Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sampling and Measurement Parameters
2.2.1. Photosynthesis and Chlorophyll Fluorescence
2.2.2. Growth and Yield Parameters
2.2.3. Nitrogen Use Efficiency (NUE)
2.2.4. Statistical Analysis
3. Results
3.1. Electrical Conductivity
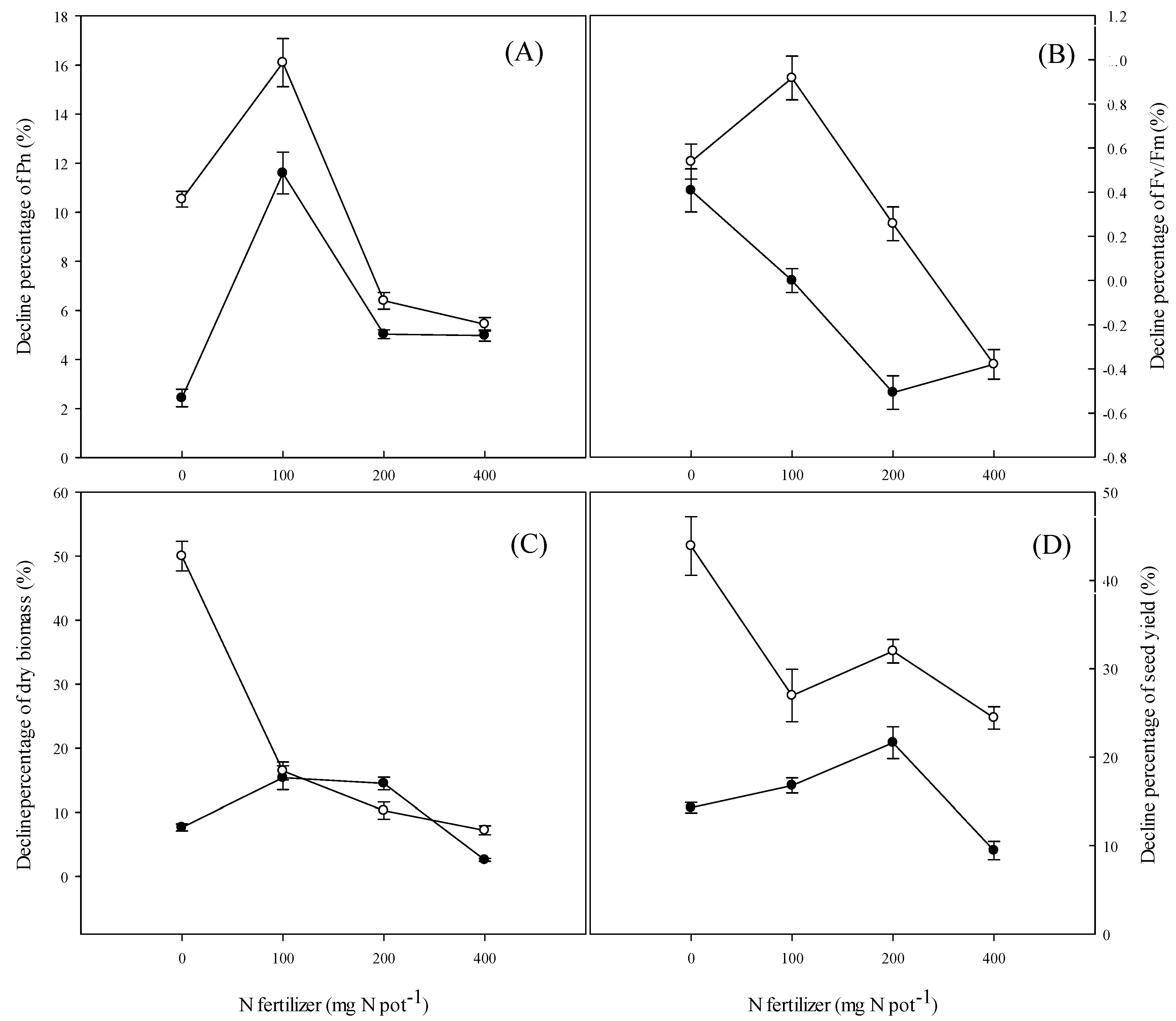
3.2. Gas Exchange
3.3. Chlorophyll Fluorescence
3.4. Nitrogen Use Efficiency
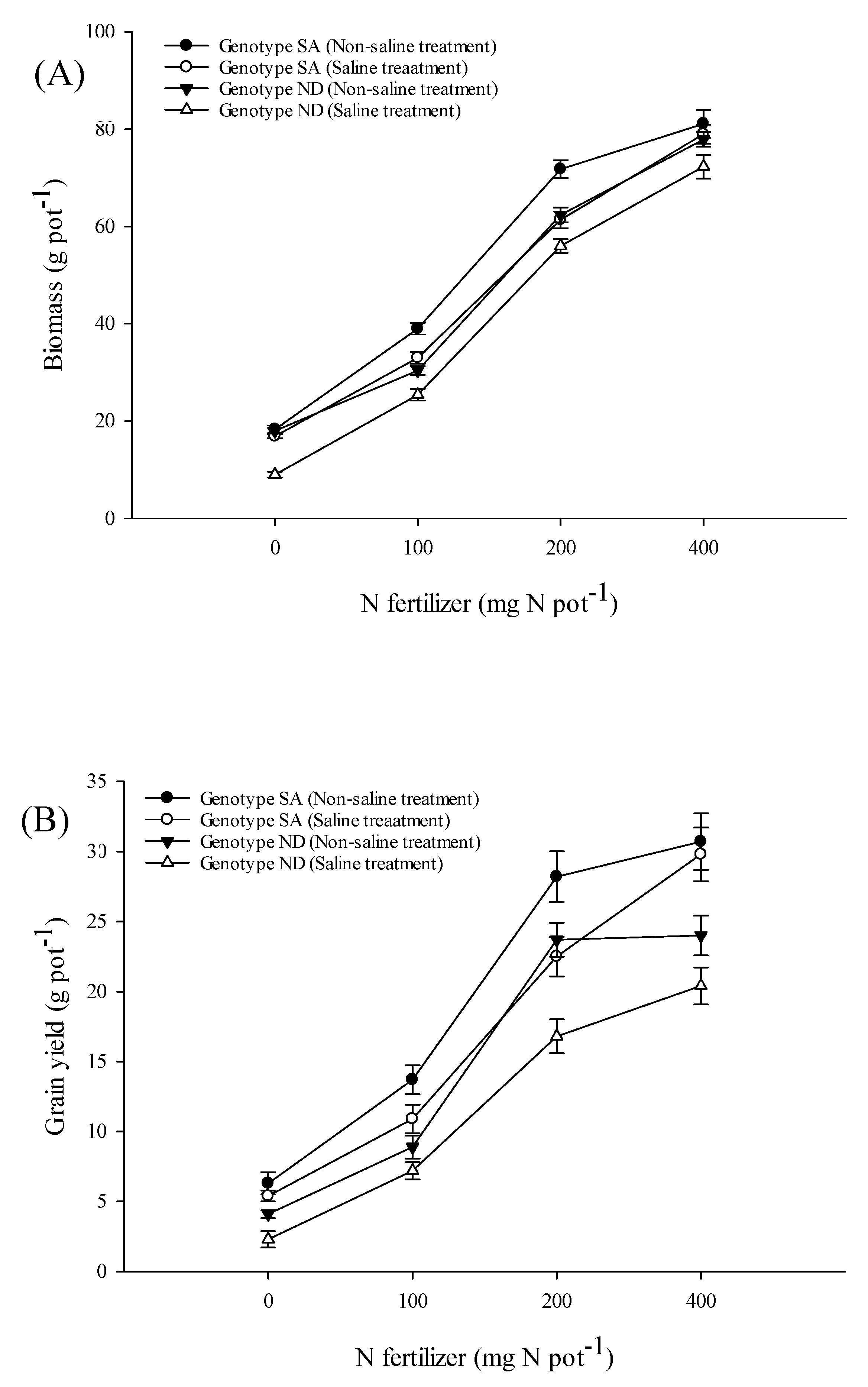
3.5. Growth and Yield Parameters
4. Discussion
4.1. Electrical Conductivity
4.2. Gas Exchange
4.3. Chlorophyll Fluorescence
4.4. Nitrogen Use Efficiency
4.5. Yield and Yield Components
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Statement
References
- Wang, W.X.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ma, B.L. Assessment of canola crop lodging under elevated temperatures for adaptation to climate change. Agric. For. Meteorol. 2018, 248, 329–338. [Google Scholar] [CrossRef]
- Pooja, S.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar]
- Rozema, J.; Flowers, T. Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Nimir, E.A.; Lu, S.Y.; Zhou, G.S.; Ma, B.L.; Wang, Y.H. Comparative effects of gibberellic acid, kinetin and salicylic acid on emergence, seedling growth and the antioxidant defense system of sweet sorghum (sorghum bicolor) under salinity and temperature stresses. Crop Pasture Sci. 2015, 66, 145–157. [Google Scholar] [CrossRef]
- Zhao, B.P.; Ma, B.L.; Hu, Y.G.; Liu, J.H. Leaf Photosynthesis, Biomass Production and Water and Nitrogen Use Efficiencies of Two Contrasting Naked Vs. Hulled Oat Genotypes Subjected to Water and Nitrogen Stresses. Plant Nutr. 2011, 34, 2139–2157. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Wakeel, A.; Kadambot, H.; Siddique, M. Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Ma, B.L.; Wu, T.Y.; Tremblay, N.; Deen, W.; McLaughlin, N.B.; Morrison, M.J.; Stewart, G. Rate and timing effects of fertilizer nitrogen application to corn on ammonia volatilization in cool and humid regions. Agron. J. 2010, 102, 134–144. [Google Scholar] [CrossRef]
- Chen, W.; Hou, Z.; Wu, L.; Liang, Y.; Wei, C. Effects of salinity and nitrogen on cotton growth in arid environment. Plant Soil 2010, 326, 61–73. [Google Scholar] [CrossRef]
- El-Sidding, K.; Ludders, P. Interactive effects of nitrogen nutrition and salinity on reproductive growth of apple trees. Gartenbauwiss 1994, 59, 127–131. [Google Scholar]
- Duan, M.; Chang, S.X. Nitrogen fertilization improves the growth of lodgepole pine and white spruce seedling under low salt stress through enhancing photosynthesis and plant nutrition. For. Ecol. Manag. 2017, 404, 197–204. [Google Scholar] [CrossRef]
- Akram, M.; Ashraf, M.Y.; Jamil, M.; Iqbal, R.M.; Nafees, M.; Khan, M.A. Nitrogen application improves gas exchange characteristics and chlorophyll fluorescence in maize hybrids under salinity conditions. Russ. J. Plant Physiol. 2011, 58, 394–401. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. Plant Physiol. 2000, 157, 54–58. [Google Scholar] [CrossRef]
- Elgharably, A.; Marschner, P.; Rengasamy, P. Wheat growth in a saline sandy loam soil as affected by N form and application rate. Plant Soil 2010, 328, 303–312. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Ma, B.L.; Ren, C.Z. Growth, gas exchange, chlorophyll fluorescence, and ion content of naked oat in response to salinity. Crop Sci. 2007, 47, 123–131. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Ma, B.L.; Ren, C.Z. Response of nitrogen uptake and partitioning to critical nitrogen supply in oat cultivars. Crop Sci. 2009, 49, 1040–1048. [Google Scholar] [CrossRef]
- Bai, J.; Yan, W.K.; Wang, Y.Q.; Yin, Q.; Liu, J.H.; Wight, C.; Ma, B.L. Screening Oat Genotypes for Tolerance to Salinity and Alkalinity. Front. Plant Sci. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.P.; Liu, J.H.; Wu, J.Y.; Liu, H.J.; Xu, H.L. Effects of salt stress on plasmalemma permeability, osmolyte accumulation and protective enzyme activities in oat plant. J. Food Agric. Environ. 2013, 11, 696–701. [Google Scholar]
- Shenker, M.; Ben-Gal, A.; Shani, U. Sweet corn response to combined nitrogen and salinity environmental stress. Plant Soil 2003, 256, 139–147. [Google Scholar] [CrossRef]
- Flores, P.; Carvajal, M.; Cerda, A.; Martinez, V. Salinity and ammonium/nitrate interactions on tomato plant development, nutrition, and metabolites. J. Plant Nutr. 2012, 4, 1561–1573. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D. Relationship between water use and urea application in salt-stressed pepper plants. J. Plant Nutr. 2003, 26, 19–30. [Google Scholar] [CrossRef]
- Albassam, B.A. Effect of nitrate nutrition on growth and nitrogen assimilation of pearl millet exposed to sodium chloride stress. J. Plant Nutr. 2001, 24, 1325–1335. [Google Scholar] [CrossRef]
- Villa-Castorena, M.; Ulery, A.L.; Catalan-Valencia, E.A.; Remmenga, M.D. Salinity and nitrogen rate effects on the growth and yield of chile pepper plants. Soil Sci. Soc. Am. J. 2003, 67, 1781–1789. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Zhu, X.K.; Zhou, G.S.; Adam, Y.A.; Ahmad, I.; Farah, G.A. Nitrogen fertilizer alleviated negative impacts of NaCl on some physiological parameters of wheat. Pak. J. Bot. 2018, 50, 2097–2104. [Google Scholar]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Delfine, S.; Alvino, A.; Villani, M.C.; Loreto, F. Restrictions to carbon dioxide conductance and photosynthesis in spinach recovering from salt stress. Plant Physiol. 1999, 119, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Fracheboud, Y.; Haldimann, P.; Stamp, P. Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J. Exp. Bot. 2010, 50, 1533–1540. [Google Scholar] [CrossRef]
- Pooja, M.; Jajoo, A.; Sudhakar, S.M. Chlorophyll fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Phy. Biochem. 2010, 48, 16–20. [Google Scholar]
- Ma, B.L.; Herath, A.W. Nitrogen fertilizer application timing and rates on canola seed quality and nitrogen use efficiency. Crop Pasture Sci. 2016, 67, 167–180. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.L.; Whalen, J.K. Enhancing rape seed tolerance to heat and drought stresses in a changing climate: perspectives for stress adaptation from root system architecture. Adv. Agron. 2018, 151, 87–159. [Google Scholar]
- Hou, S.; Hou, Z.A.; Ye, J. Cotton growth and nitrogen uptake in response to rates of water and nitrogen under drip irrigation with saline water. Xinjiang Agric. Sci. 2010, 47, 1882–1887. [Google Scholar]
- Cesar, M.C.; Sadras, V.O. Water–Nitrogen Colimitation in Grain Crop. Adv. Agron. 2018, 5, 231–247. [Google Scholar]
- Zhang, D.M.; Li, W.J.; Xin, C.S.; Tang, W.; Enejia, A.E.; Dong, H.Z. Lint yield and nitrogen use efficiency of field-grown cotton vary with soil salinity and nitrogen application rate. Field Crop Res. 2012, 138, 63–70. [Google Scholar] [CrossRef]

| Source of Variation | Pn (µmol m−2 s−1) | gs (mmol m−2 s−1) | E (mmol m−2 s−1) |
|---|---|---|---|
| Genotype (G) | ** | ** | ** |
| Salinity (S) | ** | ** | ** |
| Nitrogen (N) | ** | ** | ** |
| (G × S) | ns | * | ** |
| (G × N) | ns | ns | ** |
| (S × N) | ** | * | ns |
| (G × S × N) | ns | * | ns |
| Source of Variation | Pn (µmol m−2 s−1) | gs (mmol m−2 s−1) | E (mmol m−2 s−1) |
|---|---|---|---|
| Genotype (G) | ** | ns | * |
| Salinity (S) | ** | ** | ** |
| Nitrogen (N) | ** | ** | ** |
| (G × S) | ns | ns | ns |
| (G × N) | ** | ** | ** |
| (S × N) | ** | ns | * |
| (G × S × N) | ns | ns | ns |
| NaCl (mM) | N Rates | Pn (µmol m−2 s−1) | gs (mmol m−2 s−1) | E (mmol m−2 s−1) | |||
|---|---|---|---|---|---|---|---|
| (mg pot−1) | SA | ND | SA | ND | SA | ND | |
| 0 | 0 | 10.9 ± 0.6 c | 8.9 ± 0.4 d | 0.09 ± 0.005 b | 0.07 ± 0.005 d | 2.1 ± 0.3 d | 1.7 ± 0.1 d |
| 100 | 17.3 ± 1.2 b | 15.7 ± 0.6 c | 0.11 ± 0.007 b | 0.08 ± 0.007 c | 3.1 ± 0.1 c | 2.3 ± 0.2 c | |
| 200 | 20.5 ± 0.5 a | 18.4 ± 0.5 b | 0.13 ± 0.006 a | 0.10 ± 0.006 b | 3.4 ± 0.7 b | 3.0 ± 0.3 b | |
| 400 | 21.5 ± 1.0 a | 20.3 ± 0.7 a | 0.14 ± 0.005 a | 0.12 ± 0.001 a | 3.6 ± 0.2 a | 3.3 ± 0.2 a | |
| Mean | 17.6 A | 15.8 A | 0.12 A | 0.10 A | 3.1 A | 2.9 A | |
| 100 | 0 | 10.3 ± 0.4 d | 8.3 ± 0.4 d | 0.06 ± 0.005 c | 0.07 ± 0.010 c | 1.9 ± 0.1 c | 1.6 ± 0.2 c |
| 100 | 14.2 ± 0.6 c | 12.5 ± 0.1 c | 0.10 ± 0.002 b | 0.08 ± 0.005 b | 3.0 ± 0.2 b | 2.4 ± 0.2 b | |
| 200 | 17.9 ± 0.7 b | 14.8 ± 0.6 b | 0.12 ± 0.002 a | 0.09 ± 0.004 ab | 3.3 ± 0.1 a | 2.9 ± 0.1 a | |
| 400 | 20.4 ± 0.5 a | 17.5 ± 0.3 a | 0.12 ± 0.003 a | 0.10 ± 0.006 a | 3.5 ± 0.1 a | 3.0 ± 0.2 a | |
| Mean | 15.7 B | 13.3 B | 0.09 B | 0.09 B | 2.6 B | 2.5 B | |
| NaCl (mM) | N Rates | Pn (µmol m−2 s−1) | gs (mmol m−2 s−1) | E (mmol m−2 s−1) | |||
|---|---|---|---|---|---|---|---|
| (mg pot−1) | SA | ND | SA | ND | SA | ND | |
| 0 | 0 | 14.0 ± 0.7 d | 13.8 ± 0.1 d | 0.08 ± 0.02 b | 0.07 ± 0.01 c | 2.5 ± 0.2 c | 2.2 ± 0.1 c |
| 100 | 23.3 ± 1.3 c | 20.2 ± 0.2 c | 0.10 ± 0.01 ab | 0.11 ± 0.01 b | 3.4 ± 0.3 b | 3.2 ± 0.4 b | |
| 200 | 27.1 ± 0.4 b | 24.8 ± 0.5 b | 0.10 ± 0.01 ab | 0.12 ± 0.01 a | 3.5 ± 0.1 b | 3.6 ± 0.3 a | |
| 400 | 28.8 ± 0.6 a | 27.1 ± 0.1 a | 0.12 ± 0.01 a | 0.13 ± 0.02a | 3.8 ± 0.1a | 3.7 ± 0.2 a | |
| Mean | 23.3 A | 21.4 A | 0.10 A | 0.11 A | 3.3 A | 3.2 A | |
| 100 | 0 | 13.5 ± 0.7 d | 12.0 ± 0.5 d | 0.08 ± 0.01 b | 0.06 ± 0.01 d | 2.5 ± 0.2 b | 2.2 ± 0.1 c |
| 100 | 20.2 ± 0.2 c | 17.0 ± 0.4 c | 0.09 ± 0.01 b | 0.08 ± 0.01 c | 2.8 ± 0.1 b | 2.8 ± 0.3 b | |
| 200 | 24.8 ± 0.8 b | 23.2 ± 0.6 b | 0.11 ± 0.02 a | 0.10 ± 0.02 b | 3.4 ± 0.2 a | 3.5 ± 0.1 a | |
| 400 | 27.1 ± 0.7 a | 25.6 ± 0.4 a | 0.11 ± 0.01a | 0.12 ± 0.01 a | 3.5 ± 0.1 a | 3.4 ± 0.2 a | |
| Mean | 21.8 B | 19.4 B | 0.10 A | 0.09 B | 3.0 B | 3.0 B | |
| Source of Variation | Fv/Fm | ΦPSII | qP | NPQ | ETR |
|---|---|---|---|---|---|
| Genotype (G) | ** | * | * | ** | ns |
| Salinity (S) | ** | ** | ns | ** | ** |
| Nitrogen (N) | ** | ** | ** | ** | ** |
| (G × S) | ns | ns | ns | * | ** |
| (G × N) | ns | ns | ns | ns | ns |
| (S × N) | ns | ns | ns | ns | ns |
| (G × S × N) | ns | ns | ns | ns | ns |
| Source of Variation | Fv/Fm | ΦPSII | qP | NPQ | ETR |
|---|---|---|---|---|---|
| Genotype (G) | * | ns | * | ** | ns |
| Salinity (S) | ns | ** | ns | ** | ns |
| Nitrogen (N) | ** | ** | ** | ** | ** |
| (G × S) | ns | ** | ns | ns | ns |
| (G × N) | ** | ns | ns | ** | * |
| (S × N) | ns | * | ns | ** | ** |
| (G × S × N) | ns | ns | ns | ** | ** |
| NaCl (mM) | N Rate | Fv/Fm | ΦPSII | qP | NPQ | ETR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg pot−1) | SA | ND | SA | ND | SA | ND | SA | ND | SA | ND | |
| 0 | 0 | 0.72 ± 0.01 d | 0.71 ± 0.01 c | 0.31 ± 0.01 c | 0.30 ± 0.02 c | 0.61 ± 0.02 c | 0.61 ± 0.01 c | 1.30 ± 0.01 a | 1.43 ± 0.10 a | 25.6 ± 0.6 d | 24.8 ± 1.3 c |
| 100 | 0.74 ± 0.02 c | 0.73 ± 0.02 b | 0.33 ± 0.01 b | 0.36 ± 0.01 b | 0.65 ± 0.02 b | 0.63 ± 0.01 bc | 1.10 ± 0.10 b | 1.26 ± 0.10 b | 27.2 ± 0.6 c | 27.9 ± 1.3 b | |
| 200 | 0.76 ± 0.01 b | 0.75 ± 0.02 a | 0.43 ± 0.01 a | 0.42 ± 0.01 a | 0.70 ± 0.01 a | 0.67 ± 0.01 ab | 0.85 ± 0.01 c | 0.87 ± 0.10 c | 31.3 ± 0.6 b | 32.9 ± 0.8 a | |
| 400 | 0.77 ± 0.01 a | 0.76 ± 0.01 a | 0.44 ± 0.01 a | 0.43 ± 0.01 a | 0.67 ± 0.01 b | 0.68 ± 0.02 a | 0.76 ± 0.01 c | 0.75 ± 0.03 c | 32.7 ± 0.8 a | 33.4 ± 1.9 a | |
| Mean | 0.75 A | 0.74 A | 0.38 A | 0.37 A | 0.65 A | 0.65 A | 1.00 B | 1.08 B | 29.2 A | 29.7 A | |
| 100 | 0 | 0.72 ± 0.02 c | 0.71 ± 0.01 c | 0.30 ± 0.02 b | 0.30 ± 0.02 c | 0.60 ± 0.01 c | 0.59 ± 0.02 c | 1.46 ± 0.05 a | 1.66 ± 0.09 a | 23.8 ± 1.0 d | 22.7 ± 0.7 d |
| 100 | 0.74 ± 0.01 b | 0.72 ± 0.01 b | 0.32 ± 0.01 b | 0.34 ± 0.01 b | 0.64 ± 0.01 b | 0.62 ± 0.01 b | 1.24 ± 0.05 b | 1.37 ± 0.03 b | 26.3 ± 0.1 c | 24.8 ± 0.8 c | |
| 200 | 0.74 ± 0.01 b | 0.74 ± 0.02 a | 0.42 ± 0.01 a | 0.42 ± 0.01 a | 0.69 ± 0.02 a | 0.68 ± 0.01 a | 0.89 ± 0.02 c | 1.01 ± 0.07 c | 30.3 ± 0.6 b | 30.1 ± 1.4 b | |
| 400 | 0.76 ± 0.02 a | 0.75 ± 0.02 a | 0.43 ± 0.01 a | 0.43 ± 0.02 a | 0.69 ± 0.02 a | 0.68 ± 0.02 a | 0.76 ± 0.03 d | 0.98 ± 0.06 c | 32.2 ± 0.4 a | 32.0 ± 0.7 a | |
| Mean | 0.74 B | 0.73 B | 0.36 B | 0.36 B | 0.65 A | 0.64 A | 1.09 A | 1.26 A | 28.1 B | 27.4 B | |
| NaCl (mM) | N Rate | Fv/Fm | ΦPSII | qP | NPQ | ETR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg pot−1) | SA | ND | SA | ND | SA | ND | SA | ND | SA | ND | |
| 0 | 0 | 0.74 ± 0.01 c | 0.74 ± 0.01 d | 0.31 ± 0.02 c | 0.32 ± 0.03 c | 0.63 ± 0.05 b | 0.64 ± 0.02 b | 1.15 ± 0.04 a | 1.35 ± 0.02 a | 25.8 ± 0.7 d | 26.5 ± 0.9 c |
| 100 | 0.77 ± 0.02 b | 0.76 ± 0.04 c | 0.35 ± 0.02 b | 0.38 ± 0.02 b | 0.65 ± 0.06 b | 0.67 ± 0.02 b | 0.98 ± 0.05 b | 1.22 ± 0.04 b | 30.2 ± 0.7 c | 30.9 ± 2.0 b | |
| 200 | 0.79 ± 0.01 a | 0.78 ± 0.03 b | 0.45 ± 0.02 a | 0.45 ± 0.02 a | 0.68 ± 0.03 a | 0.74 ± 0.02 a | 0.63 ± 0.06 c | 0.81 ± 0.05 c | 33.9 ± 1.4 b | 38.1 ± 1.0 a | |
| 400 | 0.79 ± 0.03 a | 0.79 ± 0.02 a | 0.47 ± 0.01 a | 0.46 ± 0.01 a | 0.70 ± 0.05 a | 0.64 ± 0.02 b | 0.51 ± 0.04 d | 0.81 ± 0.03 c | 36.7 ± 1.3 a | 31.1 ± 2.1 b | |
| Mean | 0.77 A | 0.77 A | 0.39 A | 0.41 A | 0.66 A | 0.67 A | 0.82 B | 1.05 B | 31.7 A | 31.7 A | |
| 100 | 0 | 0.73 ± 0.01 c | 0.74 ± 0.04 d | 0.31 ± 0.01 c | 0.27 ± 0.03 c | 0.62 ± 0.03 b | 0.60 ± 0.04 c | 1.33 ± 0.04 a | 1.68 ± 0.05 a | 24.4 ± 1.3 d | 22.5 ± 1.7 b |
| 100 | 0.77 ± 0.02 b | 0.76 ± 0.03 c | 0.36 ± 0.03 b | 0.36 ± 0.05 b | 0.64 ± 0.03 b | 0.67 ± 0.01 bc | 1.13 ± 0.09 c | 1.41 ± 0.01 b | 29.1 ± 0.7 c | 27.2 ± 0.8 b | |
| 200 | 0.79 ± 0.01 a | 0.78 ± 0.09 b | 0.40 ± 0.05 b | 0.39 ± 0.07 b | 0.70 ± 0.05 a | 0.69 ± 0.03 ab | 0.85 ± 0.03 d | 0.85 ± 0.05 c | 34.2 ± 0.8 b | 34.5 ± 3.3 a | |
| 400 | 0.79 ± 0.01 a | 0.79 ± 0.07 a | 0.45 ± 0.07 a | 0.44 ± 0.04 a | 0.65 ± 0.01 ab | 0.76 ± 0.05 a | 0.52 ± 0.05 e | 0.83 ± 0.04 c | 36.1 ± 1.0 a | 38.0 ± 0.9 a | |
| Mean | 0.77 A | 0.77 A | 0.38 A | 0.36 B | 0.65 A | 0.68 A | 0.96 A | 1.19 A | 31.0 A | 30.6 A | |
| Source of Variation | aNUE (%) | pNUE (%) | ANR (%) |
|---|---|---|---|
| Genotype (G) | ** | ns | ** |
| Salinity (S) | ** | ** | ** |
| Nitrogen (N) | ** | ** | ** |
| (G × S) | ns | ns | ns |
| (G × N) | ns | ** | ** |
| (S × N) | ** | * | ** |
| (G × S × N) | ns | ns | ns |
| NaCl (mM) | N Rate | aNUE (%) | pNUE (%) | ANR (%) | |||
|---|---|---|---|---|---|---|---|
| (mg pot−1) | SA | ND | SA | ND | SA | ND | |
| 0 | 100 | 36.0 ± 1.7 b | 24.4 ± 2.0 b | 66.1 ± 1.4 a | 90.4 ± 4.3 a | 55.5 ± 3.3 b | 39.4 ± 1.7 b |
| 200 | 43.8 ± 1.9 a | 39.2 ± 2.2 a | 59.7 ± 2.6 a | 60.1 ± 3.9 b | 72.8 ± 1.3 a | 60.4 ± 2.7 a | |
| 400 | 24.4 ± 0.4 c | 19.9 ± 1.5 b | 58.4 ± 2.1 a | 46.9 ± 2.1 c | 41.8 ± 2.7 c | 41.1 ± 2.0 b | |
| Mean | 34.7 A | 27.8 A | 61.4 A | 65.8 A | 56.7 A | 47.0 A | |
| 100 | 100 | 27.8 ± 2.0 ab | 23.9 ± 2.3 b | 60.3 ± 1.9 a | 61.9 ± 3.3 a | 47.4 ± 3.3 b | 26.4 ± 1.9 c |
| 200 | 34.1 ± 1.6 a | 28.9 ± 2.2 a | 59.2 ± 3.2 a | 54.1 ± 2.8 ab | 57.5 ± 2.1 a | 53.6 ± 2.9 a | |
| 400 | 24.3 ± 1.0 b | 17.9 ± 1.5 c | 54.6 ± 4.4 a | 46.7 ± 2.0 b | 44.9 ± 2.1 b | 37.4 ± 2.5 b | |
| Mean | 28.7 B | 23.6 B | 58.0 A | 54.3 B | 49.9 B | 39.1 B | |
| Source of Variation | Plant Height | Panicle Number | Tiller Number | Seed Number | Dry Biomass | Grain Yield |
|---|---|---|---|---|---|---|
| Genotype (G) | ** | ** | ** | ** | ** | ** |
| Salinity (S) | ** | ** | ** | ** | ** | ** |
| Nitrogen (N) | ** | ** | ** | ** | ** | ** |
| (G × S) | ** | ns | ns | ns | ns | ns |
| (G × N) | ** | ns | ns | ** | ns | ** |
| (S × N) | ** | ** | ns | ** | ns | ** |
| (G × S × N) | ** | ** | ns | ns | ns | ns |
| NaCl (mM) | N Rate | Plant Height (cm) | Panicle Length (cm) | Tiller Number (pot−1) | Seed number (pot−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| (mg pot−1) | SA | ND | SA | ND | SA | ND | SA | ND | |
| 0 | 0 | 93.2 ± 2.1 c | 117.8 ± 2.5 c | 16.0 ± 0.8 b | 15.2 ± 0.4 c | 5.0 ± 1.0 c | 4.0 ± 0.0 c | 184.7 ± 12 d | 128.7 ± 8.0 d |
| 100 | 100.7 ± 2.1 b | 126.5 ± 2.0 b | 18.6 ± 0.6 a | 16.2 ± 0.2 b | 7.3 ± 0.6 b | 7.0 ± 1.0 b | 347.3 ± 17 c | 282.0 ± 12.7 c | |
| 200 | 111.2 ± 1.2 a | 132.8 ± 1.0 a | 19.4 ± 0.1 a | 18.6 ± 0.3 a | 14.0 ± 1.0 a | 12.7 ± 1.2 a | 702.0 ± 9.1 b | 669.7 ± 18.1 b | |
| 400 | 108.3 ± 3.9 a | 128.2 ± 1.9 b | 19.4 ± 0.4 a | 18.3 ± 0.3 a | 15.0 ± 1.0 a | 14.0 ± 1.0 a | 883.0 ± 18.3 a | 905.7 ± 3.8 a | |
| Mean | 103.3 A | 126.3 A | 18.4 A | 17.1 A | 10.3 A | 9.4 A | 529.3 A | 496.5 A | |
| 100 | 0 | 92.5 ± 1.5 c | 89.8 ± 3.9 c | 15.8 ± 0.3 b | 13.9 ± 0.6 d | 4.0 ± 0.0 d | 4.0 ± 0.0 d | 172.7 ± 10.9 d | 73.0 ± 5.9 d |
| 100 | 98.5 ± 1.3 b | 114.8 ± 3.1 b | 16.7 ± 0.9 b | 16.1 ± 0.8 c | 6.7 ± 1.2 c | 6.7 ± 0.6 c | 269.0 ± 14.0 c | 196.7 ± 12.4 c | |
| 200 | 107.3 ± 2.6 a | 122.7 ± 1.6 a | 18.8 ± 0.3 a | 17.8 ± 0.3 b | 12.3 ± 0.6 b | 11.7 ± 0.6 b | 575.0 ± 14.5 b | 537.7 ± 15.5 b | |
| 400 | 107.2 ± 1.8 a | 122.3 ± 0.7 a | 19.6 ± 0.3 a | 18.7 ± 0.2 a | 14.3 ± 0.6 a | 13.3 ± 0.6 a | 787.7 ± 11.8 a | 820.7 ± 18.9 a | |
| Mean | 101.4 B | 112.4 B | 17.72 B | 16.64 B | 9.45 A | 9.0 A | 451.1 B | 407.2 B | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhou, G.; Ma, B.-L.; Wu, W.; Ahmad, I.; Zhu, G.; Yan, W.; Jiao, X. Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions. Agronomy 2019, 9, 115. https://doi.org/10.3390/agronomy9030115
Song X, Zhou G, Ma B-L, Wu W, Ahmad I, Zhu G, Yan W, Jiao X. Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions. Agronomy. 2019; 9(3):115. https://doi.org/10.3390/agronomy9030115
Chicago/Turabian StyleSong, Xudong, Guisheng Zhou, Bao-Luo Ma, Wei Wu, Irshad Ahmad, Guanglong Zhu, Weikai Yan, and Xiurong Jiao. 2019. "Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions" Agronomy 9, no. 3: 115. https://doi.org/10.3390/agronomy9030115
APA StyleSong, X., Zhou, G., Ma, B.-L., Wu, W., Ahmad, I., Zhu, G., Yan, W., & Jiao, X. (2019). Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions. Agronomy, 9(3), 115. https://doi.org/10.3390/agronomy9030115







