An Integrated Yield Prediction Model for Greenhouse Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Integrated Model
- (1)
- Dividing crop organs into different age classes.
- (2)
- Calculating the amount of dry matter provided by the environment: SUPPLY.
- (3)
- Calculating the amount of dry matter required by crops: DEMAND.
- (4)
- Comparing SUPPLY and DEMAND to screen out two situations: oversupply and undersupply.
- (5)
- Obtaining the final change rate of net dry matter, according to different supply and demand situations.
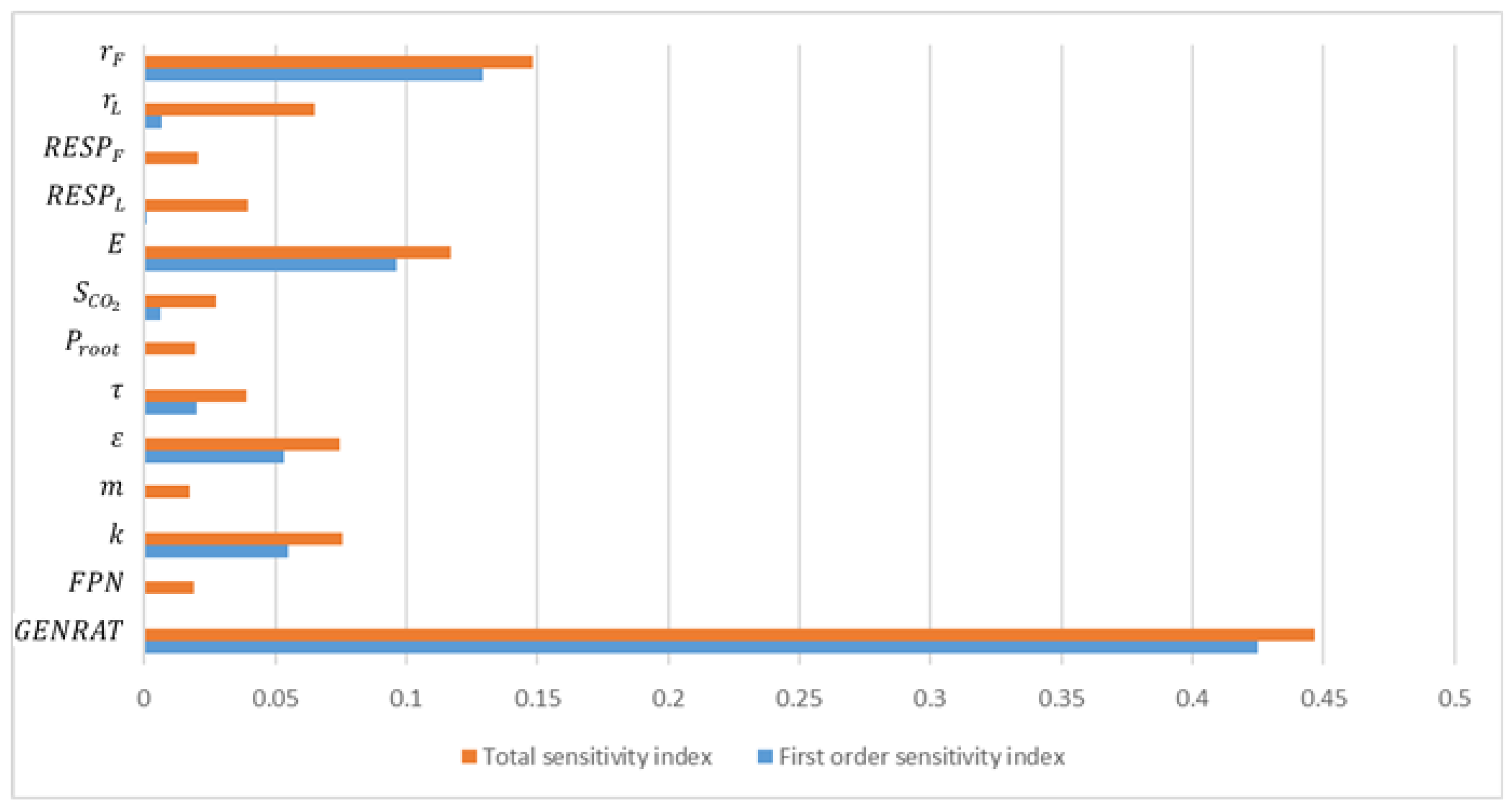
2.2. Sensitivity Analysis
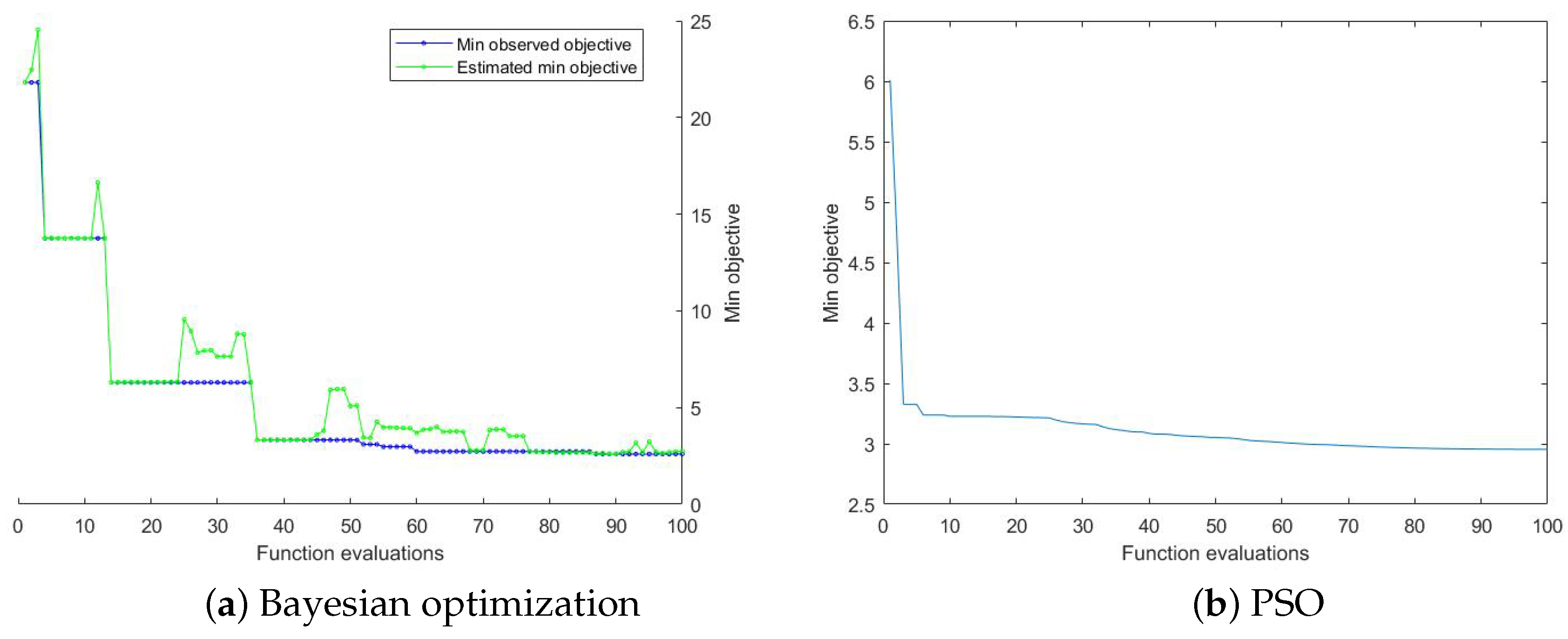
2.3. Optimization Algorithm
| Algorithm 1: Outline. Steps of Bayesian optimization algorithm. |
| Input: Microclimate data (T, , ), mature fruit yield (), and the range of variables. Output: The best vector (solution). Step 1: Randomly sample n points in the sample space, and calculate the posterior probability distribution of the first n points by Gaussian process regression to obtain the expected mean and variance of each hyperparameter at each value point. Step 2: Get the next sample point according to the acquisition function (Equation (32)), and calculate the objective function value of the sample point (Equation (32)). Step 3: Determine whether the accuracy requirement or the number of iterations is reached. If the condition is not met, add the sample points to the sample point set, repeat the above steps; and if the conditions are met, stop the iteration. |
3. Results and Discussion
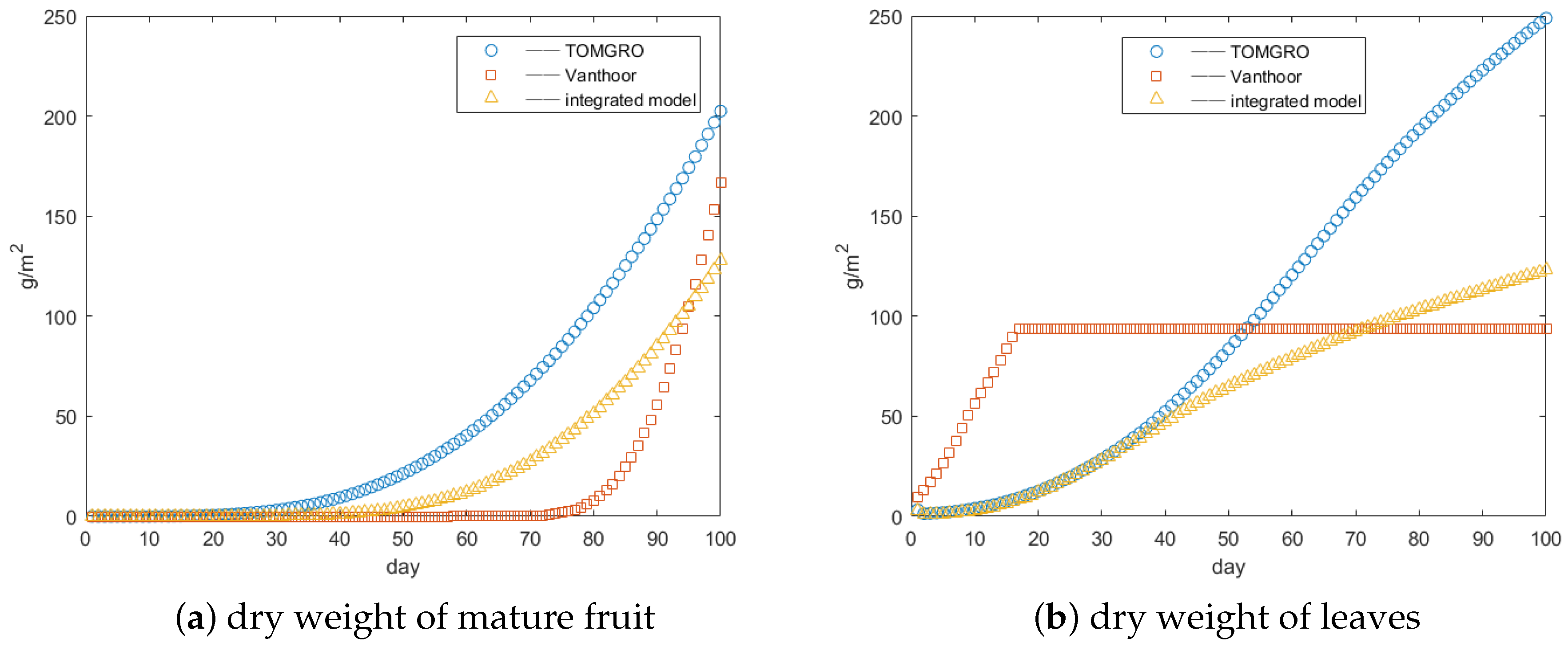
3.1. Integrated Model Validation
3.2. Parameter Analysis Results
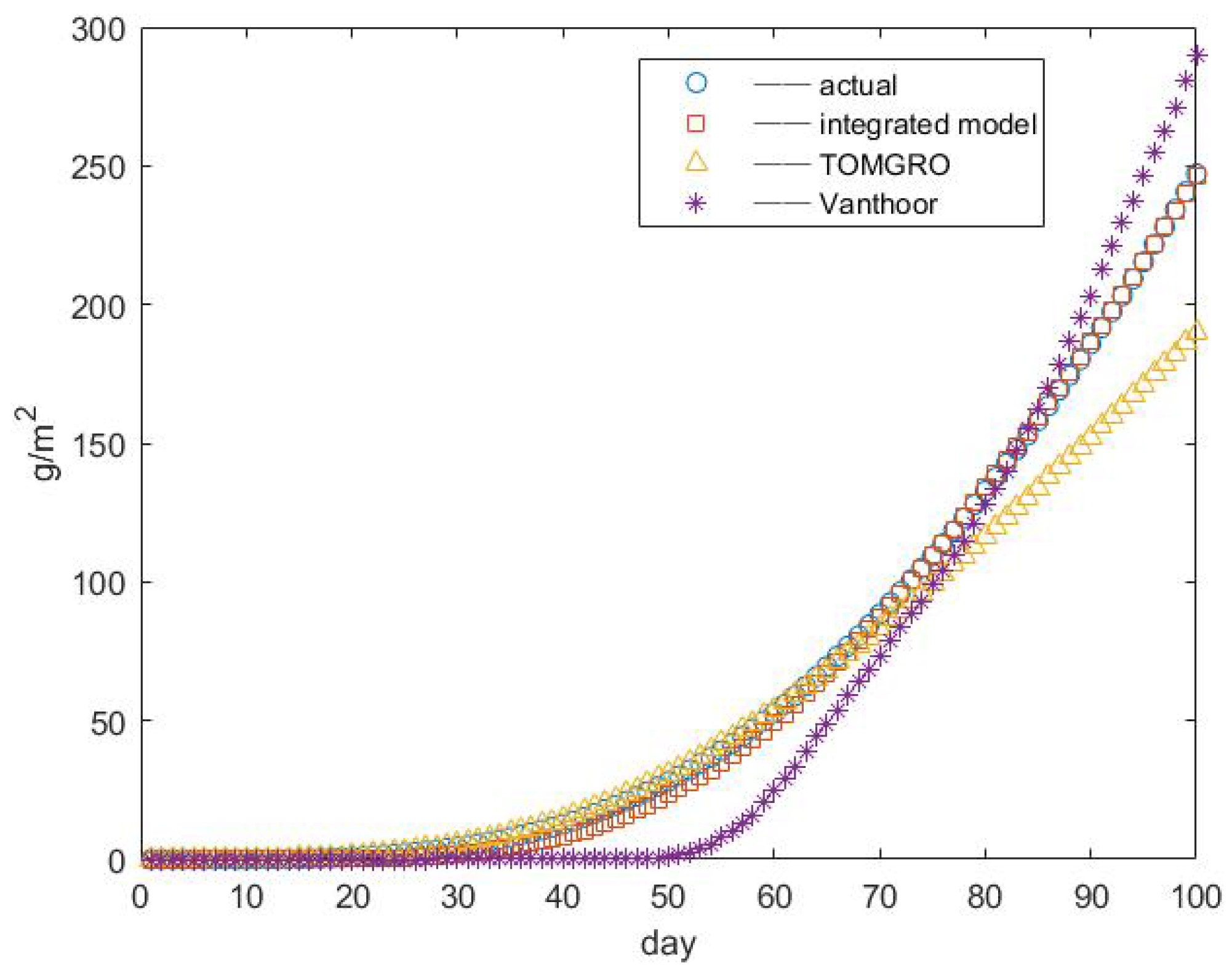
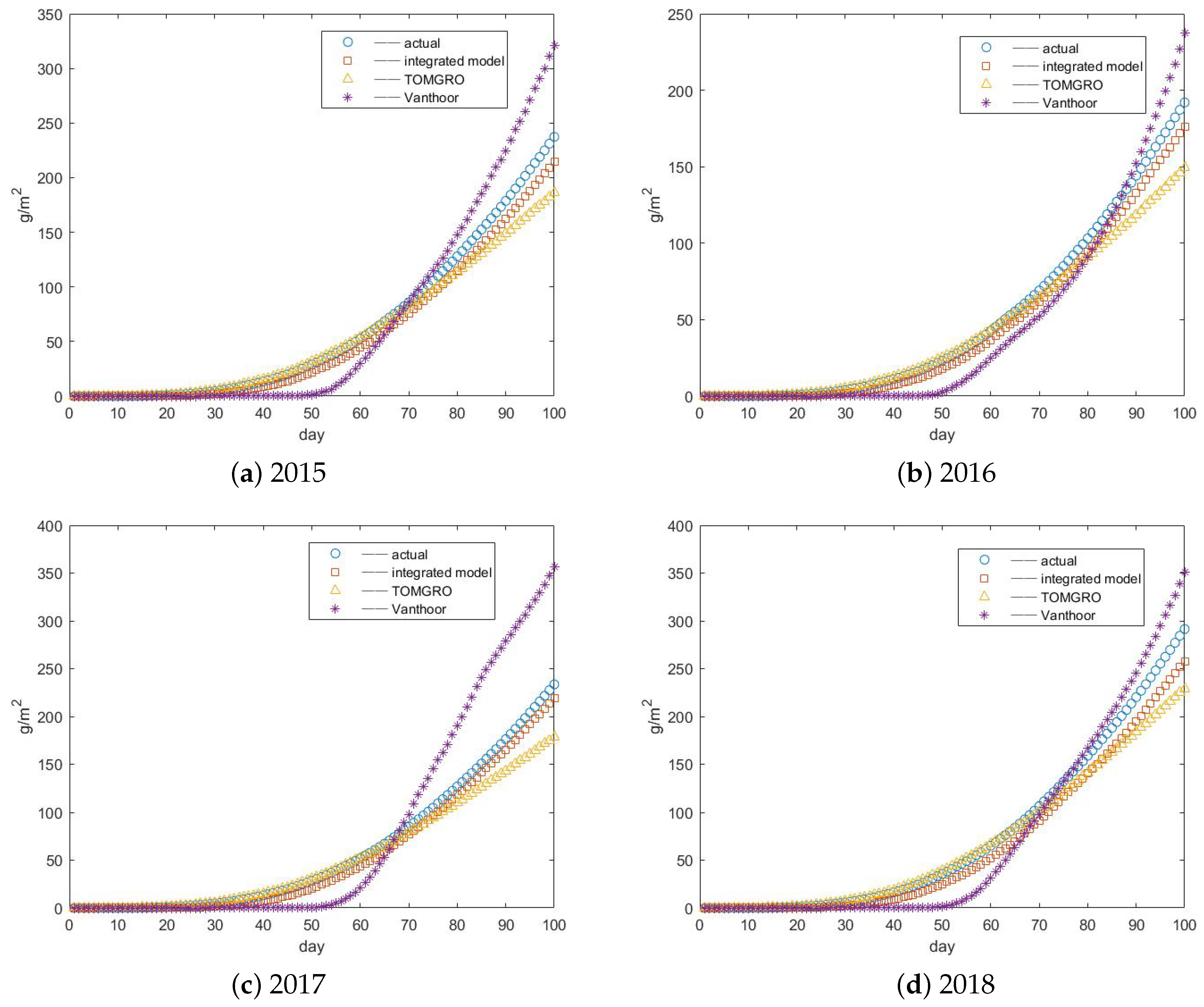
3.3. Results of Yield Prediction
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Access | Symbol | Physical Meaning | Unit | Remarks |
|---|---|---|---|---|
| Sensor | Indoor photosynthetically active radiation | - | ||
| Indoor concentration | - | |||
| T | Indoor temperature | - | ||
| variables | Fruit initiated per new node | - | - | |
| K | Light extinction coefficient | - | The values of the parameters are related to geography and greenhouse structure | |
| m | Leaf light transmission coefficient | - | ||
| Leaf quantum efficiency | ||||
| Carbon dioxide use efficiency | ||||
| Empirical formula | Temperature inhibition function | - | ||
| Potential growth rate of leaves | ||||
| Potential growth rate of fruit | ||||
| Leaves development rate | ||||
| Fruit development rate | ||||
| Maximum rate of node initiation | ||||
| Leaves mortality | 0 | |||
| Fruit mortality | ||||
| Fixed | D | Conversion efficiency | 2.593 | |
| E | Unit conversion factor | 0.75 | ||
| Sensitivity to temperature | - | 1.4 | ||
| Maximum SLA | 0.024 | |||
| Minimum SLA | 0.075 | |||
| Impact factor of concentration on SLW | 0.00085 | |||
| Impact factor of temperature on SLW | 0.085 | |||
| Number of stem segments when the first truss is formed | 12 | |||
| The number of new stem segments during the new first truss to the new first flower | 6 | |||
| Ratio of new trusses to new leaves | 0.33 | |||
| Ratio of petiole weight to blade weight | - | 0.49 | ||
| Ratio of stem segment to leaf growth rates | - | 0.33 | ||
| Relative respiration requirement for leaf | d | 0.015 | ||
| Relative respiration requirement for fruit | d | 0.01 | ||
| Artificial | Planting density | According to the actual situation of the greenhouse | ||
| Initial leaf area index | ||||
| Maximum leaf area index |
| Symbols/Acronyms | Meaning | Units |
|---|---|---|
| number of stems | ||
| number of leaves | ||
| number of fruits | ||
| appearance rate of new stems | ||
| appearance rate of new leaves | ||
| appearance rate of new fruit | ||
| number of organ age classes | - | |
| ratio of supply and demand | - | |
| A | photosynthesis rate | |
| respiration rate | ||
| maximum photosynthesis rate | ||
| leaf area index | ||
| dry matter of stems | ||
| dry matter of leaves | ||
| dry matter of fruit | ||
| dry matter demand of stems | ||
| dry matter demand of leaves | ||
| dry matter demand of fruit | ||
| leaf area change rate | ||
| actual dry matter growth rate of fruit | ||
| actual dry matter growth rate of stems | ||
| actual dry matter growth rate of leaves | ||
| extended Fourier amplitude sensitivity test | - | |
| Particle Swarm Optimization | - |
References
- Heuvelink, E.; Marcelis, L.F.M. Model application in horticultural practice: Summary of discussion groups. Acta Hortic. 1998, 456, 142–149. [Google Scholar] [CrossRef]
- Lentz, W. Model applications in horticulture: A review. Sci. Hortic. (Amst.) 1998, 74, 151–174. [Google Scholar] [CrossRef]
- Gary, C.; Jones, J.W.; Tchamitchian, M. Crop modelling in horticulture: State of the art. Sci. Hortic. 1998, 74, 3–20. [Google Scholar] [CrossRef]
- Jones, J.W.; Dayan, E.; Allen, L.H.; VanKeulen, H.; Challa, H. A dynamic tomato growth and yield model (TOMGRO). Trans. ASAE 1998, 34, 663–672. [Google Scholar] [CrossRef]
- Heuvelink, E. Evaluation of a dynamic simulation model for tomato crop growth and development. Ann. Bot. (Lond.) 1999, 83, 413–422. [Google Scholar] [CrossRef]
- Cohen, S.; Gijzen, H. The implementation of software engineering concepts in the greenhouse crop model hortisim1. Sci. Hortic. 1998, 456, 431–440. [Google Scholar] [CrossRef]
- Van Keulen, H.; Seligman, N.G.; Benjamin, R.W. Simulation of water use and herbage growth in arid regions—A re-evaluation and further development of the model ‘arid crop’. Agric. Syst. 1981, 6, 159–193. [Google Scholar] [CrossRef]
- Keulen, H.V.; Wolf, J.; Fresco, L.O.; Stroosnijder, L.; Bouma, J. Modelling of agricultural production: weather, soils and crops. Agric. Ecosyst. Environ. 1986, 30, 142–143. [Google Scholar]
- Ritchie, J.T.; Jerry, R. An expert system for a rangeland simulation model. Sci. Hortic. 1989, 46, 91–105. [Google Scholar] [CrossRef]
- Ritchie, J.T.; Nesmith, D.S. Temperature and crop development. Sci. Hortic. 1991, 74, 341–342. [Google Scholar]
- Ritchie, J.T.; Alocilja, E.C.; Singh, U.; Uehara, G. IBSNAT and the CERES-Rice model. Weather and Rice. In Proceedings of the International Workshop on the Impact of Weather Parameters on Growth and Yield of Rice, International Rice Research Institute, Manilla, Philippines, 7–10 April 1986; pp. 271–281. [Google Scholar]
- Ritchie, J.T. A User-Orientated Model of the Soil Water Balance in Wheat. In Wheat Growth and Modelling; Day, W., Atkin, R.K., Eds.; NATO ASI Science (Series A: Life Sciences); Springer: Boston, MA, USA, 1985; Volume 86. [Google Scholar]
- Bertin, N.; Gary, C. Tomato fruit-set: A case study for validation of the model tomgro. Acta Hortic. 1993, 328, 185–194. [Google Scholar] [CrossRef]
- Jones, J.W.; Kenig, A.; Vallejos, C.E. Reduced state–variable tomato growth model. Trans. ASAE 1999, 42, 255–265. [Google Scholar] [CrossRef]
- Dayan, E.; Keulen, H.V.; Jones, J.W.; Zipori, I.; Challa, H. Development, calibration and validation of a greenhouse tomato growth model: I. Description of the model. Agric. Syst. 1993, 43, 145–163. [Google Scholar] [CrossRef]
- Marcelis, L.F.M. Simulation of biomass allocation in greenhouse crops—A review. Acta Hortic. 1993, 328, 49–67. [Google Scholar] [CrossRef]
- Su, Y.; Xu, L. A greenhouse climate model for control design. In Proceedings of the 2015 IEEE 15th International Conference on Environment and Electrical Engineering (EEEIC), Rome, Italy, 10–13 June 2015. [Google Scholar]
- Vanthoor, B.H.E.; Stanghellini, C.; Henten, E.J.V.; Visser, P.H.B.D. A methodology for model-based greenhouse design: Part 1, a greenhouse climate model for a broad range of designs and climates. Biosyst. Eng. 2011, 110, 363–377. [Google Scholar] [CrossRef]
- Vanthoor, B.H.E.; Visser, P.H.B.D.; Stanghellini, C.; Henten, E.J.V. A methodology for model-based greenhouse design: Part 2, description and validation of a tomato yield model. Biosyst. Eng. 2011, 110, 378–395. [Google Scholar] [CrossRef]
- Vanthoor, B.H.E.; Henten, E.J.V.; Stanghellini, C.; Visser, P.H.B.D. A methodology for model-based greenhouse design: Part 3, sensitivity analysis of a combined greenhouse climate-crop yield model. Biosyst. Eng. 2011, 110, 396–412. [Google Scholar] [CrossRef]
- Cooman, A.; Schrevens, E. A monte carlo approach for estimating the uncertainty of predictions with the tomato plant growth model, tomgro. Biosyst. Eng. 2006, 94, 517–524. [Google Scholar] [CrossRef]
- Koning, A.N.M. Simulation of Biomass Allocation in Greenhouse Crops—A Review. Ph.D. Thesis, Wageningen Agriculture University, Wageningen, The Netherlands, 1993. [Google Scholar]
- Heuvelink, E.; Bertin, N. Dry-matter partitioning in a tomato crop: Comparison of two simulation models. J. Pomol. Hortic. Sci. 1994, 69, 19. [Google Scholar] [CrossRef]
- Lamboni, M.; Makowski, D.; Lehuger, S.; Gabrielle, B.; Monod, H. Multivariate global sensitivity analysis for dynamic crop models. Field Crops Res. 2009, 113, 312–320. [Google Scholar] [CrossRef]
- Confalonieri, R.; Bellocchi, G.; Tarantola, S.; Acutis, M.; Donatelli, M.; Genovese, G. Sensitivity analysis of the rice model warm in europe: Exploring the effects of different locations, climates and methods of analysis on model sensitivity to crop parameters. Environ. Model. Softw. 2010, 25, 479–488. [Google Scholar] [CrossRef]
- Lopez-Cruz, I.L.; Rojano-Aguilar, A.; Salazar-Moreno, R.; Ruiz-Garcia, A.; Goddard, J. A comparison of local and global sensitivity analyses for greenhouse crop models. Acta Hortic. 2012, 957, 267–273. [Google Scholar] [CrossRef]
- Vazquez-Cruz, M.A.; Guzman-Cruz, R.; Lopez-Cruz, I.L.; Cornejo-Perez, O.; Torres-Pacheco, I.; Guevara-Gonzalez, R.G. Global sensitivity analysis by means of efast and sobol’ methods and calibration of reduced state-variable tomgro model using genetic algorithms. Comput. Electron. Agric. 2014, 100, 1–12. [Google Scholar] [CrossRef]
- Morris, M.D. Factorial sampling plans for preliminary computational experiments. Biosyst. Eng. 1991, 33, 161–174. [Google Scholar] [CrossRef]
- Sobol, I.M. Estimation of the sensitivity of nonlinear mathematical models. J. Thorac. Cardiovasc. Surg. 2001, 122, 402–403. [Google Scholar]
- Cukier, R.I.; Levine, H.B.; Shuler, K.E. Nonlinear sensitivity analysis of multiparameter model systems. J. Comput. Phys. 1978, 26, 1–42. [Google Scholar] [CrossRef]
- Saltelli, A. Sensitivity analysis: Could better methods be used? J. Geophys. Res. Atmos. 1999, 104, 3789–3793. [Google Scholar] [CrossRef]
- Mulla, D.J. Mathematical models of small watershed hydrology and applications. J. Environ. Qual. 2003, 32, 374. [Google Scholar] [CrossRef]
- Marino, S.; Hogue, I.B.; Ray, C.J.; Kirschner, D.E. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 2008, 254, 178–196. [Google Scholar] [CrossRef]
- Mckay, M.D.; Beckman, R.J.; Conover, W.J. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 2000, 42, 55–61. [Google Scholar] [CrossRef]
- Singh, V.P. Computer models of watershed hydrology. Comput. Models Watershed Hydrol. 1997, 25, 443–476. [Google Scholar]
- Crosetto, M.; Tarantola, S.; Saltelli, A. Sensitivity and uncertainty analysis in spatial modelling based on gis. Agric. Ecosyst. Environ. 2000, 81, 71–79. [Google Scholar] [CrossRef]
- Xu, C.; Gertner, G. Extending a global sensitivity analysis technique to models with correlated parameters. Comput. Stat. Data Anal. 2007, 51, 5579–5590. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Lu, L.; Fang, F. Parameter sensitivity analysis of crop growth models based on the extended fourier amplitude sensitivity test method (ei). Environ. Model. Softw. 2013, 48, 171–182. [Google Scholar] [CrossRef]
- Jones, D.R.; Schonlau, M.; Welch, W.J. Efficient global optimization of expensive black-box functions. J. Glob. Optim. 1998, 13, 455–492. [Google Scholar] [CrossRef]
- Jing-Xiao, Z.; Wei, S.U. Sensitivity Analysis of Ceres-Wheat Model Parameters Based on Efast Method. J. China Agric. Univ. 2012, 17, 149–154. [Google Scholar]
| Parameters | Description | Ranges | Distribution |
|---|---|---|---|
| Maximum rate of node initiation | [0.45,0.55] | uniform | |
| Fruit initiated per new node | [0.45,0.55] | uniform | |
| K | Light extinction coefficient | [0.522,0.638] | uniform |
| m | Leaf light transmission coefficient | [0.09,0.11] | uniform |
| Leaf quantum efficiency | [0.058,0.071] | uniform | |
| Carbon dioxide use efficiency | [0.062,0.076] | uniform | |
| Supply of photosynthesis for root growth | [0.063,0.077] | uniform | |
| Effect of on new stems | [0.00027,0.00033] | uniform | |
| E | Conversion efficiency | [0.675,0.825] | uniform |
| Relative respiration requirement for leaf | [0.0135,0.0165] | uniform | |
| Relative respiration requirement for fruit | [0.009,0.011] | uniform | |
| Leaf development rate | [0.009,0.011] | uniform | |
| Fruit development rate | [0.018,0.022] | uniform |
| Parameters | First Order Sensitivity Index | Total Sensitivity Index |
|---|---|---|
| 0.4247 | 0.446388 | |
| 3.98 × 10 | 0.019022 | |
| K | 0.0549 | 0.075865 |
| m | 0.000366 | 0.017531 |
| 0.0536 | 0.074421 | |
| 0.0199 | 0.039272 | |
| 0.000436 | 0.019621 | |
| 0.0064 | 0.027562 | |
| E | 0.0966 | 0.116923 |
| 0.0011 | 0.039419 | |
| 0.000514 | 0.020610 | |
| 0.0067 | 0.065230 | |
| 0.1290 | 0.148063 |
| Types | Parameters | Approach |
|---|---|---|
| High sensitivity value, parameters to be optimized | , | need to be optimized |
| High sensitivity value, fixed parameters | E | fixed |
| Low sensitivity value, parameters to be optimized | ,K,m,,,,, | fixed |
| Low sensitivity, fixed parameters | , | ignored |
| Parameters | Default Recommended Value |
|---|---|
| 0.0009389 | |
| 0.000756 | |
| 0.779 | |
| −0.000458 | |
| 1.2653 | |
| 0.04295 | |
| 46.34 |
| Parameters | Parameter Range | Optimization Result |
|---|---|---|
| [0.00075112,0.00112668] | 0.00079752 | |
| [0.0006048,0.0009072] | 0.00086825 | |
| [0.6232,0.9348] | 0.63451 | |
| [−0.0005496,−0.0003664] | −0.00046959 | |
| [1.01224,1.51836] | 1.1745 | |
| [0.03436,0.05154] | 0.051295 | |
| [37.072,55.608] | 37.284 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Wei, R.; Xu, L. An Integrated Yield Prediction Model for Greenhouse Tomato. Agronomy 2019, 9, 873. https://doi.org/10.3390/agronomy9120873
Lin D, Wei R, Xu L. An Integrated Yield Prediction Model for Greenhouse Tomato. Agronomy. 2019; 9(12):873. https://doi.org/10.3390/agronomy9120873
Chicago/Turabian StyleLin, Dingyi, Ruihua Wei, and Lihong Xu. 2019. "An Integrated Yield Prediction Model for Greenhouse Tomato" Agronomy 9, no. 12: 873. https://doi.org/10.3390/agronomy9120873
APA StyleLin, D., Wei, R., & Xu, L. (2019). An Integrated Yield Prediction Model for Greenhouse Tomato. Agronomy, 9(12), 873. https://doi.org/10.3390/agronomy9120873





