An Evaluation of Spelt Crosses for Breeding New Varieties of Spring Spelt
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Yield Components and Lodging Tolerance
2.3. Chemical Analyses of Grain
2.4. Field Experiment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of F2 Spelt Crosses of Spring Spelt
3.2. Characteristics of F5 Breeding Lines of Spring Spelt
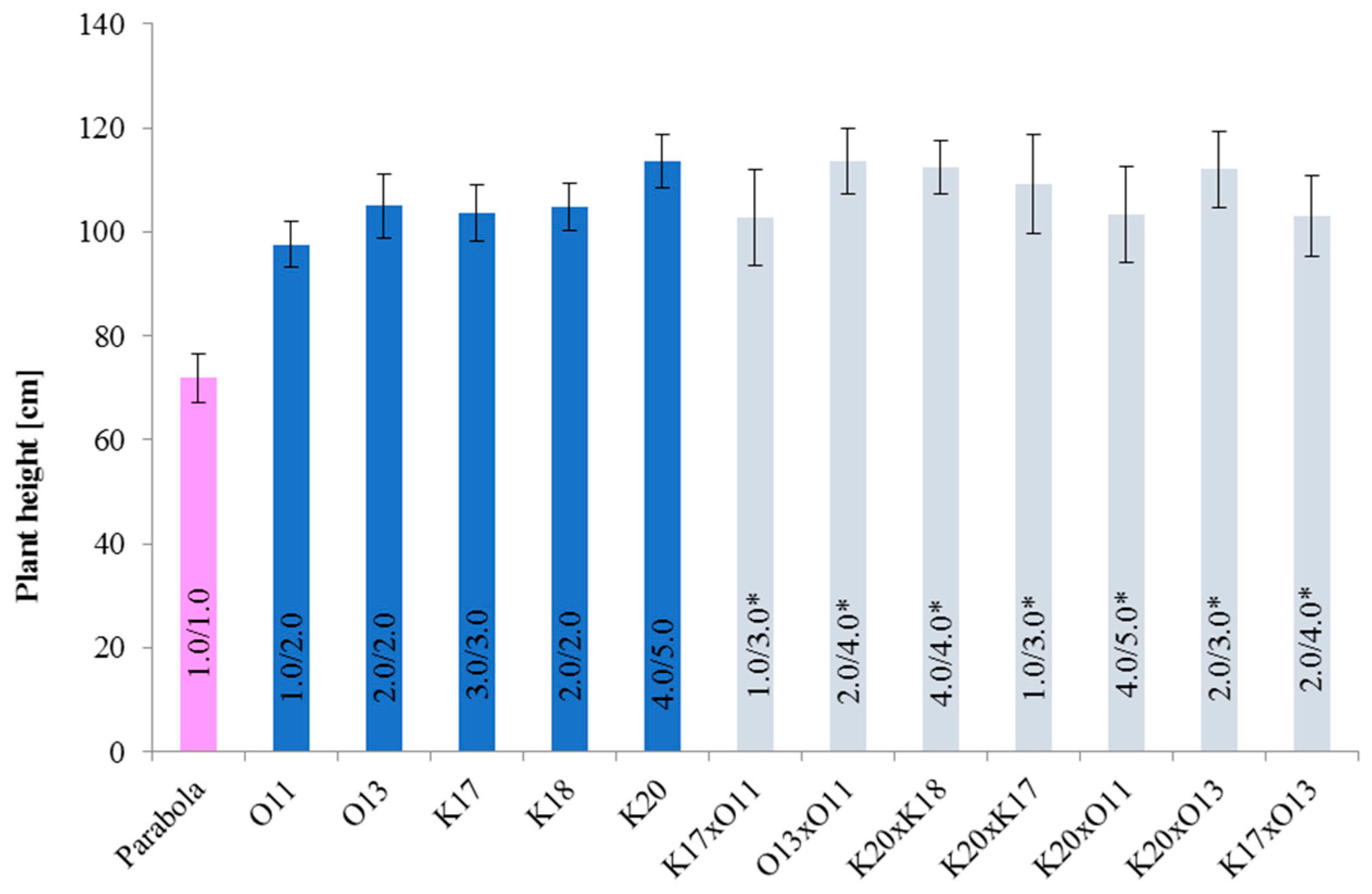
3.2.1. Plant Height and Sensitivity to Lodging
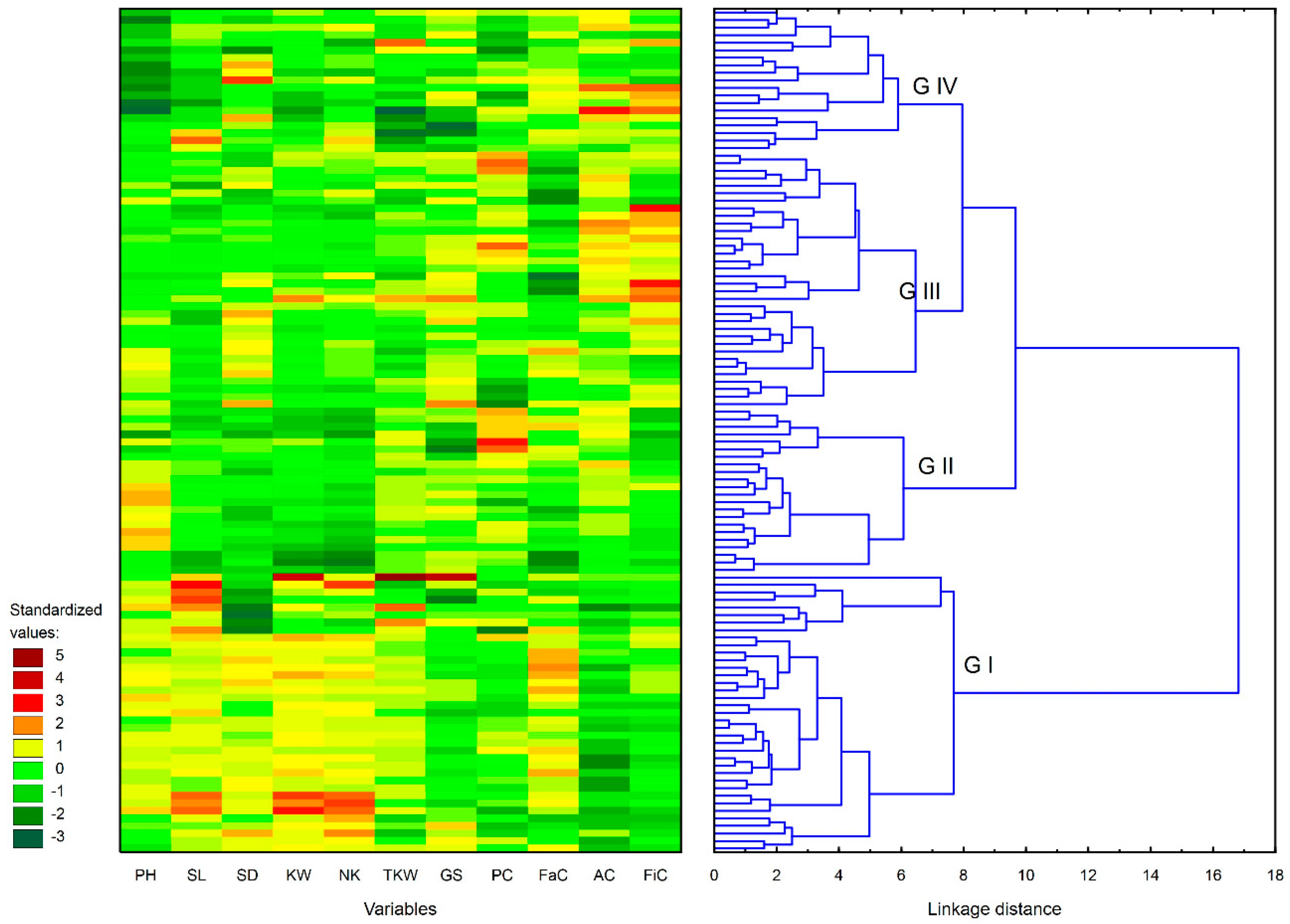
3.2.2. Grouping Spelt Breeding Lines and Parents by Cluster Analysis
4. Discussion
4.1. Yield Components
4.2. Chemical Composition of Grain
4.2.1. Protein Content
4.2.2. Fat Content
4.2.3. Ash Content
4.2.4. Fiber Content
4.3. Sensitivity to Lodging
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Andrews, A.C. The genetic origin of spelt and related wheats. Züchter 1964, 34, 17–22. [Google Scholar] [CrossRef]
- Yan, Y.; Hsam, S.L.K.; Yu, J.Z.; Jiang, Y.; Ohtsuka, I.; Zeller, F.J. HMW and LMW glutenin alleles among putative tetraploid and hexaploid European spelt wheat (Triticum spelta L.) progenitors. Theor. Appl. Genet. 2003, 107, 1321–1330. [Google Scholar] [CrossRef]
- Nesbitt, M.; Samuel, D. From staple crop to extinction? The archaeology and history of the hulled wheats. In Hulled wheat. In Proceedings of the First International Workshop on Hulled Wheats, Castelvecchio Pascoli, Tuscany, 21–22 July 1995; Padulosi, S., Hammer, K., Heller, J., Eds.; IPGRI: Roma, Italy, 1995; pp. 41–101. [Google Scholar]
- Lobiz, R. Urgetreide—Mehr Schein als Sein. Ernährung Fokus 2018, 3, 114–119. [Google Scholar]
- Ackerbauflächen je Bundesland: Ernte 2018 (inkl. Bioflächen) 2017–2018: Dinkel. Available online: https://www.ama.at/ (accessed on 12 February 2019).
- Produktionsflächen/Surface de Production 2008–2018: Dinkel. Available online: https://www.swissgranum.ch/documens/ (accessed on 12 February 2019).
- Dedkova, O.S.; Badaeva, E.D.; Mitrofanova, O.P.; Zelenin, A.V.; Pukhalskiy, V.A. Analysis of intraspecific divergence of hexaploid wheat Triticum spelta L. by C-banding of chromosomes. Russ. J. Genet. 2004, 40, 1111–1126. [Google Scholar] [CrossRef]
- Elia, M.; Moralejo, M.; Rodriguez-Quijano, M.; Molina-Cano, J.L. Spanish spelt: A separate gene pool within the spelt germplasm. Plant Breed. 2004, 123, 297–299. [Google Scholar] [CrossRef]
- Guzman, C.; Caballero, L.; Martin, L.M.; Alvarez, J.B. Waxy genes from spelt wheat: New alleles for modern wheat breeding and new phylogenetic inferences about the origin of this species. Ann. Bot. Lond. 2012, 110, 1161–1171. [Google Scholar] [CrossRef]
- Beschreibende Sortenliste: Winterdinkel 2018. Available online: https://www.bundessortenamt.de (accessed on 12 February 2019).
- Österreichische Beschreibende Sortenliste: Winterdinkel 2018. Available online: https://bsl.baes.gv.at/pdf_version (accessed on 12 February 2019).
- Liste der Empfohlenen Getreidensorten für die Ernte 2019. Available online: https://www.swissgranum.ch/sortenlisten (accessed on 12 February 2019).
- GZPK Biodynamische Pflanzenzüchtung. Available online: https://www.gzpk.ch/kulturen/dinkel (accessed on 12 February 2019).
- European Union. Common Catalogue of Varieties of Agricultural Plant Species, 37th ed.; C 13; Official Journal of European Union: Brussels, Belgium, 2019. [Google Scholar]
- Okoń, S.; Paczos-Grzęda, E.; Kraska, P.; Kwiecińska-Poppe, E.; Pałys, E. Assessment of genetic similarity of spelt (Triticum aestivum ssp. spelta L.) cultivars based on RAPD markers. Biuletyn IHAR 2009, 252, 35–41. [Google Scholar]
- Gulyas, G.; Rakszegi, M.; Bognar, Z.; Lang, L.; Bedö, Z. Evaluation of genetic diversity of spelt breeding materials based on AFLP and quality analyses. Cereal Res. Commun. 2012, 40, 185–193. [Google Scholar] [CrossRef]
- Research Center for Cultivar Testing. Polish National List of Agricultural Plant Varieties; Research Center for Cultivar Testing: Słupia Wielka, Poland, 2015. [Google Scholar]
- Wiwart, M.; Suchowilska, E.; Packa, D.; Bieńkowska, T.; Rutkowska-Łoś, A. Registration of ‘Wirtas’, a new spring spelt cultivar in Poland. J. Plant Regist. 2016, 10, 271–275. [Google Scholar] [CrossRef]
- COBORU. Lista Opisowa Odmian Roślin Rolniczych Rośliny Zbożowe. (Descriptive List of Agricultural Plant Varieties. Cereal crops.); COBORU: Słupia Wielka, Poland, 2018. (in Polish) [Google Scholar]
- Research Center for Cultivar Testing. Polish National List of Agricultural Plant Varieties; Research Center for Cultivar Testing: Słupia Wielka, Poland, 2018. [Google Scholar]
- Polish National List of Agricultural Plant Varieties. Available online: http://www.coboru.pl/English/index_eng.aspx (accessed on 21 December 2018).
- Winzeler, H.; Schmidt, J.E.; Winzeler, M. Analysis of the yield potential and components of F1 and F2 hybrids of crosses between wheat (Triticum aestivum L.) and spelt (Triticum spelta L.). Euphytica 1993, 74, 211–218. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Winzeler, M.; Winzeler, H. Analysis of disease resistance and quality characters of F1 hybrids of crosses between wheat (Triticum aestivum L.) and spelt (Triticum spelta L.). Euphytica 1994, 75, 105–110. [Google Scholar] [CrossRef]
- Zanetti, S.; Winzeler, M.; Keller, M.; Keller, B.; Messmer, M. Genetic analysis of pre-harvest sprouting resistance in wheat x spelt cross. Crop. Sci. 2000, 40, 1406–1417. [Google Scholar] [CrossRef]
- Wiwart, M.; Suchowilska, E.; Lajszner, W.; Graban, Ł. Identification of hybrids of spelt and wheat and their parental forms using shape and color descriptors. Comp. Electron. Agric. 2012, 83, 68–76. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial good products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef]
- Acquaah, G. Breeding wheat. In Principles of Plant Genetics and Breeding, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 577–590. [Google Scholar]
- NCPGR. Plant Breeding and Acclimatization Institute. Available online: http://genbank.ihar.edu.pl (accessed on 21 December 2018).
- EWDB. European Wheat Data Base. Available online: http://genbank.vurv.cz/ewdb (accessed on 21 December 2018).
- IPK. Leibniz-Institut. Available online: https://www.ipk-gatersleben.de/ (accessed on 21 December 2018).
- International Organization for Standardization. Cereals and Pulses. Determination of Nitrogen Content and Calculation of the Crude Protein Content. Kjeldahl Method; PN-EN ISO 20483:2014; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- International Organization for Standardization. Oilseed Meals. Determination of Oil Content. Extraction Method with Hexane (or Light Petroleum); PN-EN ISO 734-1:2008; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- International Organization for Standardization. Solid Fuels. Determination of Moisture Content, Volatile Parts and Ash with an Automatic Analyzer; PN-G-04560:1998; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Möller, J. Comparing Methods for Fibre Determination in Food and Feed. Available online: https://www.fossanalytics.com (accessed on 12 February 2019).
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Stanisz, A. Cluster analysis. In Przystępny Kurs Statystyki z Zastosowaniem STATISTICA PL na Przykładach z Medycyny: Analizy Wielowymiarowe; StatSoft Polska: Kraków, Poland, 2007; Tom 3; pp. 113–164. (in Polish) [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. Available online: http://statistica.io (accessed on 21 December 2018).
- Abdel-Aal, E.S.M.; Hucl, P.; Sosulski, F.W. Compositional and nutritional characteristics of spring einkorn and spelt wheats. Cereal Chem. 1995, 72, 621–624. [Google Scholar]
- Grela, E.R. Nutrient composition and content of antinutritional factors in spelt (Triticum spelta L.) cultivars. J. Sci. Food Agric. 1996, 71, 399–404. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Rizzi, R.; Pannacciulli, E.; Della Gatta, C. Mineral composition in hulled wheat grains: A comparison between emmer (Triticum dicoccon Schrank) and spelt (T. spelta L.) accessions. Int. J. Food Sci. Nutr. 1997, 48, 381–386. [Google Scholar] [CrossRef]
- Gomez-Becerra, H.F.; Erdem, H.; Yazici, A.; Tutus, Y.; Torun, B.; Ozturk, L.; Cakmak, I. Grain concentrations of protein and mineral nutrients in large collection of spelt wheat grown under different environments. J. Cereal Sci. 2010, 52, 342–349. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Krska, R. A comparison of macro- and microelement concentrations in the grain of four Triticum species. Plant Soil Environ. 2012, 58, 141–147. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Ziegler, J.; Schweiggert, R.; Koehler, P.; Carle, R.; Würschum, T. Comparative study of hulled (einkorn, emmer, and spelt) and naked wheats (durum and bread wheat): Agronomic performance and quality traits. Crop Sci. 2016, 56, 302–311. [Google Scholar] [CrossRef]
- Wiwart, M.; Szafrańska, A.; Wachowska, U.; Suchowilska, E. Quality parameters and rheological dough properties of fifteen spelt (Triticum spelta L.) varieties cultivated today. Cereal Chem. 2017, 94, 1037–1044. [Google Scholar]
- Rapp, M.; Beck, H.; Gütler, H.; Heilig, W.; Starck, N.; Römer, P.; Cuendet, C.; Uhlig, F.; Kurz, H.; Würschum, T.; et al. Spelt: Agronomy, quality, and flavor of its breads from 30 varieties tested across multiple environments. Crop Sci. 2017, 57, 739–747. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Würschum, T. Genetic variability, heritability and correlation among agronomic and disease resistance traits in a diversity panel and elite breeding material of spelt wheat. Plant Breed. 2014, 133, 459–464. [Google Scholar] [CrossRef]
- Kema, G.H.J. Resistance in spelt wheat to yellow rust. Euphytica 1992, 63, 225–231. [Google Scholar]
- Sulewska, H.; Nita, Z.; Kruczek, A. Variability of grain quality characters among spelt wheat genotypes. Biuletyn IHAR 2005, 235, 65–74. [Google Scholar]
- Simon, M.R.; Khlestkina, E.K.; Castillo, N.S.; Börner, A. Mapping quantitative resistance to Septoria tritici blotch in spelt wheat. Eur. J. Plant Pathol. 2010, 128, 317–324. [Google Scholar] [CrossRef]
- Mohler, V.; Singh, D.; Singrün, C.; Park, R.F. Characterization and mapping of Lr65 in spelt wheat ‘Altgold Rotkorn’. Plant Breed. 2012, 131, 252–257. [Google Scholar] [CrossRef]
- Kolomiets, T.M.; Pankratova, L.F.; Pakholkova, T.M. Wheat (Triticum, L.) cultivars from GRIN collection (USA) selected for durable resistance Septoria tritici and Stagonospora nodorum Blotch. Sel’skokhozyaistvennaya Biol. Agric. Biol. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Rüegger, A.; Winzeler, H. Performance of spelt (Triticum spelta L.) and wheat (Triticum aestivum L.) at two different seeding rates and nitrogen levels under contrasting environmental conditions. J. Agron. Crop Sci. 1993, 170, 289–295. [Google Scholar] [CrossRef]
- Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P.; Pałys, E. Yield of winter cultivars of spelt wheat (Triticum aestivum ssp. spelta L.) cultivated under diversified conditions of mineral fertilization and chemical protection. Acta Sci. Pol. Agric. 2011, 10, 5–14. [Google Scholar]
- Andruszczak, S. Reaction of winter spelt cultivars to reduced tillage system and chemical plant protection. Zemdirbyste 2017, 104, 15–22. [Google Scholar] [CrossRef]
- Sobczyk, A.; Pycia, K.; Stankowski, S.; Jaworska, G.; Kuźniar, P. Evaluation of rheological properties of dough and quality of bread made with the flour obtained from old cultivars and modern breeding lines of spelt (Triticum aestivum ssp. spelta). J. Cereal Sci. 2017, 77, 35–41. [Google Scholar] [CrossRef]
- Varieties of Crop Kinds Registered in Canada. Available online: http://www.inspection.gc.ca/active/netapp/regvar/regvar_lookupe.aspx (accessed on 21 December 2018).
- Dorval, I.; Vanasse, A.; Pageau, D.; Dion, Y. Seeding rate and cultivar effects on yield, yield components and grain quality of spring spelt in eastern Canada. Can. J. Plant Sci. 2015, 95, 841–849. [Google Scholar] [CrossRef]
- Sulewska, H. Characterization of 22 spelt (Triticum aestivum ssp. spelta) genotypes relating to some features. Biuletyn IHAR 2004, 231, 43–53. [Google Scholar]
- Konvalina, P.; Capouchová, I.; Stehno, Z.; Moudrý, J. Agronomic characteristics of the spring forms of the wheat landraces (einkorn, emmer, spelt, intermediate bread wheat) grown in organic farming. J. Agrobiol. 2010, 27, 9–17. [Google Scholar] [CrossRef]
- Lacko-Bartosova, M.; Korczyk-Szabo, J.; Razny, R. Triticum spelta—Specialty grain for ecological farming systems. Res. J. Agric. Sci. 2010, 42, 143–147. [Google Scholar]
- Packa, D.; Załuski, D.; Graban, Ł.; Lajszner, W.; Hościk, M. The response of diploid, tetraploid and hexaploid wheats to Fusarium culmorum (W.G. Smith) Sacc. inoculation. Pol. J. Agron. 2013, 12, 38–48. [Google Scholar]
- Escarnot, E.; Jacquemin, J.-M.; Agneessens, R.; Paquot, M. Comparative study of the content and profiles of macronutrients in spelt and wheat, a review. Biotechnol. Agron. Soc. 2012, 16, 243–256. [Google Scholar]
- Boros, D. The content of nutrients and bioactive components in the grain of common wheat varieties. AgroSerwis 2011, 5, 57–66. (in Polish). [Google Scholar]
- Oury, F.X.; Berard, P.; Brancourt-Hulmel, M.; Depatureaux, C.; Doussinault, G.; Galic, N.; Giraud, A.; Heumez, E.; Lecomte, C.; Pluchard, P.; et al. Yield and grain protein concentration in bread wheat: A review and a study of multi-annual data from a French breeding program. J. Genet. Breed. 2003, 57, 59–68. [Google Scholar]
- Capouchová, I. Technological quality of spelt (Triticum spelta L.) from ecological growing system. Sci. Agric. Bohemica 2001, 32, 307–322. [Google Scholar]
- Cacak-Pietrzak, G.; Gondek, E.; Jończyk, K. Comparison of internal structure and milling properties of spelt and bread wheat from ecological farming. Zeszyty Problemowe PNR 2013, 574, 3–10. [Google Scholar]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their content of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Biel, W.; Stankowski, S.; Jaroszewska, A.; Pużyński, S.; Bośko, P. The influence of selected agronomic factors on the chemical composition of spelt wheat (Triticum aestivum ssp. spelta) grain. J. Integr. Agric. 2016, 15, 1763–1769. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Fotiadis, S.; Damalas, C.A. Biomas and nitrogen accumulation and translocationin spelt (Triticum spelta) grown in Mediterraneum area. Field Crop Res. 2012, 127, 1–8. [Google Scholar] [CrossRef]
- Berry, P.M.; Sterling, M.; Spink, J.H.; Baker, C.J.; Sylvester-Bradley, R.; Mooney, S.J.; Tams, A.R.; Ennos, A.R. Understanding and reducing lodging in cereals. Adv. Agron. 2004, 84, 217–271. [Google Scholar]
- Khobra, L.; Sereen, S.; Meena, B.K.; Kumar, A.; Tivari, V.; Singh, G.P. Exploring the traits for lodging tolerance in wheat genotypes: A review. Physiol. Mol. Biol. Plants 2019. [Google Scholar] [CrossRef]
- Packa, D.; Wiwart, M.; Suchowilska, E.; Bieńkowska, T. Morpho-anatomical traits of two lowest internodes related to lodging resistance in selected genotypes of Triticum. Int. Agrophys. 2015, 29, 475–483. [Google Scholar] [CrossRef]
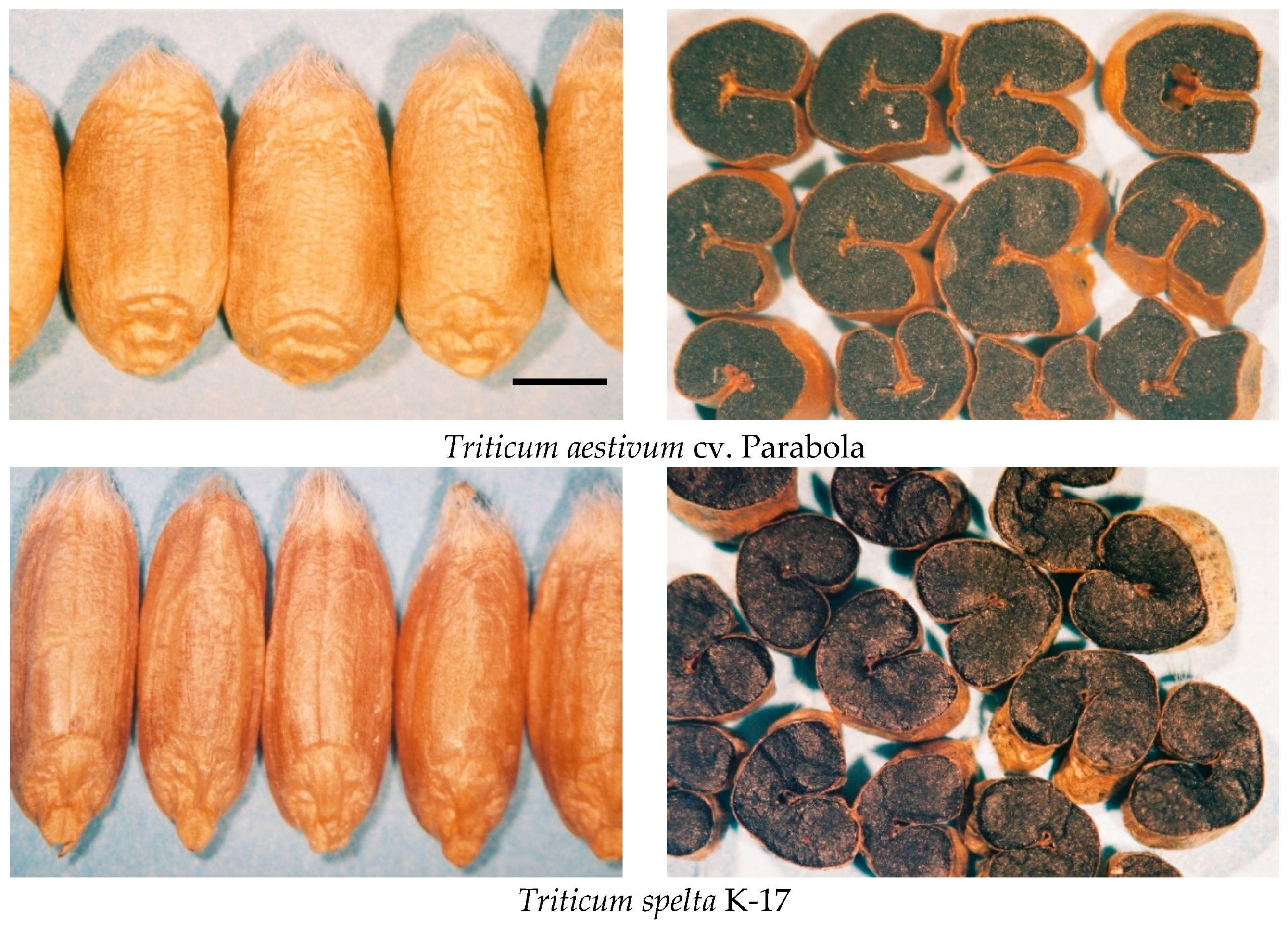

| Parental Forms | Variety | Gene Bank | Designation/Accession/EWDB Number | Region of Origin |
|---|---|---|---|---|
| K17 | var. arduini (Mazz.) Koern | NCPGR Radzików | 2-1257/PL 21981/EWDB 45624 | - |
| K18 | - | IPK Gatersleben | -/TRI 17506/EWDB 76243 | Spain |
| K20 | var. caeruleum (Alef.) Koern | IPK Gatersleben | Schwarzer Bartspelz/TRI 3419/EWDB 62904 | Germany |
| O11 | var. album (Alef.) Koern | NCPGR Radzików | Spelz aus Tzari Brod/PL 21805/EWDB 53925 | - |
| O13 | var. arduini (Mazz.) Koern | NCPGR Radzików | Weisser Grannen Spelz/PL 21806/EWDB 55245 | Germany |
| Years | Selection Cycle | Generation |
|---|---|---|
| 2007 | Crossing of spring spelt: K17 × O11; O13 × O11; K20 × K18; K20 × K17; K20 × O11; K20 × O13; K17 × O13 | |
| 2008 | First | F1—collection of all spikes without selection |
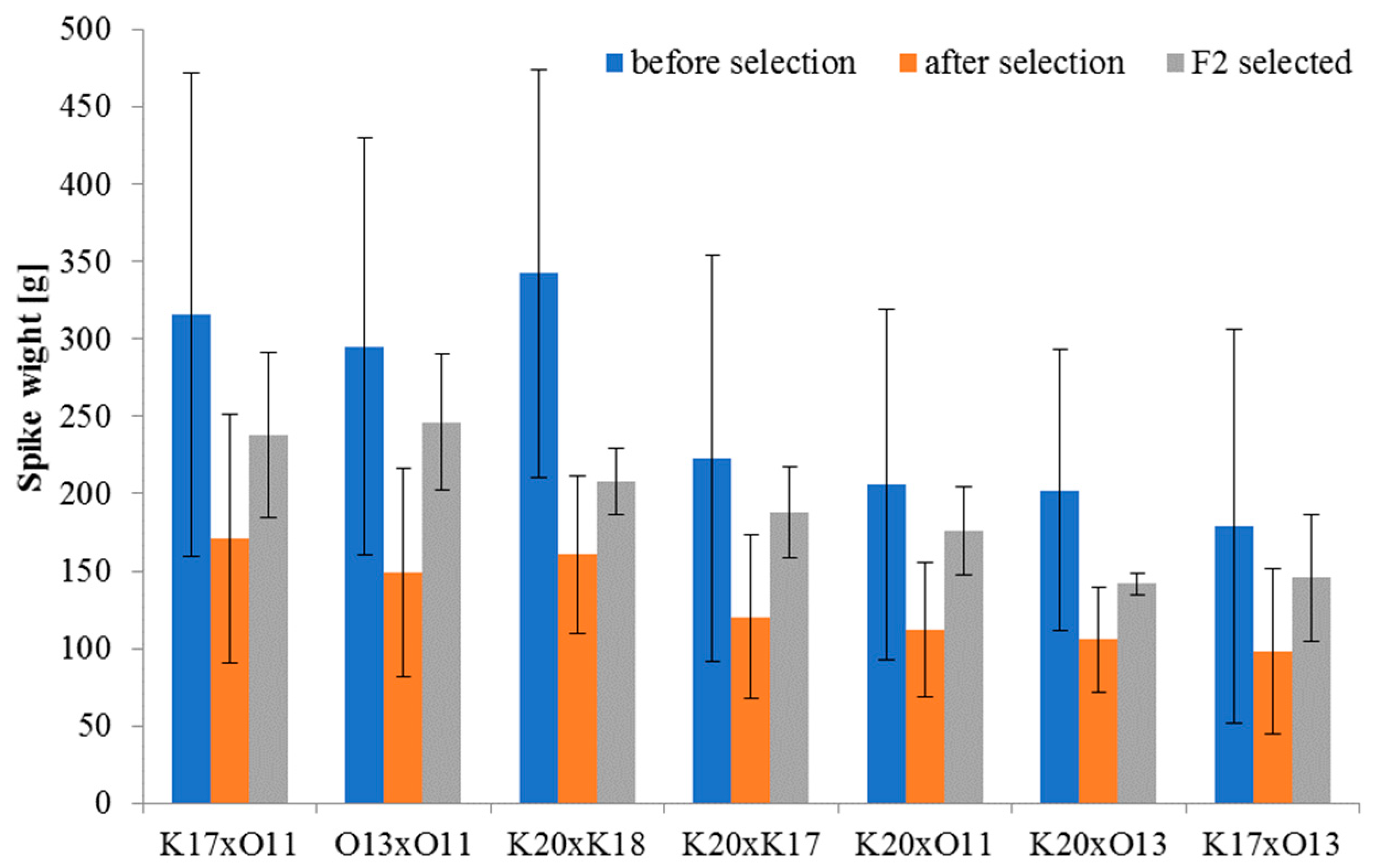
| 2009 | First | F2—collection, evaluation, selection, and qualification of spelt crosses for further breeding: K17 × O11 14/6; O13 × O11 27/7; K20 × K18 29/8; K20 × K17 26/6; K20 × O11 37/7; K20 × O13 23/7; K17 × O13 16/5, in all 172/46 |
| 2011 | First | F3—selection, evaluation, and qualification of individuals for further breeding (184) |
| 2013 | First | F4—field and laboratory evaluation and qualification for further breeding |
| 2014 | Second | F3—selection, evaluation, and qualification of individuals for further breeding (from reserve) (80) |
| 2015 | Second | F4—field and laboratory evaluation and qualification for further breeding |
| 2016 | First and second | F5—field and laboratory evaluation of selected breeding lines (107) and their parents (5) |
| Protein (% DM) | Crude Fat (% DM) | Ash (% DM) | Crude Fiber (% DM) | |
|---|---|---|---|---|
| K17 × O11 (n = 14)1) | ||||
| Mean ± SD | 16.81 ± 0.84 | 1.75 ± 0.15 | 2.32 ± 0.18 | 1.69 ± 0.21 |
| Min./Max. | 15.77/18.61 | 1.51/1.95 | 1.51/1.95 | 1.48/2.20 |
| CV (%) | 5.0 | 8.5 | 8.0 | 12.7 |
| O13 × O11 (n = 27) | ||||
| Mean ± SD | 16.29 ± 0.75 | 1.58 ± 0.22 | 2.17 ± 0.13 | 1.55 ± 0.24 |
| Min./Max. | 14.93/17.85 | 1.08/2.05 | 1.94/2.42 | 1.24/2.09 |
| CV (%) | 4.6 | 13.7 | 5.9 | 15.2 |
| K20 × K18 (n = 29) | ||||
| Mean ± SD | 15.48 ± 0.81 | 1.91 ± 0.20 | 2.04 ± 0.10 | 1.33 ± 0.26 |
| Min./Max. | 14.08/17.38 | 1.53/2.28 | 1.80/2.25 | 0.77/1.82 |
| CV (%) | 5.2 | 10.7 | 4.7 | 19.6 |
| K20 × K17 (n = 26) | ||||
| Mean ± SD | 16.36 ± 0.74 | 2.02 ± 0.24 | 2.13 ± 0.12 | 1.55 ± 0.23 |
| Min./Max. | 15.20/18.00 | 1.67/2.47 | 1.89/2.38 | 1.11/1.81 |
| CV (%) | 4.5 | 12.0 | 5.6 | 14.5 |
| K20 × O11 (n = 37) | ||||
| Mean ± SD | 16.03 ± 0.84 | 1.99 ± 0.20 | 2.01 ± 0.20 | 1.47 ± 0.19 |
| Min./Max. | 14.56/17.70 | 1.66/2.49 | 1.66/2.53 | 1.12/1.91 |
| CV (%) | 5.2 | 9.9 | 10.0 | 13.0 |
| K20 × O13 (n = 23) | ||||
| Mean ± SD | 15.79 ± 0.75 | 1.75 ± 0.18 | 2.14 ± 0.26 | 1.41 ± 0.19 |
| Min./Max. | 14.80/17.37 | 1.20/2.18 | 1.79/2.80 | 1.12/1.79 |
| CV (%) | 4.7 | 10.0 | 12.2 | 13.3 |
| K17 × O13 (n = 16) | ||||
| Mean ± SD | 16.97 ± 0.95 | 1.50 ± 0.22 | 2.19 ± 0.13 | 1.41 ± 0.19 |
| Min./Max. | 15.66/18.64 | 1.04/1.73 | 2.04/2.45 | 1.13/1.66 |
| CV (%) | 5.6 | 14.7 | 5.8 | 13.3 |
| Parents (O11, O13, K17, K18, K20) | ||||
| Mean ± SD | 17.20 ± 0.55 | 2.20 ± 0.27 | 2.32 ± 0.12 | 1.81 ± 0.14 |
| Min./Max. | 16.23/17.93 | 1.72/2.56 | 2.17/2.52 | 1.62/2.07 |
| CV (%) | 3.2 | 12.5 | 5.1 | 7.9 |
| T. aestivum cv. Parabola | ||||
| Mean | 14.35 | 1.65 | 2.10 | 2.29 |
| Group | n | Parents | Characteristics |
|---|---|---|---|
| Group I | 35 | K18, K20 | tall plants with satisfactory yield components, lower content of protein, ash, and fiber relative to the remaining groups, high fat content, and high sensitivity to lodging |
| Group II | 22 | - | tall plants with unsatisfactory yield components, higher content of protein and ash relative to the remaining groups, lower content of fiber and fat, and high sensitivity to lodging |
| Group III | 32 | O13, K17 | shorter plants with moderate yield components, high content of protein, ash, and fiber, low fat content, and moderate sensitivity to lodging |
| Group IV | 18 | O11 | shortest plants with moderate yield components, moderate content of fat, ash, and fiber, lower protein content relative to the remaining groups, and moderate sensitivity to lodging |
| Group | PH 1 | SL 2 | SD 3 | KW 4 | NK 5 | TKW 6 | GS 7 | PC 8 | FaC 9 | AC 10 | FiC 11 | LO (1–5) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G I CV (%) | 111.5 ± 4.1 3.7 | 12.8 ± 0.6 4.9 | 13.2 ± 0.8 6.1 | 1.56 ± 0.12 7.9 | 31.9 ± 2.3 7.0 | 48.9 ± 3.3 6.8 | 70.5 ± 1.9 2.7 | 16.60 ± 0.77 4.6 | 2.23 ± 0.21 9.3 | 2.08 ± 0.09 4.4 | 1.97 ± 0.18 9.4 | 3/4 |
| G II CV (%) | 111.5 ± 7.2 6.5 | 11.4 ± 0.5 4.4 | 12.6 ± 0.3 2.6 | 1.24 ± 0.09 7.6 | 25.7 ± 1.8 6.9 | 48.3 ± 1.2 2.5 | 70.7 ± 1.7 2.4 | 17.52 ± 0.94 5.3 | 1.99 ± 0.22 11.0 | 2.27 ± 0.08 3.6 | 1.89 ± 0.18 9.3 | 3/4 |
| G III CV (%) | 107.9 ± 3.9 3.6 | 11.3 ± 0.4 3.9 | 13.3 ± 0.5 4.0 | 1.34 ± 0.09 7.0 | 28.6 ± 1.7 6.1 | 46.9 ± 2.1 4.5 | 71.9 ± 1.2 1.7 | 17.07 ± 1.01 5.9 | 1.96 ± 0.23 11.9 | 2.32 ± 0.09 3.9 | 2.32 ± 0.32 13.8 | 2/3 |
| G IV CV (%) | 96.8 ± 5.3 5.5 | 11.6 ± 0.9 7.6 | 13.2 ± 0.7 5.3 | 1.30 ± 0.11 8.2 | 29.0 ± 2.5 8.5 | 44.9 ± 3.8 8.5 | 70.2 ± 2.1 3.0 | 16.40 ± 0.90 5.5 | 2.19 ± 0.14 6.2 | 2.30 ± 0.15 6.4 | 2.23 ± 0.32 14.6 | 2/3 |
| G II-IV CV (%) | 106.1 ± 7.8 7.3 | 11.4 ± 0.6 5.2 | 13.1 ± 0.6 4.6 | 1.30 ± 0.10 8.0 | 27.8 ± 2.4 8.5 | 46.8 ± 2.7 5.9 | 71.1 ± 1.8 2.5 | 17.03 ± 1.03 6.1 | 2.03 ± 0.23 11.2 | 2.30 ± 0.11 4.6 | 2.17 ± 0.34 15.6 | 2/3 |
| T. aestivum Parabola | 71.9 | 8.8 | 17.2 | 2.10 | 41.7 | 50.0 | 79.3 | 15.29 | 1.52 | 2.12 | 2.35 | 1/1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Packa, D.; Załuski, D.; Graban, Ł.; Lajszner, W. An Evaluation of Spelt Crosses for Breeding New Varieties of Spring Spelt. Agronomy 2019, 9, 167. https://doi.org/10.3390/agronomy9040167
Packa D, Załuski D, Graban Ł, Lajszner W. An Evaluation of Spelt Crosses for Breeding New Varieties of Spring Spelt. Agronomy. 2019; 9(4):167. https://doi.org/10.3390/agronomy9040167
Chicago/Turabian StylePacka, Danuta, Dariusz Załuski, Łukasz Graban, and Waldemar Lajszner. 2019. "An Evaluation of Spelt Crosses for Breeding New Varieties of Spring Spelt" Agronomy 9, no. 4: 167. https://doi.org/10.3390/agronomy9040167
APA StylePacka, D., Załuski, D., Graban, Ł., & Lajszner, W. (2019). An Evaluation of Spelt Crosses for Breeding New Varieties of Spring Spelt. Agronomy, 9(4), 167. https://doi.org/10.3390/agronomy9040167





