of mineral nutritional status on shoot-root partitioning of photo-assimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Kurdali, F.; Al-Ain, F.; Al-Shamma, M. Nodulation, dry matter production, and N2 fixation by fababean and chickpea as affected by soil moisture and potassium fertilizer. J. Plant Nutr. 2002, 25, 355–368. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Taha, A.A.; Omar, M.M.; Hadeer, R.K. Effect of different sources and levels of potassium on growth, yield and chemical composition of faba bean. J. Soil Sci. Agric. Eng. Mansoura Univ. 2016, 7, 243–248. [Google Scholar]
- Gremigni, P.; Wong, M.; Edwards, N.; Harris, D.; Hamblin, J. Potassium nutrition effects on seed alkaloid concentrations, yield and mineral content of lupins (Lupinus angustifolius). Plant Soil 2001, 234, 131–142. [Google Scholar] [CrossRef]
- Mona, A.M.; Sabah, M.A.; Rehab, A.M. Influence of potassium sulphate on faba bean yield and quality. Aust. J. Basic Appl. Sci. 2011, 5, 87–95. [Google Scholar]
- Scherer, H.W. Sulfur in soils. J. Plant Nutr. Soil. Sci. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Tabe, L.; Higgins, T. Engineering plant protein composition for improved nutrition. Trends Plant Sci. 1998, 3, 282–296. [Google Scholar] [CrossRef]
- Neugschwandtner, R.; Ziegler, K.; Kriegner, S.; Wagentristl, H.; Kaul, H.P. Nitrogen yield and nitrogen fixation of winter faba beans. Acta Agric. Scand. Sect. B 2015, 65, 658–666. [Google Scholar] [CrossRef]
- Głowacka, A.; Gruszecki, T.; Szostak, B.; Michałek, S. The response of common bean to sulphur and molybdenum fertilization. Int. J. Agron. 2019, 2019, 3830712. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Mendel, R.R.; Bittner, F. Cell biology of molybdenum. Biochim. Biophys. Acta 2006, 1763, 621–635. [Google Scholar] [CrossRef]
- Cazzato, E.; Tufarelli, V.; Ceci, E.; Stellacci, A.M.; Laudadio, V. Quality, yield and nitrogen fixation of faba bean seeds as affected by sulphur fertilization. Acta Agric. Scand. Sect. B 2012, 62, 732–738. [Google Scholar] [CrossRef]
- Zhou, L.I.; Cao, J.; Zhang, F.S.; Li, L. Rhizosphere acidification of faba bean, soybean and maize. Sci. Total Environ. 2009, 407, 4359–4362. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Barłóg, P.; Borowiak, K.; Wolna-Maruwka, A. The effect of sulphur and potassium fertilisation on the nitrogenase and microbial activity in soil under broad bean (Vicia faba L.) cultivation. Fresenius Environ. Bull. 2015, 24, 723–732. [Google Scholar]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Faba bean yield and growth dynamics in response to soil potassium availability and sulfur application. Field Crop Res. 2018, 219, 87–97. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Wang, T.L.; Domoney, C.; Hedley, C.L.; Casey, R.; Grusak, M.A. Can we improve the nutritional quality of legume seeds? Plant Physiol. 2003, 131, 886–891. [Google Scholar] [CrossRef]
- Elscheikh, E.A.E.; Elzidany, A.A. Effect of Rhizobium inoculation, organic and chemical fertilizers on proximate composition, in vitro protein digestibility, tannin and sulphur content of faba beans. Food Chem. 1997, 59, 41–45. [Google Scholar] [CrossRef]
- Jansman, A.J.M. Tannins in feedstuffs for simple stomached animals. Nutr. Res. Rev. 1993, 6, 209–236. [Google Scholar] [CrossRef]
- Delimont, N.M.; Haub, M.D.; Lindshield, B.L. The impact of tannin consumption on iron bioavailability and status: A narrative review. Curr. Dev. Nutr. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Turco, I.; Ferretti, G.; Bacchetti, T. Review of the health benefits of Faba bean (Vicia faba L.) polyphenols. J. Food Nutr. Res. 2016, 55, 283–293. [Google Scholar]
- Burstin, J.; Gallardo, K.; Mir, R.R.; Varshney, R.K.; Duc, G. Improving protein content and nutrition quality. In Biology and Breeding of Food Legumes; Pratap, A., Kumar, J., Eds.; CAB International: Wallingford, UK, 2011; pp. 314–328. [Google Scholar]
- Lisiewska, Z.; Kmiecik, W.; Słupski, J. Content of amino acids in raw and frozen broad beans (Vicia faba var. major) seeds at milk maturity stage, depending on the processing method. Food Chem. 2007, 105, 1468–1473. [Google Scholar] [CrossRef]
- Burbano, C.; Cuadrado, C.; Muzquiz, M.; Cubero, J.I. Variation of favism—Inducing factors (vicine, convicine and L-Dopa) during pod development in Vicia faba L. Plant Food Hum. Nut. 1995, 47, 265–275. [Google Scholar] [CrossRef]
- WRB. World reference base for soil resources 2014. In Word Soil Resources Reports, 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Meier, U. Phenological growth stages and BBCH-identification keys of faba bean (Vicia faba L.). In Growth Stages of Mono- and Dicotyledonous Plants, 2nd ed.; Meier, U., Ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin and Braunschweig, Germany, 2001. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Herlich, K., Ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Kuhla, S.; Ebmeier, C. Untersuchungen zum Tanningehalt in Ackerbohnen. Arch. Tierernahr. 1981, 31, 573–588. [Google Scholar] [CrossRef]
- Oser, B.L. An integrated essential amino acid index for predicting the biological value of proteins. In Protein and Amino Acid Nutrition; Albanese, A.A., Ed.; Academic Press: New York, NY, USA, 1959; pp. 295–311. [Google Scholar]
- FAO/WHO/UNU. Protein and amino acid requirements in human nutrition. In WHO Technical Report Series; No. 935; Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition, Food and Agriculture Organization of the United Nations, World Health Organization and United Nations University: Geneva, Switzerland, 2007. [Google Scholar]
- StatSoft Inc. Electronic Statistics Textbook; StatSoft Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Lafiandra, D.; Polignano, G.B.; Filippetti, A.; Porceddu, E. Genetic variability for protein content and S-aminoacids in broad-beans (Vicia faba L.). Kulturpflanze 1981, 29, 115–127. [Google Scholar] [CrossRef]
- Avola, G.; Gresta, F.; Abbate, V. Diversity examination based on physical, technological and chemical traits in a locally grown landrace of faba bean (Vicia faba L. var. major). Int. J. Food Sci. Technol. 2009, 44, 2568–2576. [Google Scholar] [CrossRef]
- Schumacher, H.; Paulsen, H.M.; Gau, A.E.; Link, W.; Jörgens, H.U.; Sassand, O.; Dietrich, R.D. Seed protein amino acid composition of important local grain legumes Lupinus angustifolius L., Lupinus luteus L., Pisum sativum L. and Vicia faba L. Plant Breed. 2011, 30, 156–164. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Chen, C.; Walia, M.K.; Torrion, J.A.; McVay, J.; Lamb, P.; Miller, P.; Eckhoff, J.; Miller, J.; Khan, Q. Dry pea (Pisum sativum L.) protein, starch, and ash concentrations as affected by cultivar and environment. Can. J. Plant Sci. 2018, 98, 1188–1198. [Google Scholar] [CrossRef]
- Alghamdi, S.S. Chemical Composition of Faba Bean (Vicia faba L.) Genotypes under Various Water Regimes. Pak. J. Nutr. 2009, 8, 477–482. [Google Scholar]
- El Fiel, H.E.A.; El Tinay, A.H.; Elscheikh, E.A.E. Effect of nutritional status of faba bean (Vicia faba L.) on protein solubility profiles. Food Chem. 2002, 76, 219–223. [Google Scholar] [CrossRef]
- Foroud, N.; Mûndel, H.H.; Saindon, G.; Entz, T. Effect of level and timing of moisture stress on soybean yield, protein, and oil responses. Field Crop Res. 1993, 31, 195–209. [Google Scholar] [CrossRef]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 1999, 9, 273–293. [Google Scholar] [CrossRef]
- Barłóg, P.; Niewiadomska, A.; Ambroży-Deręgowska, K. Effect of sulphur fertilization on seed yield and yield components of broad bean on the background of different levels of potassium content in soil. Fragm. Agron. 2014, 31, 7–17. [Google Scholar]
- Singh, N.; Kuhad, M.S. Role of Potassium in Alleviating the Effect of Water Stress on Yield and Seed Quality in Chickpea (Cicer arietinum L.). Bull. Natl. Inst. Ecol. 2005, 15, 219–225. [Google Scholar]
- Fan, S.; Lifang, H.; Jin, H.; Li, Z. Improvement of Root Nodule Nitrogen Fixation and Soil Fertility by Balanced Fertilization of Broad Beans. Better Crops Int. 1997, 11, 22–23. [Google Scholar]
- Habtemichial, K.H.; Singh, B.R.; Aune, J.B. Wheat response to N2 fixed by faba bean (Vicia faba L.) as affected by sulfur fertilization and rhizobial inoculation in semi-arid Northern Ethiopia. J. Plant Nutr. Soil Sci. 2007, 170, 412–418. [Google Scholar] [CrossRef]
- Pacyna, S.; Schulz, M.; Scherer, H. Influence of sulphur supply on glucose and ATP concentrations of inoculated broad beans (Vicia faba minor L.). Biol. Fertil. Soils 2006, 42, 324–329. [Google Scholar] [CrossRef]
- Zhao, F.; Wood, A.; McGrath, S. Effects of sulphur nutrition on growth and nitrogen fixation of pea (Pisum sativum L.). Plant Soil 1999, 212, 209–219. [Google Scholar] [CrossRef]
- Hussain, K.; Islam, M.; Siddique, M.T.; Hayat, R.; Mohsan, S. Soybean growth and nitrogen fixation as affected by sulfur fertilization and inoculation under rainfed conditions in Pakistan. Int. J. Agric. Biol. 2011, 13, 951–955. [Google Scholar]
- Pandurangan, S.; Sandercock, M.; Beyaert, R.; Conn, K.L.; Hou, A.; Marsolais, F. Differential response to sulfur nutrition of two common bean genotypes differing in storage protein composition. Front. Plant Sci. 2015, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Baginsky, C.; Peña-Neira, A.; Cáceres, A.; Hernández, T.; Estrella, I.; Morales, H.; Pertuze, R. Phenolic compound composition in immature seeds of fava bean (Vicia faba L.) varieties cultivated in Chile. J. Food Compos. Anal. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Legumes as a source of antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 855–878. [Google Scholar] [CrossRef]

| Soil Depth, m | K Treatment | pH 1 M KCl | Nutrient Content, mg kg−1 | |||
|---|---|---|---|---|---|---|
| P 1 | K 1 | Mg 2 | S-SO4 3 | |||
| 0–0.3 | K1 | 6.16 ± 0.54 | 101.8 ± 19.3 | 87.0 ± 27.2 | 53.4 ± 14.6 | 8.5 ± 7.7 |
| K2 | 6.23 ± 0.54 | 108.3 ± 15.8 | 131.8 ± 35.1 | 57.0 ± 19.5 | 10.0 ± 9.3 | |
| K3 | 6.28 ± 0.50 | 103.2 ± 24.8 | 108.3 ± 29.6 | 52.2 ± 13.3 | 7.2 ± 5.3 | |
| K4 | 6.34 ± 0.48 | 107.3 ± 23.0 | 167.3 ± 34.6 | 57.6 ± 18.7 | 6.6 ± 4.3 | |
| 0.3–0.6 | K1 | 5.98 ± 0.47 | 82.1 ± 25.1 | 97.4 ± 33.9 | 59.7 ± 22.3 | 5.9 ± 4.2 |
| K2 | 6.00 ± 0.49 | 88.1 ± 32.9 | 108.1 ± 26.6 | 67.9 ± 17.2 | 7.9 ± 6.9 | |
| K3 | 6.18 ± 0.57 | 86.7 ± 41.7 | 107.0 ± 32.5 | 63.9 ± 10.4 | 7.0 ± 5.9 | |
| K4 | 6.28 ± 0.61 | 87.8 ± 37.0 | 125.7 ± 38.5 | 62.8 ± 18.7 | 7.2 ± 5.6 | |
| Factor | Degr. of Freedom | CP | CPY | ||
|---|---|---|---|---|---|
| GS | MS | GS | MS | ||
| Y | 3 | 33.1 *** | 93.3 *** | 30.0 *** | 42.6 *** |
| K | 3 | 0.2 | 1.5 | 2.7 * | 18.5 *** |
| S | 2 | 0.0 | 1.0 | 1.7 | 4.9 ** |
| Y × K | 9 | 0.4 | 1.3 | 0.5 | 2.8 ** |
| Y × S | 6 | 0.3 | 1.0 | 0.5 | 0.8 |
| K × S | 6 | 0.2 | 0.6 | 1.7 | 2.6 * |
| Y × K × S | 18 | 0.2 | 0.5 | 0.5 | 0.7 |
| Error | 144 | ||||
| Factor | CP g kg−1 | CPY kg ha−1 | ||
|---|---|---|---|---|
| GS | MS | GS | MS | |
| Year | ||||
| 2010 | 312.0 a | 296.3 a | 412.7 b | 520.5 b |
| 2011 | 274.9 b | 270.2 b | 346.3 b | 464.3 b |
| 2012 | 266.9 c | 256.8 c | 633.5 a | 772.1 a |
| 2013 | 270.4 bc | 266.9 b | 656.5 a | 730.9 a |
| K treatments | ||||
| K1 | 280.6 | 275.3 | 468.5 b | 490.1 b |
| K2 | 280.8 | 271.9 | 491.4 ab | 667.8 a |
| K3 | 284.8 | 274.3 | 551.2 a | 634.0 a |
| K4 | 281.0 | 268.7 | 537.9 a | 696.0 a |
| S treatments | ||||
| S0 | 281.4 | 273.4 | 480.9 | 586.6 b |
| S25 | 279.5 | 271.9 | 517.7 | 611.7 ab |
| S50 | 284.5 | 272.4 | 538.2 | 667.6 a |
| Seed Maturity | K Treatment | Year | Mean | |||
|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | |||
| GS | K1 | 0.356 | 0.284 | 0.487 | 0.743 | 0.467 |
| K2 | 0.385 | 0.242 | 0.379 | 0.679 | 0.421 | |
| K3 | 0.389 | 0.281 | 0.464 | 0.712 | 0.462 | |
| K4 | 0.328 | 0.219 | 0.350 | 0.712 | 0.402 | |
| Mean | 0.364 b | 0.256 c | 0.420 b | 0.711 a | ||
| MS | K1 | 0.401 | 0.384 | 0.618 | 0.524 | 0.482 a |
| K2 | 0.396 | 0.355 | 0.378 | 0.474 | 0.401 b | |
| K3 | 0.336 | 0.376 | 0.537 | 0.464 | 0.428 ab | |
| K4 | 0.410 | 0.362 | 0.407 | 0.414 | 0.398 b | |
| Mean | 0.376 a | 0.369 a | 0.535 b | 0.469 ab | ||
| Factor | Asp | Tre | Ser | Glu | Pro | Cys | Gli | Ala | Wal | Met | Ile | Leu | Tyr | Fen | His | Liz | Arg | TAA 1 | EAA 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | |||||||||||||||||||
| 2010 | 8.29 a | 3.04 b | 3.96 b | 16.1 a | 5.12 a | 0.94 b | 3.41 b | 3.59 c | 3.76 a | 0.50 b | 3.13 c | 6.05 c | 2.99 a | 3.61 b | 2.74 a | 4.83 c | 7.47 b | 79.6 c | 31.6 b |
| 2011 | 8.58 b | 2.80 c | 3.76 c | 16.2 a | 4.93 a | 1.05 a | 3.48 b | 3.77 b | 3.77 b | 0.60 b | 3.26 b | 6.39 b | 2.57 c | 3.21 c | 2.30 c | 5.04 b | 6.36 d | 78.1 c | 31.0 b |
| 2012 | 9.55 a | 3.23 a | 4.33 a | 13.2 c | 4.04 b | 0.70 d | 3.89 a | 3.76 b | 4.49 a | 0.72 a | 3.71 a | 6.64 a | 2.91 ab | 4.03 a | 2.54 b | 6.58 a | 9.38 a | 83.7 a | 35.6 a |
| 2013 | 8.37 ab | 2.63 d | 3.44 d | 14.1 b | 2.78 c | 0.85 c | 3.21 c | 4.41 a | 3.66 b | 0.57 b | 3.06 c | 5.51 d | 2.72 bc | 3.05 c | 2.29 c | 4.68 c | 7.02 c | 72.3 c | 29.0 c |
| F3,44 | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| K treatments | |||||||||||||||||||
| K1 | 8.65 | 2.92 | 3.87 | 14.79 | 4.27 | 0.92 | 3.50 | 3.83 | 3.89 | 0.58 | 3.31 | 6.12 | 2.84 | 3.48 | 2.45 | 5.26 | 7.58 | 78.3 | 31.8 |
| K2 | 8.69 | 2.90 | 3.88 | 14.99 | 4.27 | 0.90 | 3.49 | 3.95 | 4.12 | 0.60 | 3.31 | 6.15 | 2.83 | 3.50 | 2.48 | 5.31 | 7.55 | 78.9 | 32.1 |
| K3 | 8.69 | 2.95 | 3.88 | 14.93 | 4.17 | 0.86 | 3.51 | 3.83 | 3.79 | 0.58 | 3.23 | 6.13 | 2.74 | 3.43 | 2.45 | 5.22 | 7.50 | 77.9 | 31.4 |
| K4 | 8.76 | 2.93 | 3.86 | 14.98 | 4.15 | 0.84 | 3.50 | 3.93 | 3.87 | 0.63 | 3.30 | 6.18 | 2.79 | 3.49 | 2.49 | 5.34 | 7.60 | 78.6 | 31.9 |
| F3,44 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| S treatments | |||||||||||||||||||
| S0 | 8.63 | 2.93 | 3.87 | 14.9 | 4.18 | 0.88 | 3.49 | 3.86 | 4.04 | 0.63 | 3.31 | 6.16 | 2.84 | 3.54 | 2.50 | 5.32 | 7.63 | 78.7 | 32.2 |
| S25 | 8.70 | 2.90 | 3.85 | 14.8 | 4.25 | 0.87 | 3.49 | 3.91 | 3.85 | 0.60 | 3.27 | 6.11 | 2.76 | 3.40 | 2.41 | 5.22 | 7.39 | 77.8 | 31.4 |
| S50 | 8.77 | 2.94 | 3.89 | 15.0 | 4.22 | 0.89 | 3.52 | 3.87 | 3.87 | 0.56 | 3.28 | 6.17 | 2.80 | 3.48 | 2.49 | 5.31 | 7.65 | 78.7 | 31.8 |
| F2,45 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Factor | Asp | Tre | Ser | Glu | Pro | Cys | Gli | Ala | Wal | Met | Ile | Leu | Tyr | Fen | His | Liz | Arg | TAA 1 | EAA 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | |||||||||||||||||||
| 2010 | 8.43 c | 3.15 b | 3.95 c | 15.9 b | 5.27 a | 1.25 b | 3.56 b | 3.46 b | 3.64 b | 0.72 | 3.11 b | 6.08 c | 2.70 b | 3.82 | 3.18 b | 5.65 b | 8.71 b | 82.6 ab | 33.3 b |
| 2011 | 9.12 bc | 3.27 ab | 4.36 a | 16.6 a | 5.49 a | 1.65 a | 3.87 a | 3.81 a | 2.63 c | 0.72 | 2.08 c | 5.84 d | 3.83 a | 3.91 | 4.08 a | 5.61 b | 7.28 c | 84.1 a | 33.6 b |
| 2012 | 9.40 ab | 3.31 a | 4.15 b | 13.0 c | 3.91 b | 0.69 c | 3.82 a | 3.72 a | 4.13 a | 0.68 | 3.52 a | 6.41 b | 2.72 a | 3.83 | 2.52 c | 6.43 a | 9.36 a | 81.6 b | 34.2 ab |
| 2013 | 9.70 a | 3.41 a | 4.28ab | 13.4 c | 4.04 b | 0.71 c | 3.94 a | 3.84 a | 4.27 a | 0.70 | 3.63 a | 6.61 a | 2.81 b | 3.95 | 2.60 c | 6.63 a | 9.65 a | 84.2 a | 35.3 a |
| F3,44 | *** | *** | *** | *** | *** | *** | *** | *** | *** | n.s. | *** | *** | *** | n.s. | *** | *** | *** | ** | *** |
| K treatments | |||||||||||||||||||
| K1 | 9.06 | 3.29 | 4.16 | 14.5 | 4.73 | 1.07 | 3.74 | 3.65 | 3.72 | 0.67 | 3.15 | 6.16 | 3.04 | 3.64 | 2.97 | 6.00 | 8.63 | 82.2 | 33.7 |
| K2 | 9.28 | 3.36 | 4.27 | 14.9 | 4.56 | 1.08 | 3.82 | 3.75 | 3.77 | 0.73 | 3.15 | 6.31 | 2.96 | 3.99 | 2.91 | 6.12 | 8.78 | 83.8 | 34.4 |
| K3 | 9.17 | 3.22 | 4.19 | 14.8 | 4.73 | 1.10 | 3.83 | 3.73 | 3.56 | 0.69 | 3.04 | 6.26 | 3.09 | 3.91 | 3.23 | 6.10 | 8.89 | 83.5 | 34.2 |
| K4 | 9.14 | 3.27 | 4.11 | 14.7 | 4.69 | 1.06 | 3.79 | 3.69 | 3.62 | 0.74 | 3.01 | 6.22 | 2.96 | 3.97 | 3.29 | 6.11 | 8.71 | 83.1 | 34.2 |
| F3,44 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| S treatments | |||||||||||||||||||
| S0 | 9.15 | 3.27 | 4.18 | 14.7 | 4.69 | 1.09 | 3.83 | 3.70 | 3.65 | 0.72 | 3.07 | 6.24 | 3.15 | 3.82 | 3.05 | 6.11 | 8.76 | 83.2 | 34.2 |
| S25 | 9.11 | 3.26 | 4.14 | 14.6 | 4.69 | 1.06 | 3.75 | 3.69 | 3.62 | 0.70 | 3.05 | 6.18 | 2.96 | 3.81 | 3.15 | 5.99 | 8.69 | 82.5 | 33.8 |
| S50 | 9.22 | 3.32 | 4.23 | 14.8 | 4.66 | 1.09 | 3.81 | 3.73 | 3.73 | 0.70 | 3.14 | 6.29 | 2.93 | 4.00 | 3.08 | 6.13 | 8.81 | 83.7 | 34.4 |
| F2,45 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Factor | CSSAA | CSVal | CSLeu | EAAI | |||||
|---|---|---|---|---|---|---|---|---|---|
| GS | MS | GS | MS | GS | MS | GS | MS | ||
| Year | |||||||||
| 2010 | 55.5 b | 75.9 b | 89.6 b | 86.7 b | 96.0 c | 96.5 c | 90.2 b | 94.3 a | |
| 2011 | 63.2 a | 88.2 a | 89.7 b | 62.6 c | 101.4 b | 92.8 d | 92.6 a | 87.4 c | |
| 2012 | 54.6 b | 52.8 c | 106.9 a | 98.4 a | 105.4 a | 101.7 b | 92.6 a | 92.0 b | |
| 2013 | 54.4 b | 54.5 c | 87.1 b | 101.6 a | 87.5 d | 105.0 a | 87.7 c | 92.6 ab | |
| F3,44 | *** | *** | *** | *** | *** | *** | *** | *** | |
| K treatments | |||||||||
| K1 | 57.8 | 63.7 | 92.7 | 88.6 | 97.1 | 97.8 | 91.2 | 91.5 | |
| K2 | 57.6 | 68.1 | 98.1 | 89.7 | 97.6 | 100.1 | 91.1 | 92.3 | |
| K3 | 55.6 | 68.8 | 90.3 | 84.9 | 97.3 | 99.4 | 90.0 | 91.1 | |
| K4 | 56.6 | 69.2 | 92.2 | 86.2 | 98.2 | 98.7 | 90.6 | 91.4 | |
| F3,44 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| K × S interaction | |||||||||
| K1 | S0 | 61.7 | 65.7 | 93.1 | 90.8 | 97.8 | 98.3 | 92.2 | 92.1 |
| S25 | 55.3 | 61.6 | 92.1 | 85.2 | 96.7 | 95.2 | 90.9 | 90.1 | |
| S50 | 56.4 | 63.8 | 92.8 | 90.0 | 96.9 | 99.8 | 91.0 | 91.4 | |
| K2 | S0 | 57.5 | 66.0 | 108.4 | 89.7 | 97.3 | 100.0 | 91.3 | 91.9 |
| S25 | 57.3 | 67.6 | 93.1 | 88.2 | 97.6 | 99.4 | 90.7 | 92.3 | |
| S50 | 58.1 | 70.7 | 92.9 | 91.2 | 98.1 | 100.9 | 91.2 | 92.7 | |
| K3 | S0 | 56.9 | 67.8 | 91.6 | 83.3 | 98.2 | 97.9 | 91.0 | 90.2 |
| S25 | 56.1 | 69.4 | 89.1 | 84.0 | 96.1 | 98.7 | 89.5 | 91.4 | |
| S50 | 53.9 | 69.2 | 90.2 | 87.3 | 97.7 | 101.4 | 89.6 | 91.7 | |
| K4 | S0 | 56.7 | 69.1 | 92.0 | 84.3 | 98.1 | 99.8 | 90.4 | 90.9 |
| S25 | 58.0 | 72.2 | 92.1 | 87.4 | 97.5 | 99.2 | 90.5 | 92.4 | |
| S50 | 55.2 | 66.1 | 92.5 | 86.7 | 99.0 | 97.0 | 90.9 | 91.0 | |
| F6,36 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Amino Acids | Immature Seeds (GS) | Mature Seeds (MS) | ||||||
|---|---|---|---|---|---|---|---|---|
| SY | SW | CP | T | SY | SW | CP | T | |
| Asp | 0.43 ** | 0.50 *** | −0.51 *** | −0.17 | 0.42 ** | 0.64 *** | −0.67 *** | 0.25 |
| Tre | 0.04 | −0.06 | 0.11 | −0.42 ** | 0.20 | 0.40 ** | −0.42 ** | 0.07 |
| Se | 0.02 | 0.01 | 0.02 | −0.47 ** | −0.07 | 0.37 * | −0.40 ** | 0.05 |
| Glu | −0.83 *** | −0.42 ** | 0.62 *** | −0.56 *** | −0.77 *** | −0.51 *** | 0.56 *** | −0.57 *** |
| Pro | −0.80 *** | −0.44 ** | 0.57 *** | −0.87 *** | −0.71 *** | −0.47 ** | 0.56 *** | −0.59 *** |
| Cys | −0.74 *** | −0.23 | 0.32 * | −0.40 ** | −0.71 *** | −0.39 ** | 0.44 ** | −0.57 *** |
| Gli | 0.16 | 0.31 * | −0.28 | −0.42 ** | 0.25 | 0.59 *** | −0.60 *** | 0.14 |
| Ala | 0.56 *** | 0.40 ** | −0.50 *** | 0.77 *** | 0.12 | 0.52 *** | −0.59 *** | 0.11 |
| Val | 0.18 | 0.17 | −0.25 | −0.16 | 0.54 *** | 0.18 | −0.21 | 0.49 *** |
| Met | 0.34 * | 0.43 ** | −0.50 *** | −0.07 | −0.23 | −0.12 | 0.02 | −0.17 |
| Ile | 0.27 | 0.39 ** | −0.45 ** | −0.31 * | 0.56 *** | 0.16 | −0.18 | 0.50 *** |
| Leu | −0.16 | 0.22 | −0.15 | −0.71 *** | 0.53 *** | 0.37 * | −0.38 ** | 0.37 ** |
| Tyr | 0.01 | −0.40 ** | 0.28 | −0.00 | −0.37 * | 0.10 | −0.13 | −0.30 * |
| Fen | 0.12 | −0.05 | 0.02 | −0.33 * | 0.03 | 0.12 | −0.08 | −0.05 |
| His | −0.21 | −0.51 *** | 0.59 *** | −0.31 * | −0.49 *** | −0.14 | 0.24 | −0.46 ** |
| Liz | 0.39 ** | 0.38 ** | −0.45 ** | −0.21 | 0.65 *** | 0.54 *** | −0.56 *** | 0.46 ** |
| Arg | 0.51 *** | 0.13 | −0.20 | 0.05 | 0.57 *** | 0.20 | −0.17 | 0.47 ** |
| TAA 1 | −0.11 | 0.01 | 0.04 | −0.59 *** | −0.23 | 0.08 | −0.01 | −0.20 |
| EAA 2 | 0.13 | 0.15 | −0.18 | −0.40 ** | 0.31 * | 0.39 ** | −0.37 ** | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barłóg, P.; Grzebisz, W.; Łukowiak, R. The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.). Agronomy 2019, 9, 209. https://doi.org/10.3390/agronomy9040209
Barłóg P, Grzebisz W, Łukowiak R. The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.). Agronomy. 2019; 9(4):209. https://doi.org/10.3390/agronomy9040209
Chicago/Turabian StyleBarłóg, Przemysław, Witold Grzebisz, and Remigiusz Łukowiak. 2019. "The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.)" Agronomy 9, no. 4: 209. https://doi.org/10.3390/agronomy9040209
APA StyleBarłóg, P., Grzebisz, W., & Łukowiak, R. (2019). The Effect of Potassium and Sulfur Fertilization on Seed Quality of Faba Bean (Vicia faba L.). Agronomy, 9(4), 209. https://doi.org/10.3390/agronomy9040209







