Evaluation of Nematicidal Activity of Fluensulfone against Non-Target Free-Living Nematodes under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fields
2.2. Chemicals
2.3. Soils and Roots
2.4. Nematodes
2.5. Galls on Root Systems
2.6. Statistical Analysis
3. Results
3.1. Galling on Tomato and Sweet Potato Roots
3.2. Free-Living Nematode Assemblage
3.3. Diversity of Free-Living Nematodes
3.3.1. Shannon-Wiener Index (H’) and Simpson’s D
3.3.2. Species Richness and Evenness J’
3.3.3. Maturity Indices
3.3.4. Acrobeloides sp., and Fungivorous and Omnivorous Nematodes
3.3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Cook, R.; Yeates, G.W.; Denton, C.S. The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 1999, 212, 23–33. [Google Scholar] [CrossRef]
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.C. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- De Ruiter, P.C.; Moore, J.C.; Zwart, K.B.; Bouwman, L.A.; Hassink, J.; Bloem, J.; De Vos, J.A.; Marinissen, J.C.Y.; Didden, W.A.M.; Lebrink, G.; et al. Simulation of nitrogen mineralization in the below-ground food webs of two winter wheat fields. J. Appl. Ecol. 1993, 30, 95–106. [Google Scholar] [CrossRef]
- Yeates, G.W.; Wardle, D.A. Nematodes as predators and prey: Relationships to biological control and soil processes. Pedobiologia 1996, 40, 43–50. [Google Scholar]
- Yeates, G.W.; Bongers, T. Nematode diversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 113–135. [Google Scholar] [CrossRef]
- Mulder, C.; Zwart, D.; Van Wijnen, H.J.; Schouten, A.J.; Breure, A.M. Observational and simulated evidence of ecological shifts within the soil nematode community of agroecosystems under conventional and organic farming. Funct. Ecol. 2003, 17, 516–525. [Google Scholar] [CrossRef]
- Sasser, J.N.; Freckman, D.W. A world perspective on nematology: The role of the society. In Vistas on Nematology; Veech, J.A., Dickson, D.W., Eds.; Society of Nematologists: Hyattsville, MD, USA, 1987; pp. 7–14. [Google Scholar]
- Ciancio, A.; Colagiero, M.; Pentimone, I.; Rosso, L.C. Formulation of Pochonia chlamydosporia for plant and nematode management. In Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: Berlin, Germany, 2016; pp. 177–197. [Google Scholar] [CrossRef]
- Protocol, Montreal. Montreal Protocol on Substances that Deplete the Ozone Layer; US Government Printing Office: Washington, DC, USA, 1987; Volume 26, pp. 128–136. [Google Scholar]
- Chelinho, S.; Sautter, K.D.; Cachada, A.; Abrantes, I.; Brown, G.; Duarte, A.C.; Sousa, J.P. Carbofuran effects in soil nematode communities: Using trait and taxonomic based approaches. Ecotox. Environ. Safety 2011, 74, 2002–2012. [Google Scholar] [CrossRef]
- Wada, S.; Toyota, K.; Takada, A. Effects of the nematicide imicyafos on soil nematode community structure and damage to radish caused by Pratylenchus penetrans. J. Nematol. 2011, 43, 1–6. [Google Scholar]
- Smolik, J.D. Effect of nematicide treatments on nontarget nematode populations associated with corn. Plant Dis. 1983, 67, 28–31. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Chen, S. Influence of long-term corn-soybean crop sequences on soil ecology as indicated by the nematode community. Appl. Soil Ecol. 2016, 100, 172–185. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Vetsch, J.A.; Chen, S. Swine manure, nematicides, and long-term tillage change soil ecology in corn and soybean production. Agron. J. 2018, 110, 2288–2301. [Google Scholar] [CrossRef]
- Mueller, J.D.; Noling, J.W. Standardization of reporting procedures for nematicide efficacy testing: A research and extension perspective. Supp. J. Nematol. 1996, 28, 575–585. [Google Scholar]
- Cáceres, T.; Megharaj, M.; Naidu, R. Degradation of fenamiphos in soils collected from different geographical regions: The influence of soil properties and climatic conditions. J. Environ. Sci. Health B 2008, 43, 314–322. [Google Scholar]
- Pattison, A.B.; Stanton, J.M.; Cobon, J.A. Bioassay for enhanced biodegradation of nematicides in soil. Australas. Plant Path. 2000, 29, 52–58. [Google Scholar] [CrossRef]
- Read, D.C. Greatly accelerated microbial degradation of aldicarb in re-treated field soil, in flooded soil and in water. J. Econ. Entomol. 1987, 80, 156–163. [Google Scholar] [CrossRef]
- Karpouzas, D.G.; Karanasios, E.; Menkissoglu-Spiroudi, U. Enhanced microbial degradation of cadusafos in soils from potato monoculture: Demonstration and characterization. Chemosphere 2004, 56, 549–559. [Google Scholar] [CrossRef]
- Osborn, R.K.; Haydock, P.P.J.; Edwards, S.G. Isolation and identification of oxamyl-degrading bacteria from UK agricultural soils. Soil Biol. Biochem. 2010, 42, 998–1000. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Lagos, S.; Spentza, F.; Vidiadakis, E.; Karas, P.A.; Klitsinaris, T.; Karpouzas, D.G. The dissipation of fipronil, chlorpyrifos, fosthiazate and ethoprophos in soils from potato monoculture areas: First evidence for the enhanced biodegradation of fosthiazate. Pest Manag. Sci. 2015, 72, 1040–1050. [Google Scholar] [CrossRef]
- Noling, J.; Becker, J.O. The challenge of research and extension to define and implement alternatives to methyl bromide. J. Nematol. 1994, 26, 573–586. [Google Scholar]
- Kearn, J.; Ludlow, E.; Dillon, J.; O’Connor, V.; Holden-Dye, L. Fluensulfone is a nematicide with a mode of action distinct from anticholinesterases and macrocyclic lactones. Pestic. Biochem. Phys. 2014, 109, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Shuker, S.; Tkachi, N. Nematicidal efficacy of MCW-2, a new nematicide of the fluoroalkenyl group, against the root-knot nematode Meloidogyne javanica. Pest Manag. Sci. 2009, 65, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Shuker, S.; Tkachi, N. Systemic nematicidal activity of fluensulfone against the root-knot nematode Meloidogyne incognita on pepper. Pest Manag. Sci. 2011, 68, 268–275. [Google Scholar] [CrossRef]
- Oka, Y.; Shuker, S.; Tkachi, N. Influence of soil environments on nematicidal activity of fluensulfone against Meloidogyne javanica. Pest Manag. Sci. 2013, 69, 1225–1234. [Google Scholar] [CrossRef]
- Morris, K.A.; Langston, D.B.; Dickson, D.W.; Davis, R.F.; Timper, P.; Noe, J.P. Efficacy of fluensulfone in a tomato–cucumber double cropping system. J. Nematol. 2015, 47, 310–315. [Google Scholar]
- Castillo, G.X.; Ozores-Hampton, M.; Gine, P.A.N. Effects of fluensulfone combined with soil fumigation on root-knot nematodes and fruit yield of drip-irrigated fresh-market tomatoes. Crop Prot. 2017, 98, 166–171. [Google Scholar] [CrossRef]
- Jones, J.G.; Kleczewski, N.M.; Desaeger, J.; Meyer, S.L.F.; Johnson, G.C. Evaluation of nematicides for southern root-knot nematode management in lima bean. Crop Prot. 2017, 96, 151–157. [Google Scholar] [CrossRef]
- Oka, Y. Nematicidal activity of fluensulfone against some migratory nematodes under laboratory conditions. Pest Manag. Sci. 2014, 70, 1850–1858. [Google Scholar] [CrossRef]
- Norshie, P.M.; Grove, I.G.; Back, M.A. Field evaluation of the nematicide fluensulfone for control of the potato cyst nematode Globodera pallida. Pest Manag. Sci. 2016, 72, 2001–2007. [Google Scholar] [CrossRef]
- Grabau, Z.J.; Noling, J.W.; Gine, P.A.N. Fluensulfone and 1,3-dichloropropene for plant-parasitic nematode management in potato production. J. Nematol. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Cabrera-Hidalgo, A.; Valadez, M.; Marbán, M. Effect of fluensulfone on the mobility in vitro, and reproduction and root galling of Nacobbus aberrans in microplots. Nematropica 2015, 45, 59–71. [Google Scholar]
- Waldo, B.D.; Grabau, Z.J.; Mengistu, T.M.; Crow, W.T. Nematicide effects on non-target nematodes in bermudagrass. J. Nematol. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, C. Nematodes. In The Sweetpotato; Loebenstein, G., Thottappilly, G., Eds.; Springer: Berlin, Germany, 2009; pp. 135–159. [Google Scholar] [CrossRef]
- Seid, A.; Fininsa, C.; Mekete, T.; Decraemer, W.; Wesemael, W.M. Tomato (Solanum lycopersicum) and root-knot nematodes (Meloidogyne spp.)—A century-old battle. Nematology 2015, 17, 995–1009. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar]
- Neher, D.A.; Peck, S.L.; Rawlings, J.; Campbell, C.L. Measures of nematode community structure and sources of variability among and within agricultural fields. Plant Soil 1995, 170, 167–181. [Google Scholar] [CrossRef]
- Powers, T.O.; Harris, T.S. A polymerase chain reaction method for identification of five major Meloidogyne species. J. Nematol. 1993, 25, 1–6. [Google Scholar]
- Shishida, Y. Nematoda. In Pictorial Keys to Soil Animal of Japan; Aoki, J., Ed.; Tokai University Press: Tokyo, Japan, 1999; pp. 15–38. [Google Scholar]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Smith, A.L.; Taylor, A.L. Field methods of testing for root-knot infestation. Phytopathology 1947, 37, 85–93. [Google Scholar]
- Ito, K. Evaluation of nematode’s damage. In Nematology Experimental Methods; The Japanese Nematological Society: Tsukuba, Japan, 2004; pp. 103–105. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Korthals, G. The Maturity Index, an Instrument to Monitor Changes in the Nematode Community Structure. In Proceedings of the 45th International Symposium on Crop Protection Ghent, Belgium; Wageningen University & Research: Droevendaalsesteeg, The Netherlands, 1993; p. 80. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R.-project.org/ (accessed on 30 July 2019).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Magurran, A.E. Diversity indices and species abundance models. In Ecological Diversity and Its Measurement; Springer: Berlin, Germany, 1988; pp. 7–45. [Google Scholar] [CrossRef]
- Pullen, M.P.; Fortnum, B.A. Fosthiazate controls Meloidogyne arenaria and M. incognita in flue-cured tobacco. J. Nematol. 1999, 31, 694–699. [Google Scholar] [PubMed]
- Ingham, R.E.; Hamm, P.B.; Williams, R.E.; Swanson, W.H. Control of Meloidogyne chitwoodi in potato with fumigant and nonfumigant nematicides. J. Nematol. 2000, 32, 556–565. [Google Scholar] [PubMed]
- Radwan, M.A.; Farrag, S.A.A.; Abu-Elamayem, M.M.; Ahmed, N.S. Efficacy of some granular nematicides against root-knot nematode, Meloidogyne incognita associated with tomato. Pakistan J. Nematol. 2012, 30, 41–47. [Google Scholar]
- Wada, S.; Toyota, K. Effect of three organophosphorous nematicides on non-target nematodes and soil microbial community. Microbes Environ. 2008, 23, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A.V.; Kimpinski, J. Effects of fosthiazate and aldicarb on populations of plant-growth-promoting bacteria, root-lesion nematodes and bacteria-feeding nematodes in the root zone of potatoes. Plant Pathol. 1999, 48, 26–32. [Google Scholar] [CrossRef]
- Ettema, C.H.; Bongers, T. Characterization of nematode colonization and succession in disturbed soil using the maturity index. Biol. Fert. Soils 1993, 16, 79–85. [Google Scholar] [CrossRef]
- Yeates, G.W.; van der Meulen, H. Recolonization of methyl-bromide sterilized soils by plant and soil nematodes over 52 months. Biol. Fert. Soils 1996, 21, 1–6. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Jiménez, L.; Alonso-Prados, J.L.; García-Baudin, J.M. Nematodes as indicators of fumigant effects on soil food webs in strawberry crops in southern Spain. Ecol. Indic. 2010, 10, 148–156. [Google Scholar] [CrossRef]
- Timper, P.; Davis, R.; Jagdale, G.; Herbert, J. Resiliency of a nematode community and suppressive service to tillage and nematicide application. Appl. Soil Ecol. 2012, 59, 48–59. [Google Scholar] [CrossRef]
- Watson, T.T.; Desaeger, J.A. Evaluation of non-fumigant chemical and biological nematicides for strawberry production in Florida. Crop Prot. 2019, 117, 100–107. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Sørensen, S.J.; Bååth, E.; Bloem, J.; De Ruiter, P.C.; et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity-ecosystem function relationship. Oikos 2000, 90, 279–294. [Google Scholar] [CrossRef]
- Okada, H.; Harada, H.; Kadota, I. Application of diversity indices and ecological indices to evaluate nematode community changes after soil fumigation. Nematol. Res. 2004, 34, 89–98. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fert. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Stirling, G.; Wilsey, B. Empirical relationships between species richness, evenness, and proportional diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]

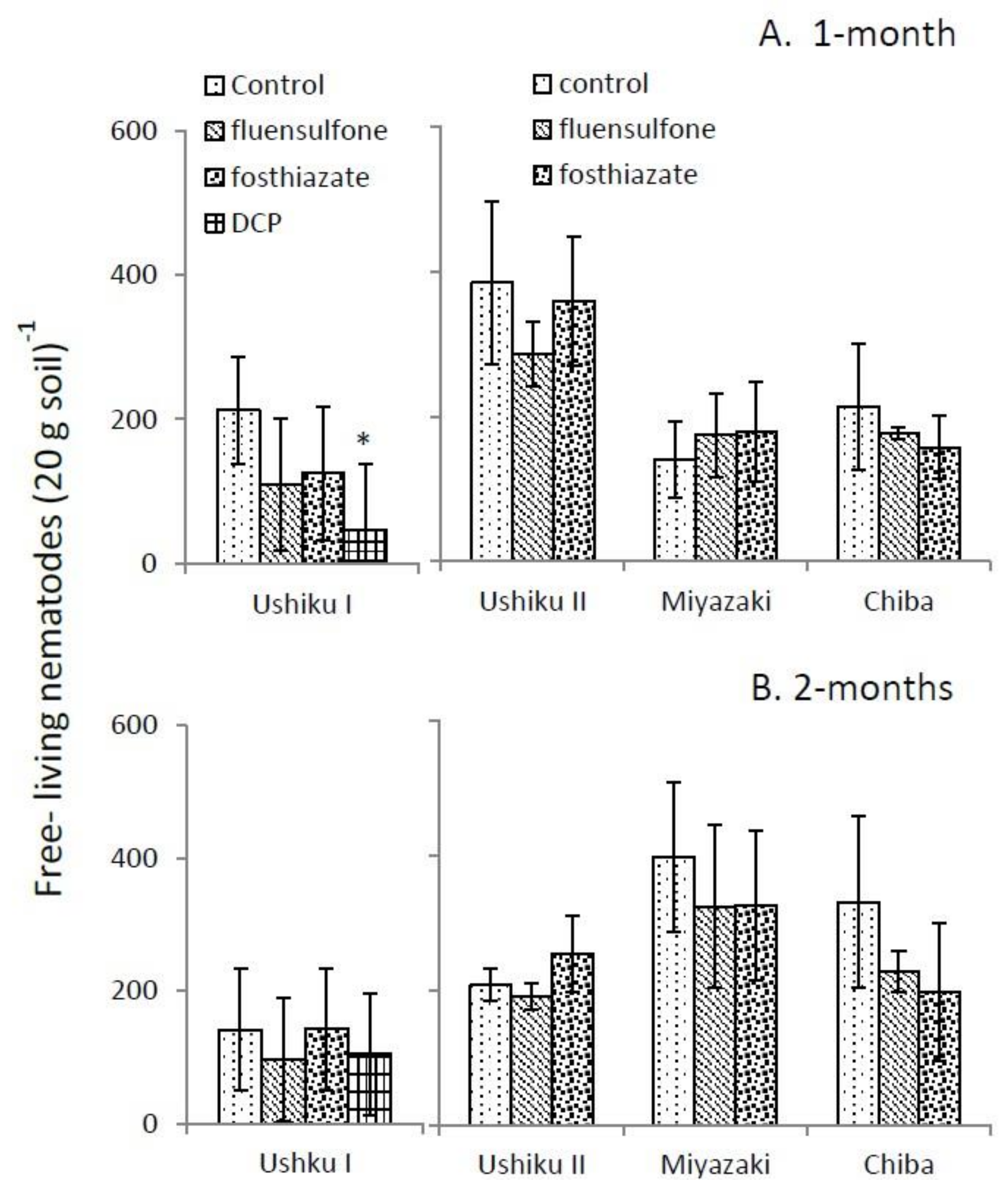
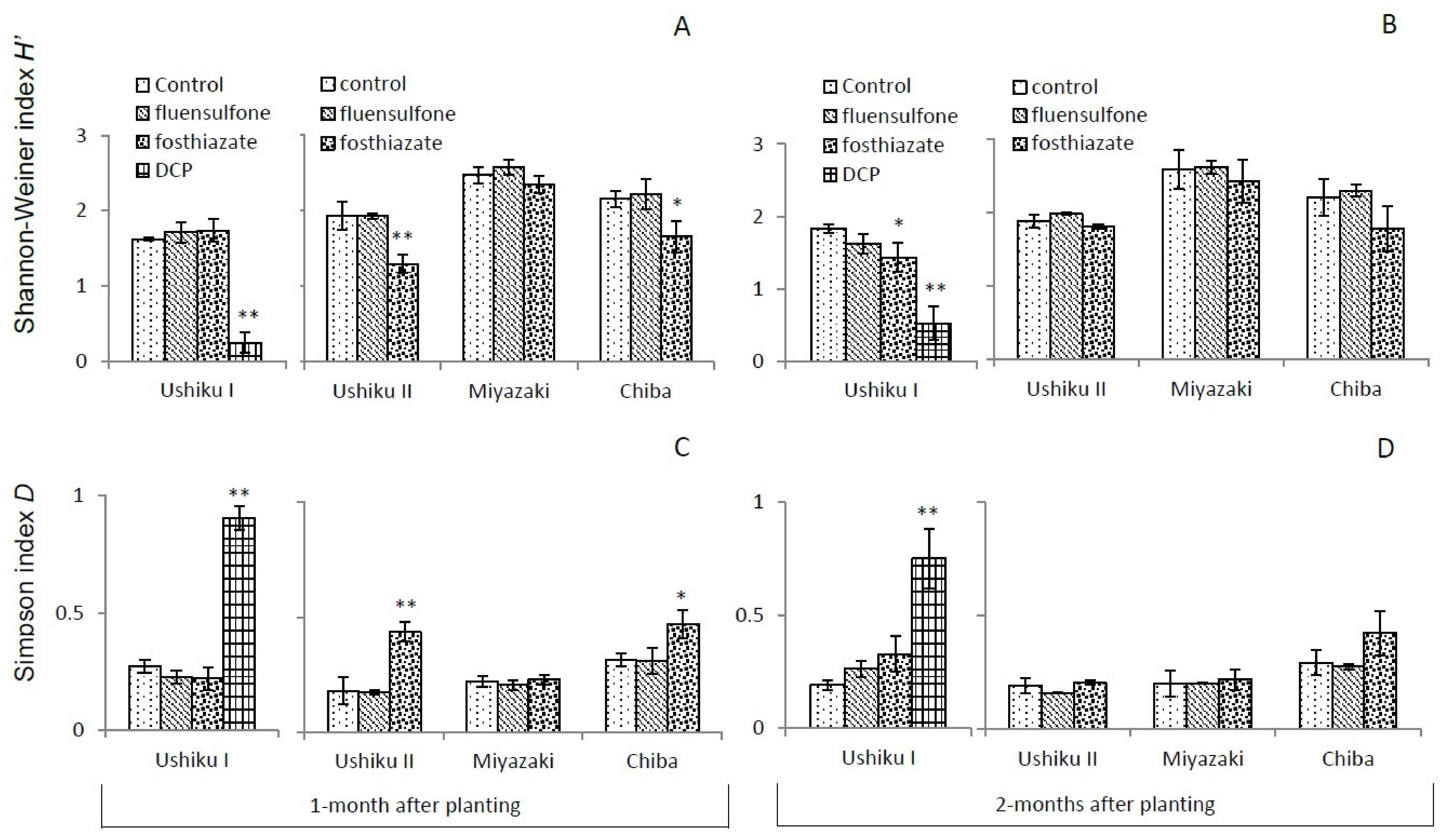
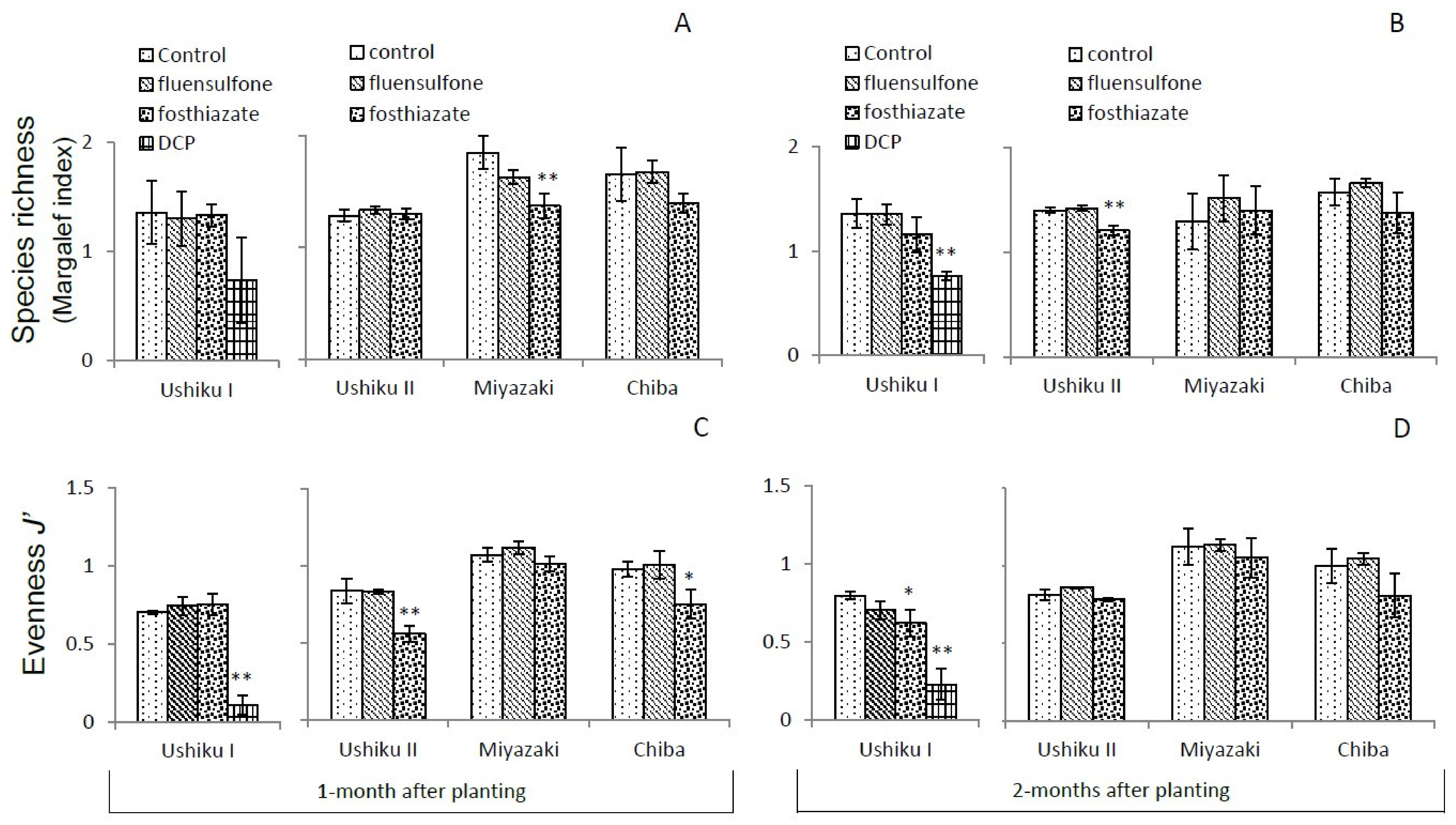


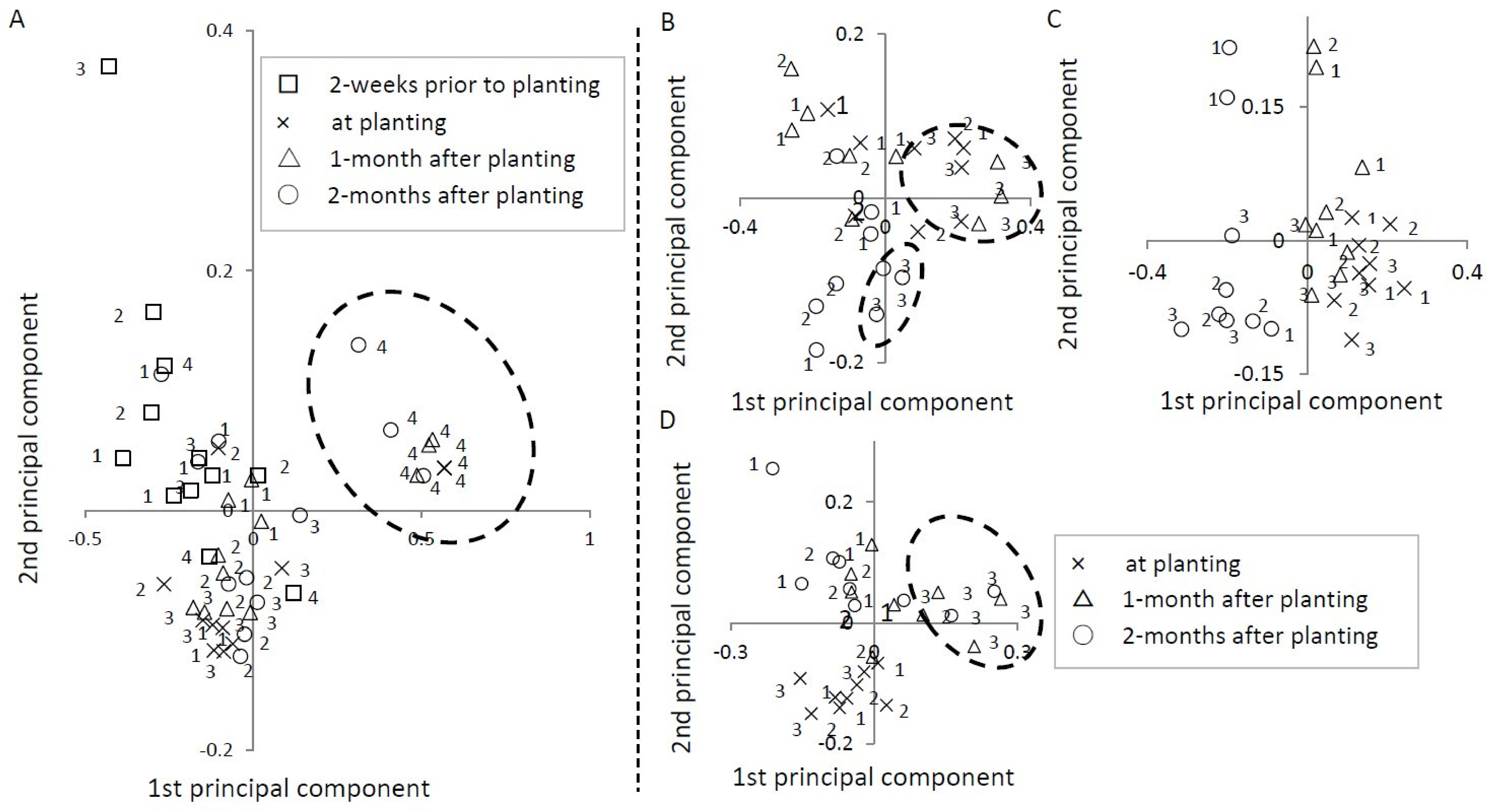
| Ushiku I | Ushiku II | Miyazaki | Chiba | |||||
|---|---|---|---|---|---|---|---|---|
| Principal Components | Principal Components | Principal Components | Principal Components | |||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| Component ratio | 0.81 | 0.09 | 0.69 | 0.16 | 0.52 | 0.20 | 0.52 | 0.34 |
| Cumulative component ratio | 0.81 | 0.91 | 0.69 | 0.86 | 0.52 | 0.72 | 0.52 | 0.86 |
| Loading factor | ||||||||
| Acrobeloides sp. | 1.00 | 0.03 | 1.00 | 0.05 | −0.50 | −0.41 | 0.98 | 0.17 |
| Other Cephalobidae | −0.64 | 0.76 | −0.44 | 0.19 | −0.68 | 0.06 | −0.45 | 0.89 |
| Rhabditis sp. | −0.74 | −0.42 | −0.28 | −0.84 | 0.99 | 0.04 | −0.39 | −0.77 |
| Other Rhabditidae | −0.61 | −0.24 | −0.27 | −0.54 | 0.00 | −0.80 | −0.29 | −0.30 |
| Other bacterivore | −0.59 | −0.41 | −0.67 | −0.51 | −0.47 | 0.18 | −0.28 | −0.63 |
| Aphelenchus sp. | −0.45 | −0.07 | −0.37 | 0.57 | 0.43 | −0.31 | −0.41 | −0.57 |
| Aphelenchoides sp. | −0.09 | −0.27 | −0.60 | 0.70 | 0.31 | 0.06 | −0.20 | −0.41 |
| Filenchus sp. | −0.30 | −0.08 | −0.53 | 0.52 | −0.53 | 0.39 | −0.32 | 0.01 |
| Ditylenchus sp. | −0.08 | −0.11 | 0.23 | 0.16 | −0.40 | 0.22 | - | - |
| Dorylaimida | −0.61 | −0.25 | −0.34 | −0.82 | −0.20 | 0.81 | −0.31 | −0.26 |
| Other | - | - | - | - | 0.36 | −0.13 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawanobe, M.; Toyota, K.; Fujita, T.; Hatta, D. Evaluation of Nematicidal Activity of Fluensulfone against Non-Target Free-Living Nematodes under Field Conditions. Agronomy 2019, 9, 853. https://doi.org/10.3390/agronomy9120853
Kawanobe M, Toyota K, Fujita T, Hatta D. Evaluation of Nematicidal Activity of Fluensulfone against Non-Target Free-Living Nematodes under Field Conditions. Agronomy. 2019; 9(12):853. https://doi.org/10.3390/agronomy9120853
Chicago/Turabian StyleKawanobe, Masanori, Koki Toyota, Tomonori Fujita, and Daisuke Hatta. 2019. "Evaluation of Nematicidal Activity of Fluensulfone against Non-Target Free-Living Nematodes under Field Conditions" Agronomy 9, no. 12: 853. https://doi.org/10.3390/agronomy9120853
APA StyleKawanobe, M., Toyota, K., Fujita, T., & Hatta, D. (2019). Evaluation of Nematicidal Activity of Fluensulfone against Non-Target Free-Living Nematodes under Field Conditions. Agronomy, 9(12), 853. https://doi.org/10.3390/agronomy9120853





