Evaluation of Somaclonal and Ethyl Methane Sulfonate-Induced Genetic Variation of Mexican Oregano (Lippia graveolens H.B.K.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
Conditions for In Vitro Germination of Mexican Oregano
2.2. Micropropagation of Mexican Oregano
2.3. Chemical Mutagenesis in Axillary Buds of L. graveolens
2.4. Analysis of the Genetic Variability Using RAMP Markers
2.5. Data Analysis
3. Results
3.1. In Vitro Propagation
3.2. Mutagenesis
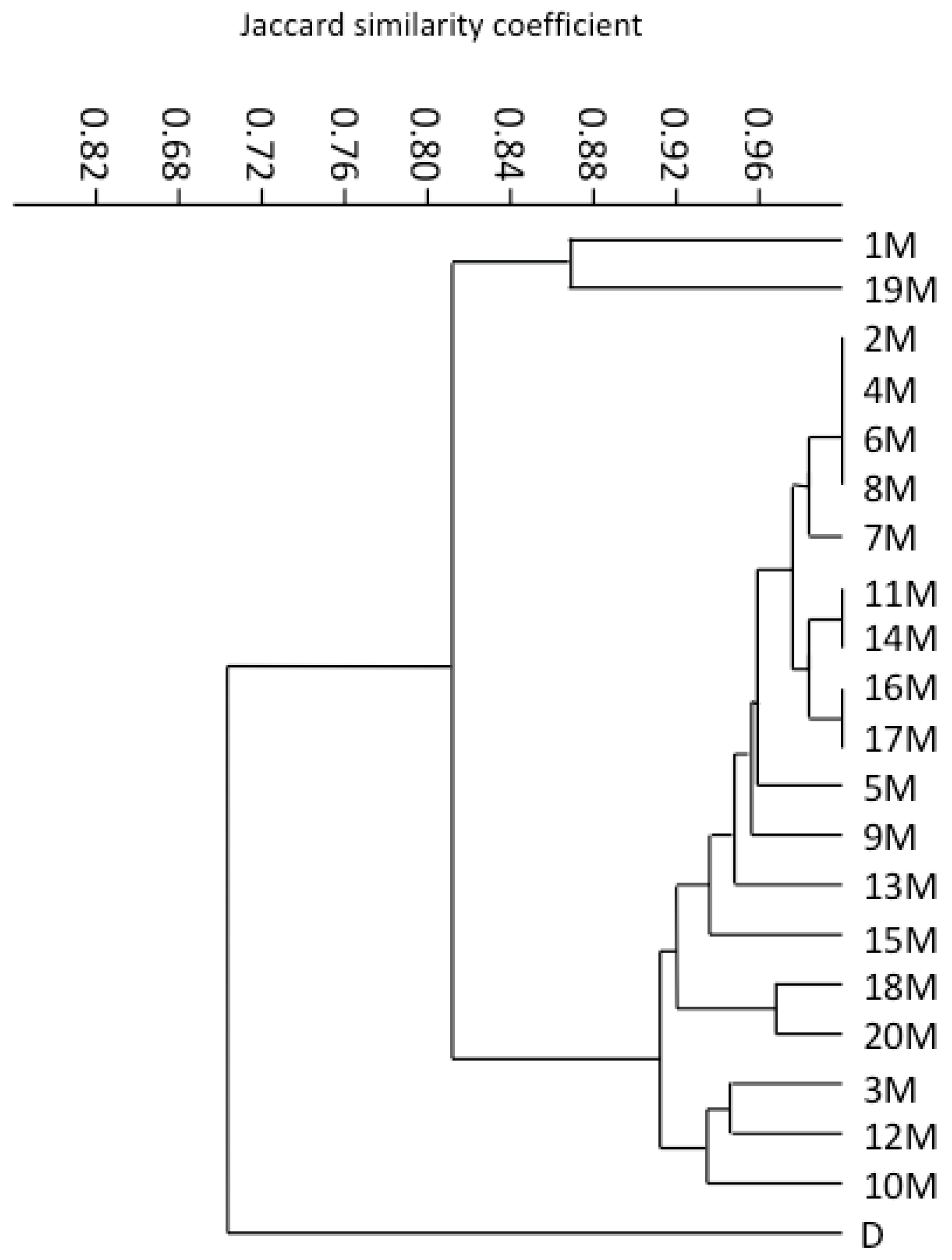
3.3. RAMP Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alamgir, A.N.M. Pharmacognostical Botany: Classification of medicinal and aromatic plants (MAPs), botanical taxonomy, morphology, and anatomy of drug plants. In Therapeutic Use of Medicinal Plants and Their Extract: 1. Progress in Drug Research; Springer: Cham, Switzerland, 2017; Volume 73, pp. 177–293. ISBN 978-3-319-6386-1. [Google Scholar]
- Villavicencio-Gutiérrez, E.; Cano-Pineda, A.; Garcia-Cuevas, X. Metodología para determinar las existencias de orégano (Lippia graveolens H.B.K.) en rodales naturales de parras de la fuente Coahuila; Instituto Nacional de Investigaciones Agrícolas y Pecuarias, Ed.; Instituto Nacional de Investigaciones Agrícolas y Pecuarias: Saltillo, Mexico, 2010; p. 42. ISBN 978-607-425-295-8. [Google Scholar]
- Güereca, M.C.G.; Hernandez, M.S.; Kite, G.; Vazquez, M.M. Antioxidant activity of flavonoids from the stem of the Mexican oregano (Lippia graveolens HBK var. berlandiefi Schauer). Rev. Fitotec. Mex. 2007, 30, 43–49. [Google Scholar]
- Muñoz-Acevedo, A.; Torres, E.A.; Gutierrez, R.G.; Cortes, S.B.; Cervantes-Diaz, M.; Tafurt-Garcia, G. Some Latin American plants promising for the cosmetic, perfume and flavor industries. In Therapeutic Medicinal Plants: From Lab to the Market; Duarte, M.C.T., Rai, M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; Volume 1, pp. 279–330. [Google Scholar] [CrossRef]
- Bueno-Durán, A.Y.; Cervantes-Martínez, J.; Obledo-Vázquez, E.N. Composition of essential oil from Lippia graveolens: Relationship between spectral light quality and thymol and carvacrol content. J. Essent Oil Res. 2014, 3, 153–160. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Hernández-Sánchez, L.Y.; Muñoz-Ocotero, V.; Dorazco-González, A.; Guevara-Fefer, P.; Aguirre-Hernández, E. Pharmacological evaluation of the anxiolytic-like effects of Lippia graveolens and bioactive compounds. Pharm. Biol. 2017, 55, 1569–1576. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Regalado-González, C.; Vázquez-Landaverde, P.; Guerrero-Legarreta, I.; García-Almendárez, B.E. Microencapsulation, Chemical Characterization, and Antimicrobial Activity of Mexican (Lippia graveolens H.B.K.) and European (Origanum vulgare L.) Oregano Essential Oils. Sci. World J. 2014, 12. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Gastélum-Franco, M.G.; Camacho-Dávila, A.; Torres-Muños, J.V.; Nevárez-Moorillón, G. Extracts of Mexican Oregano (Lippia berlandieri Schauer) with Antioxidant and Antimicrobial Activity. Food Bioprocess Technol. 2010, 3, 434–440. [Google Scholar] [CrossRef]
- Martínez-Rocha, A.; Puga, R.; Hernández-Sandoval, L.; Loarca-Piña, G.; Mendoza, S. Antioxidant and Antimutagenic Activities of Mexican Oregano (Lippia graveolens Kunth). Plant. Foods Hum. Nutr. 2008, 63, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.; Moctezuma, E.; Gutierrez-Pacheco, M.; Tapia-Rodriguez, M.; Ortega-Ramirez, L.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Calpouzos, L. Botanical aspects of Oregano. Econ. Bot. 1954, 8, 222–233. [Google Scholar] [CrossRef]
- Ocampo-Velázquez, R.V.; Malda-Barrera, G.X.; Suárez-Ramos, G. Reproductive biology of Mexican oregano (Lippia graveolens Kunth) in three exploitation conditions. Agrociencia 2009, 43, 475–482. [Google Scholar]
- Costa, A.S.; Arrigoni-Blank, M.F.; Blank, A.F.; Mendonza, A.B.; Amancio, V.F.; Ledo, A.S. Estabelecimento de alecrim-pimenta in vitro. Hortic. Bras. 2007, 25, 068–072. [Google Scholar] [CrossRef]
- Castellanos-Hernández, O.A.; Acevedo-Hernández, G.J.; Torres-Morán, M.I.; Zurita, F.; Gutiérrez-Lomelí, M.; Del Toro-Sánchez, C.L.; Rodríguez-Sahagún, A. In vitro Clonal Propagation and Regeneration of the Commercially Important Plant Mexican Oregano (Lippia graveolens). In Vitro Cell. Dev. Biol. Plant 2013, 49, 620–625. [Google Scholar] [CrossRef]
- Ahloowalia, B.; Maluszynski, M. Induced mutations—A new paradigm in plant breeding. Euphytica 2001, 118, 167–173. [Google Scholar] [CrossRef]
- Yolmeh, M.; Khomeiri, M. Effect of mutagenesis treatment on antimicrobial and antioxidant activities of pigments extracted from Rhodotorula glutinis. Biocatal. Agric. Biotechnol. 2017, 10, 285–290. [Google Scholar] [CrossRef]
- Bahl, J.R.; Sinha, S.; Naqvi, A.A.; Bansal, R.P.; Gupta, A.K.; Kumar, S. Linalool-rich essential oil quality variants obtained from irradiated stem nodes in Lippia alba. Flavour Fragr. J. 2002, 17, 127–132. [Google Scholar] [CrossRef]
- Khalil, F.; Naiyan, X.; Tayyab, M.; Pinghua, C. Screening of EMS-Induced Drought-Tolerant Sugarcane Mutants Employing Physiological, Molecular and Enzymatic Approaches. Agronomy 2018, 8, 226. [Google Scholar] [CrossRef]
- Srivastava, D.; Gayatri, M.C.; Sarangi, S.K. In vitro mutagenesis and characterization of mutants through morphological and genetic analysis in orchid Aerides crispa Lindl. Indian J. Exp. Biol. 2018, 56, 385–394. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hewitt, G.M. Special DNA Extraction Methods for Some Animal Species. In Molecular Tools for Screening Biodiversity: Plants and Animals; Karp, A., Isaac, P.G., Ingram, D.S., Eds.; Chapman and Hall: London, UK, 1998; pp. 25–31. ISBN 9789400900196. [Google Scholar]
- Pu, Z.E.; Hou, Y.C.; Xu, X.X.; Yan, Z.H.; Wei, Y.M.; Lan, X.J.; Zheng, Y.L. Genetic Diversity Among Barley Populations from West China Based on RAMP and RAPD Markers. Asian J. Plant. Sci. 2009, 8, 111–119. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc Numerical Taxonomy and Multivariate Analysis System, version 2.00; Exeter Software Publications: Setauket, NY, USA, 1997; p. 31. Available online: http://www.exetersoftware.com/cat/ntsyspc/ntsyspc.html (accessed on 12 December 2018).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Cao, H.; Yang, J.; Peng, Z.S.; Kang, C.Y.; Chen, D.C.; Gong, Z.C.; Tan, X. Micropropagation of Penthorum chinense Through Axillary Bud. In Vitro Cell. Dev. Biol. Plant 2007, 43, 149–153. [Google Scholar] [CrossRef]
- Islam, M.R.; Khan, R.; Hossain, S.N.; Ahmed, G.; Hakim, L. In vitro Clonal Propagation of Vitex negundo L. An important Medicinal Plant. Plant Tissue Cult. Biotechnol. 2009, 19, 113–117. [Google Scholar] [CrossRef]
- Zucchi, M.I.; Arizono, H.; Morais, V.A.; Pelegrinelli, M.H.; Carneiro, M.L. Genetic instability of sugarcane plants derived from meristem cultures. Genet. Mol. Biol. 2002, 25, 91–96. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Razdan, M.K.; Bhojwani, S.S. In vitro clonal propagation of an adult tree of neem (Azadirachta indica A. Juss.) by forced axillary branching. Plant Sci. 2004, 166, 501–506. [Google Scholar] [CrossRef]
- Junaid, A.; Mujib, A.; Sharma, M.P. Effect of growth regulators and ethyl methane sulphonate on growth, and chlorophyll, sugar and proline contents in Dracaena sanderiana cultured in vitro. Biol. Plant. 2008, 52, 569–572. [Google Scholar] [CrossRef]
- Chikelu, M.; Rownak, A.; Souleymane, B.; Shri-Mohan, J. Induced mutagenesis in plants using physical and chemical agents. In Plant Cell Culture: Essential Methods; Davey, M.R., Anthony, P., Eds.; John Wiley & Son, Ltd.: Hoboken, NJ, USA, 2010; pp. 111–127. ISBN 9780470686515. [Google Scholar]
- Glandorf, B.; de Loose, M.; Davies, H. Evaluation of changes in the genome of plants through application of new plant breeding techniques. Annex 15. In New Plant Breeding Techniques. State-of-the-Art and Prospects for Commercial Development; Lusser, M., Parisi, C., Plan, D., Rodríguez-Cerezo, E., Eds.; EUR 24760 EN; European Commission, Joint Research Centre: Brussels, Belgium, 2011; pp. 141–155. [Google Scholar]
- Hoang, T.M.L.; De Filippis, L.F.; Le, X.T. Salt Tolerance and Screening for Genetic Changes in Rice Mutants After Gamma Irradiation using RAPD and Microsatellite (RAMP) markers. Open Hortic. J. 2009, 2, 62–69. [Google Scholar] [CrossRef]
- Xi, M.; Sun, L.; Qiu, S.; Liu, J.; Xu, J.; Shi, J. In vitro Mutagenesis and Identification of Mutants via ISSR in Lily (Lilium longiflorum). Plant Cell Rep. 2012, 31, 1043–1051. [Google Scholar] [CrossRef]
- Martins, M.; Sarmento, D.; Oliveira, M.M. Genetic stability of micropropagated almond plantlets, as Assessed by RAPD and ISSR Markers. Plant Cell Rep. 2004, 23, 492–496. [Google Scholar] [CrossRef]
- Chittora, M. Assessment of genetic fidelity of long term micropropagated shoot cultures of Achras sapota L. var. ‘Cricket Ball’ as assessed by RAPD and ISSR markers. Indian J. Biotechnol. 2018, 17, 492–497. [Google Scholar]
- Rathore, M.S.; Yadav, P.; Mastan, S.G.; Prakash, C.R.; Singh, A.; Agarwal, P.K. Evaluation of Genetic Homogeneity in Tissue Culture Regenerates of Jatropha curcas L. Using Flow Cytometer and DNA-based Molecular Markers. Appl. Biochem. Biotechnol. 2014, 172, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, M.; Guangze, C.; Pan, T.; Shan, C. Assessment of the Genetic Diversity and Genetic Relationships of Pomegranate (Punicagranatum L.) in China Using RAMP Markers. Sci. Hortic. 2013, 151, 63–67. [Google Scholar] [CrossRef]
- Falk, L.; Biswas, K.; Boeckelmann, A.; Lane, A.; Mahmoud, S.S. An Efficient Method for Micropropagation of Lavenders: Regeneration of a Unique Mutant. J. Essent Oil Res. 2009, 21, 225–228. [Google Scholar] [CrossRef]




| Primer RAPD | Primer ISSR | Combination |
|---|---|---|
| OPB-07 | ARS-01 | 1 |
| OPB-08 | ARS-01 | 2 |
| OPB-12 | ARS-01 | 3 |
| OPB-07 | ARS-02 | 4 |
| OPB-08 | ARS-02 | 5 |
| OPB-12 | ARS-02 | 6 |
| OPB-07 | ARS-08 | 7 |
| OPB-08 | ARS-08 | 8 |
| OPB-12 | ARS-08 | 9 |
| EMS Concentration (%) | Exposure Time (h) | No. of Shoots per Explant * | Length of the Shoots (cm) * |
|---|---|---|---|
| 0.1 | 1 | 2.9 ± 0.3a | 2.1 ± 0.2x |
| 0.1 | 2 | 2.0 ± 0.4a | 2.0 ± 0.1x |
| 0.1 | 3 | 1.2 ± 0.1b | 0.8 ± 0.1y |
| 0.3 | 1 | 1.5 ± 0.4b | 1.9 ± 0.4x |
| 0.3 | 2 | 1.0 ± 0.0b | 1.6 ± 0.3x |
| 0.3 | 3 | 0.0 ± 0.0c | 0.0 ± 0.0z |
| 0.5 | 1 | 1.3 ± 0.1b | 1.7 ± 0.1x |
| 0.5 | 2 | 0.2 ± 0.1c | 0.9 ± 0.2y |
| 0.5 | 3 | 0.0 ± 0.0c | 0.0 ± 0.0z |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Miranda, L.A.; Rodríguez-Sahagún, A.; Acevedo Hernández, G.J.; Cruz-Martínez, V.O.; Torres-Morán, M.I.; Lépiz-Ildefonso, R.; Aarland, R.C.; Castellanos-Hernández, O.A. Evaluation of Somaclonal and Ethyl Methane Sulfonate-Induced Genetic Variation of Mexican Oregano (Lippia graveolens H.B.K.). Agronomy 2019, 9, 166. https://doi.org/10.3390/agronomy9040166
Muñoz-Miranda LA, Rodríguez-Sahagún A, Acevedo Hernández GJ, Cruz-Martínez VO, Torres-Morán MI, Lépiz-Ildefonso R, Aarland RC, Castellanos-Hernández OA. Evaluation of Somaclonal and Ethyl Methane Sulfonate-Induced Genetic Variation of Mexican Oregano (Lippia graveolens H.B.K.). Agronomy. 2019; 9(4):166. https://doi.org/10.3390/agronomy9040166
Chicago/Turabian StyleMuñoz-Miranda, Luis A., Araceli Rodríguez-Sahagún, Gustavo J. Acevedo Hernández, Victor O. Cruz-Martínez, Martha I. Torres-Morán, Rogelio Lépiz-Ildefonso, Rayn C. Aarland, and Osvaldo A. Castellanos-Hernández. 2019. "Evaluation of Somaclonal and Ethyl Methane Sulfonate-Induced Genetic Variation of Mexican Oregano (Lippia graveolens H.B.K.)" Agronomy 9, no. 4: 166. https://doi.org/10.3390/agronomy9040166
APA StyleMuñoz-Miranda, L. A., Rodríguez-Sahagún, A., Acevedo Hernández, G. J., Cruz-Martínez, V. O., Torres-Morán, M. I., Lépiz-Ildefonso, R., Aarland, R. C., & Castellanos-Hernández, O. A. (2019). Evaluation of Somaclonal and Ethyl Methane Sulfonate-Induced Genetic Variation of Mexican Oregano (Lippia graveolens H.B.K.). Agronomy, 9(4), 166. https://doi.org/10.3390/agronomy9040166





